Enrichment of Geobacter on Anode Biofilms from Domestic Wastewater without Posing Anode Potential in Microbial Electrochemical Cells
Abstract
1. Introduction
2. Materials and Methods
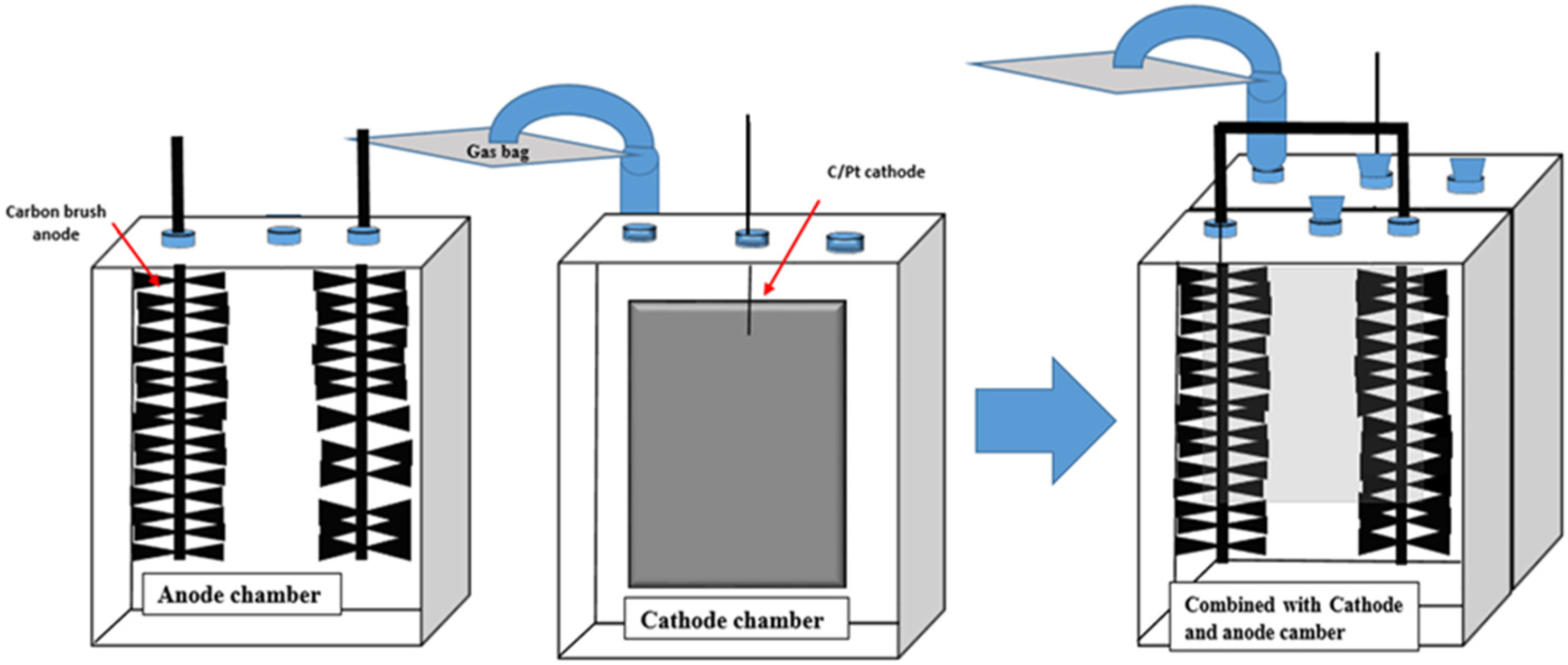
2.1. Reactor Configuration and Operation
2.2. Measurements and Analysis
2.3. Biofilm Community Analysis
3. Results and Discussions
3.1. Current Density and COD Removal
3.2. Hydrogen Production
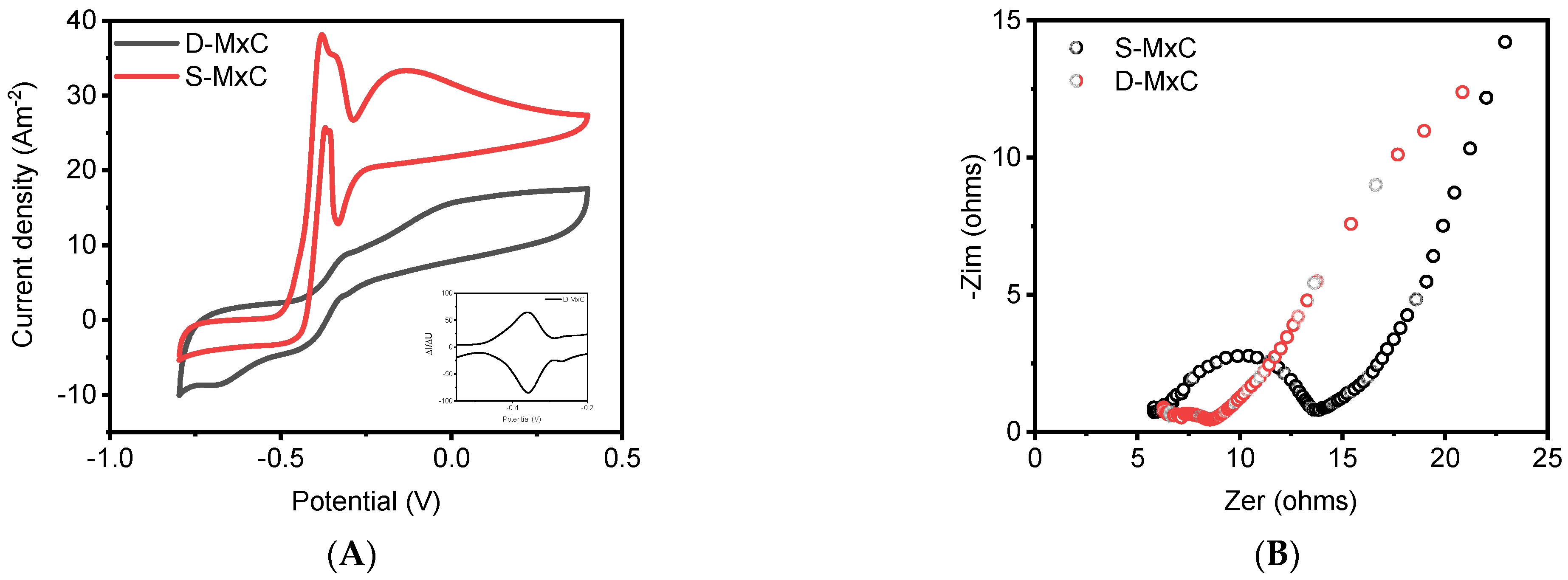
3.3. Electrochemical Analysis
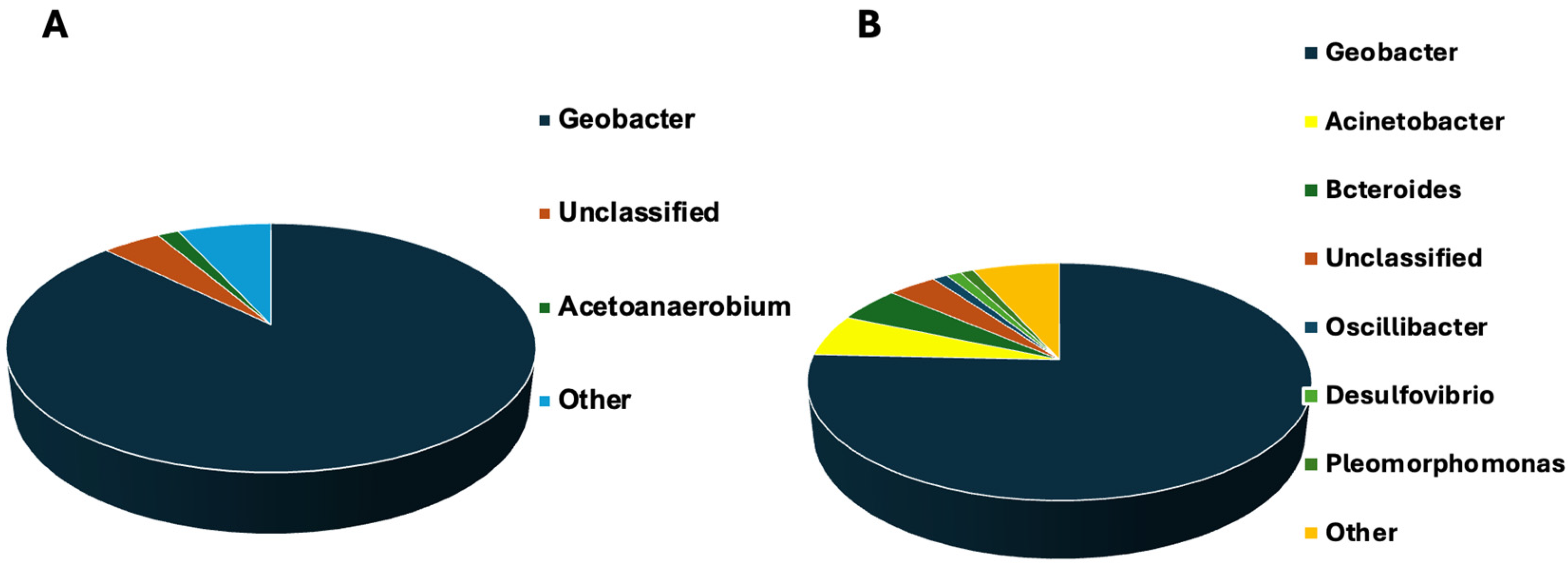
3.4. Microbial Community Analysis
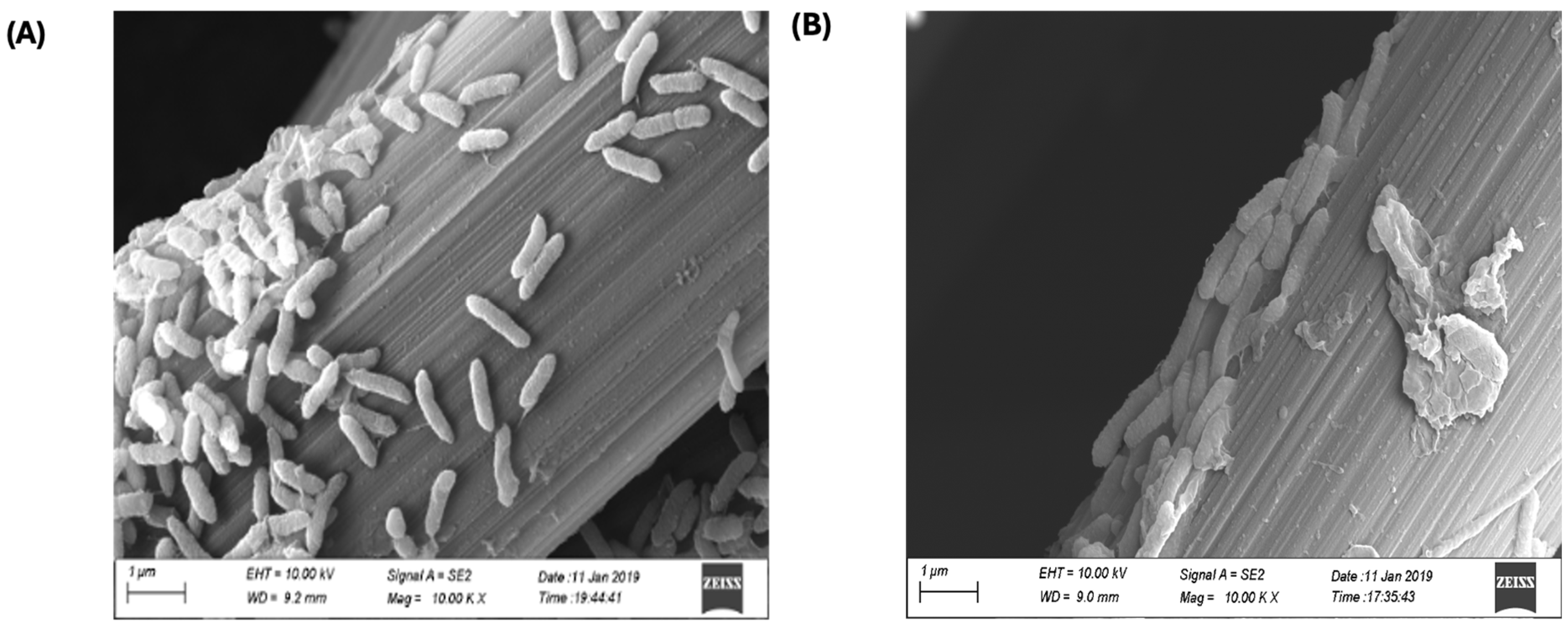
3.5. Anode Biofilm Imagining
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, P.; Wang, L.; Li, X.; Zhang, J.; Jiang, H. Geobacter grbiciae—A New Electron Donor in the Formation of Co-Cultures via Direct Interspecies Electron Transfer. Microbiol. Res. 2023, 14, 1774–1787. [Google Scholar] [CrossRef]
- Lee, H.S. Electrokinetic analyses in biofilm anodes: Ohmic conduction of extracellular electron transfer. Bioresour. Technol. 2018, 256, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Sarangi, P.K.; Vivekanandm, V.; Nidhi, P.; Khasim, B.S.; Sanjukta, S. Microbial fuel cells for waste nutrients minimization: Recent process technologies and inputs of electrochemical active microbial system. Microbiol. Res. 2022, 265, 127216. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Juan, A.Z.; Bian, Y.; Sun, D.; Yan, Y.; Chen, X.; Zhu, J.; Harold, D.M. Scale-up and techno-economic analysis of microbial electrolysis cells for hydrogen production from wastewater. Water Res. 2023, 241, 120139. [Google Scholar] [CrossRef]
- Mohammad, H.K.M.; Sepehr, D.; Seyed, A.M.R.; Ana, M.B. Optimal 4E evaluation of an innovative solar-wind cogeneration system for sustainable power and fresh water production based on integration of microbial desalination cell, humidification–dehumidification, and reverse osmosis desalination. Energy 2024, 297, 131256. [Google Scholar]
- Ao, T.; Zhao, X.; Muhammad, A.M.; Wang, N.; Zhu, H.; Liu, C.; Bai, F. A double-chamber microbial electrolysis cell improved the anaerobic digestion efficiency and elucidated the underlying bio-electrochemical mechanism. Chem. Eng. J. 2023, 471, 144228. [Google Scholar] [CrossRef]
- Oscar, G.; Juan, A.B.; Albert, G. Enhancing bioelectrochemical hydrogen production from industrial wastewater using Ni-foam cathodes in a microbial electrolysis cell pilot plant. Water Res. 2024, 256, 121616. [Google Scholar]
- He, K.; Li, W.; Tang, L.; Li, W.; Lv, S.; Xing, D. Suppressing Methane Production to Boost High-Purity Hydrogen Production in Microbial Electrolysis Cells. Environ. Sci. Technol. 2022, 56, 11931–11951. [Google Scholar] [CrossRef]
- Tahseena, N.; Ankit, K.; Anusha, V.; Nupur, S.; Soumya, P.; Pankaj, G.; Sokhee, P.J. Recent advances in biological approaches towards anode biofilm engineering for improvement of extracellular electron transfer in microbial fuel cells. Environ. Eng. Res. 2023, 28, 220666. [Google Scholar]
- Jiang, J.; He, P.C.; Luo, Y.; Peng, Z.; Jiang, Y.; Hu, Y.; Qi, L.; Dong, X.; Dong, Y.; Shi, L. The varied roles of pilA-N, omcE, omcS, omcT, and omcZ in extracellular electron transfer by Geobacter sulfurreducens. Front. Microbiol. 2023, 14, 1251346. [Google Scholar] [CrossRef]
- Hu, L.; Yang, Y.; Fu, Q.; Zhang, L.; Zhu, X.; Li, J.; Liao, Q. In Situ Probing the Mass Transport Property Inside an Imitated Three-Dimensional Porous Bioelectrode. Environ. Sci. Technol. 2023, 57, 6159–6168. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xia, X.; Nie, W.; Qin, B.; Hou, Y.; Lin, A.; Yao, S.; Zhuang, L. Bidirectional extracellular electron transfer pathways of Geobacter sulfurreducens biofilms: Molecular insights into extracellular polymeric substances. Environ. Res. 2024, 245, 118038. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.I.; Krajmalnik-Brown, R.; Parameswaran, P.; Marcus, A.K.; Wanger, G.; Gorby, Y.A.; Rittmann, B.E. Selecting Anode-Respiring Bacteria Based on Anode Potential: Phylogenetic, Electrochemical, and Microscopic Characterization. Environ. Sci. Technol. 2009, 43, 9519–9524. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Chen, Y.-C.; Yu, C.-P. Microbial community dynamics in electroactive biofilms across time under different applied anode potentials. Sustain. Environ. Res. 2022, 32, 19. [Google Scholar] [CrossRef]
- De La Fuente, M.J.; Daille, L.K.; De la Iglesia, R.; Walczak, M.; Armijo, F.; Pizarro, G.E.; Vargas, I.T. Electrochemical bacterial enrichment from natural seawater and its implications in biocorrosion of stainless-steel electrodes. Materials 2020, 13, 2327. [Google Scholar] [CrossRef]
- Lim, S.S.; Kim, B.H.; Li, D.; Feng, Y.; Daud, W.R.W.; Scott, K.; Yu, E.H. Effects of applied potential and reactants to hydrogen-producing biocathode in a microbial electrolysis cell. Front. Chem. 2018, 6, 318. [Google Scholar] [CrossRef]
- Szydlowski, L.; Sorokin, A.; Vasieva, O.; Fedorovich, V.; Goryanin, I. Evolutionary dynamics of microbial communities in bioelectrochemical systems. BioRxiv 2019, 13, 725580. [Google Scholar]
- Feng, Y.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of carbon fiber brush anodes for improving power generation in air–cathode microbial fuel cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]
- Dhar, B.R.; Ren, H.; Chae, J.; Lee, H.S. Recoverability of electrical conductivity of a Geobacter-enriched biofilm. J. Power Sources 2018, 402, 198–202. [Google Scholar] [CrossRef]
- Dhar, B.R.; Ryu, H.; Ren, H.; Domingo, J.W.S.; Chae, J.; Lee, H.S. High Biofilm Conductivity Maintained Despite Anode Potential Changes in a Geobacter-Enriched Biofilm. ChemSusChem 2016, 9, 3485–3502. [Google Scholar] [CrossRef]
- Lee, H.S.; Torres, C.I.; Rittmann, B.E. Effects of Substrate Diffusion and Anode Potential on Kinetic Parameters for Anode-Respiring Bacteria. Environ. Sci. Technol. 2009, 43, 7571–7577. [Google Scholar] [CrossRef] [PubMed]
- Dhar, B.R.; Park, J.H.; Park, H.D.; Lee, H.S. Hydrogen-based syntrophy in an electrically conductive biofilm anode. Chem. Eng. J. 2019, 359, 208–216. [Google Scholar] [CrossRef]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct Biological Conversion of Electrical Current into Methane by Electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
- Alrashed, W.; Lee, J.; Park, J.; Rittmann, B.E.; Tang, Y.; Neufeld, J.D.; Lee, H.S. Hypoxic Methane Oxidation Coupled to Denitrification in a Membrane Biofilm. Chem. Eng. J. 2018, 348, 745–753. [Google Scholar] [CrossRef]
- Lusk, B.G.; Colin, A.; Parameswaran, P.; Rittmann, B.E.; Torres, C.I. Simultaneous fermentation of cellulose and current production with an enriched mixed culture of thermophilic bacteria in a microbial electrolysis cell. Microb. Biotechnol. 2018, 11, 63–73. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. High hydrogen production rate of microbial electrolysis cell (MEC) with reduced electrode spacing. Bioresour. Technol. 2011, 102, 3571–3574. [Google Scholar] [CrossRef]
- Commault, A.S.; Lear, G.; Weld, R.J. Maintenance of Geobacter -dominated biofilms in microbial fuel cells treating synthetic wastewater. Bioelectrochemistry 2015, 106 Pt A, 150–158. [Google Scholar] [CrossRef]
- Chung, T.H.; Meshref, M.N.; Dhar, B.R. A review and roadmap for developing microbial electrochemical cell-based biosensors for recalcitrant environmental contaminants, emphasis on aromatic compounds. Chem. Eng. J. 2021, 424, 130245. [Google Scholar] [CrossRef]
- Escapa, A.; San-Martin, M.I.; Mateos, R.; Moran, A. Scaling-up of membraneless microbial electrolysis cells (MECs) for domestic wastewater treatment: Bottlenecks and limitations. Bioresour. Technol. 2015, 180, 72–78. [Google Scholar] [CrossRef]
- Mei, X.X.; Xing, D.F.; Yang, Y.; Liu, Q.; Zhou, H.H.; Guo, C.H.; Ren, N.Q. Adaptation of microbial community of the anode biofilm in microbial fuel cells to temperature. Bioelectrochemistry 2017, 117, 29–33. [Google Scholar] [CrossRef]
- Lu, L.; Hou, D.; Wang, X.; Jassby, D.; Ren, Z.J. Active H2 Harvesting Prevents Methanogenesis in Microbial Electrolysis Cells. Environ. Sci. Technol. Lett. 2016, 3, 286–290. [Google Scholar] [CrossRef]
- Lu, L.; Ren, N.; Zhao, X.; Wang, H.; Wu, D.; Xing, D. Hydrogen production, methanogen inhibition and microbial community structures in psychrophilic single-chamber microbial electrolysis cells. Energy Environ. Sci. 2011, 4, 1329–1336. [Google Scholar] [CrossRef]
- Sim, J.; An, J.; Elbeshbishy, E.; Ryu, H.; Lee, H.S. Characterization and optimization of cathodic conditions for H2O2 synthesis in microbial electrochemical cells. Bioresour. Technol. 2015, 195, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Ullery, M.L.; Logan, B.E. Anode acclimation methods and their impact on microbial electrolysis cells treating fermentation effluent. Int. J. Hydrog. Energy 2015, 40, 6782–6791. [Google Scholar] [CrossRef]
- Call, D.; Logan, B.E. Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ. Sci. Technol. 2008, 42, 3401. [Google Scholar] [CrossRef]
- Qian, X.L.; Reguera, G.; Mester, T.; Lovley, D.R. Evidence that OmcB and OmpB of Geobacter sulfurreducens are outer membrane surface proteins. FEMS Microbiol. Lett. 2007, 277, 21–27. [Google Scholar] [CrossRef]
- Fricke, K.; Harnisch, F.; Schröder, U. On the use of cyclic voltammetry for the study of anodic electron transfer in microbial fuel cells. Energy Environ. Sci. 2008, 1, 144–147. [Google Scholar] [CrossRef]
- Abdullah, A.; Akintunde, O.B.; Mishari, K.; Gordon, W.; Mohammad, A. Microbial community structure of anode electrodes in microbial fuel cells and microbial electrolysis cells. J. Water Process Eng. 2020, 34, 101140. [Google Scholar]


| Inoculum | Substrate (Applied Voltage) | Output | Acclimation Time for Steady-State Current/Power Density | Dominant Exoelectrogens (Geobacter’s Population) | References |
|---|---|---|---|---|---|
| Domestic wastewater | Acetate (air-cathode) | 894 ± 48.6 mW/m2 | 2 months | Geobacter (11.77%) | [30] |
| Domestic wastewater | Acetate (air-cathode) | 7.98 A/m2 | 15–17 d | Geobacter (73%) | [32] |
| MFC effluent | Acetate (posed anode potential −0.4 V) | 8 A/m2 | 2 weeks | -- | [33] |
| MFC effluent | Acetate (applied potential 0.7 V) | 3 A/m2 | 2 months | -- | [34] |
| Domestic wastewater | Acetate (applied voltage 0.7 V) | 0.45–0.55 A/m2 | 7–25 d | Geobacter dominated | [29] |
| Domestic Wastewater | Acetate | 4–10 A/m2 | 10–15 d | Geobacter (75–87%) | This study |
| MEC | Sequence Number | OTU Number | ACE | Chao1 |
|---|---|---|---|---|
| D-MxCs | 55413 | 277 | 436 | 1150 |
| S-MxCs | 57646 | 407 | 620 | 561 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, R.S.; He, W.; Liang, D.; Li, C.; Yu, Y.; Feng, Y. Enrichment of Geobacter on Anode Biofilms from Domestic Wastewater without Posing Anode Potential in Microbial Electrochemical Cells. Microbiol. Res. 2024, 15, 1859-1869. https://doi.org/10.3390/microbiolres15030124
Yadav RS, He W, Liang D, Li C, Yu Y, Feng Y. Enrichment of Geobacter on Anode Biofilms from Domestic Wastewater without Posing Anode Potential in Microbial Electrochemical Cells. Microbiology Research. 2024; 15(3):1859-1869. https://doi.org/10.3390/microbiolres15030124
Chicago/Turabian StyleYadav, Ravi Shankar, Weihua He, Dandan Liang, Chao Li, Yanling Yu, and Yujie Feng. 2024. "Enrichment of Geobacter on Anode Biofilms from Domestic Wastewater without Posing Anode Potential in Microbial Electrochemical Cells" Microbiology Research 15, no. 3: 1859-1869. https://doi.org/10.3390/microbiolres15030124
APA StyleYadav, R. S., He, W., Liang, D., Li, C., Yu, Y., & Feng, Y. (2024). Enrichment of Geobacter on Anode Biofilms from Domestic Wastewater without Posing Anode Potential in Microbial Electrochemical Cells. Microbiology Research, 15(3), 1859-1869. https://doi.org/10.3390/microbiolres15030124








