Factors Influencing Central Venous Catheter-Associated Bloodstream Infections in COVID-19 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Defining the Outcome of Catheter-Associated Bloodstream Infection
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Porto, A.P.M.; Borges, I.C.; Buss, L.; Machado, A.; Bassetti, B.R.; Cocentino, B.; Bicalho, C.S.; Carrilho, C.M.; Rodrigues, C.; Neto, E.A.S.; et al. Healthcare-associated infections on the intensive care unit in 21 Brazilian hospitals during the early months of the coronavirus disease 2019 (COVID-19) pandemic: An ecological study. Infect. Control Hosp. Epidemiol. 2023, 44, 284–290. [Google Scholar] [CrossRef]
- Kwon, J.H.; Nickel, K.B.; Reske, K.A.; Stwalley, D.; Dubberke, E.R.; Lyons, P.G.; Michelson, A.; McMullen, K.; Sahrmann, J.M.; Gandra, S.; et al. Risk factors for hospital-acquired infection during the SARS-CoV-2 pandemic. J. Hosp. Infect. 2023, 133, 8–14. [Google Scholar] [CrossRef]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast during an Emergency Response. JAMA 2020, 323, 1545–1546. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- Lai, C.-C.; Wang, C.-Y.; Hsueh, P.-R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Fernandes, G.H.; de Freitas, B.S.; de Barros, A.L.S.; Ferreira, T.R.L.; de Almeida, W.S.; Lima, V.P.; Ramos, M.M.F.; da Costa, A.B.F. Infecções de corrente sanguínea por bactérias gram negativas em pacientes internados na unidade de terapia intensiva de um hospital universitário em 2021 e 2022: Características epidemiológicas, tempo de ocorrência e desfecho da internação. Braz. J. Infect. Dis. 2023, 27, 102851. [Google Scholar] [CrossRef]
- Bento, L.F.; Fram, D.S.; Ferreira, D.B.; Tauffer, J.; Escudero, D.V.d.S.; Matias, L.d.O.; Medeiros, E.A.S. Impacto da pandemia de COVID-19 nas infecções de corrente sanguínea em unidades de terapia intensiva de um hospital universitário. Braz. J. Infect. Dis. 2022, 26, 101796. [Google Scholar] [CrossRef]
- De Francesco, M.A.; Signorini, L.; Piva, S.; Pellizzeri, S.; Fumarola, B.; Corbellini, S.; Piccinelli, G.; Simonetti, F.; Carta, V.; Mangeri, L.; et al. Bacterial and Fungal Superinfections Are Detected at Higher Frequency in Critically Ill Patients Affected by SARS-CoV-2 Infection than Negative Patients and Are Associated to a Worse Outcome. J. Med. Virol. 2023, 95, e28892. [Google Scholar] [CrossRef]
- Fernandes, T.P.; de Abreu, C.M.; Rocha, J.O.; Bianchetti, L.d.O.; Sales, L.d.A.; Alves, M.Q.; Prates, M.E.; Lemes, N.M.; Vieira, S.D.; Corrêa, M.I. Infecções secundárias em pacientes internados por COVID-19: Consequências e particularidades associadas. Rev. Eletrônica Acervo Científico 2021, 34, e8687. [Google Scholar] [CrossRef]
- Osme, S.; Almeida, A.; Lemes, M.; Barbosa, W.; Arantes, A.; Mendes-Rodrigues, C.; Filho, P.G.; Ribas, R. Costs of healthcare-associated infections to the Brazilian public Unified Health System in a tertiary-care teaching hospital: A matched case–control study. J. Hosp. Infect. 2020, 106, 303–310. [Google Scholar] [CrossRef]
- CDC/NHSN National Heathcare Safety Network. CDC. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-central Line Associated Bloodstream Infection) Table of Contents. 2021; pp. 1–50. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf (accessed on 27 January 2024).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Lapchik, M.S.; Carvalho, V.B.; Neubauer, I.W.; Souza, M.C.; Valente, M.G. Incidência de Infecções Primárias da Corrente Sanguínea em UTI Adulto Causados por Candida SPP em Hospitais Públicos e Privados no Município de São Paulo: Análise no Ano 2019 e Durante a Pandemia de COVID-19. Braz. J. Infect. Dis. 2022, 26, 102524. Available online: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-2007519 (accessed on 1 April 2024). [CrossRef]
- Freire, M.P.; de Assis, D.B.; Tavares, B.d.M.; Brito, V.O.; Marinho, I.; Lapchik, M.; Guedes, A.R.; Madalosso, G.; Oliveira, M.S.; de Lima, A.C.P.; et al. Impact of COVID-19 on healthcare-associated infections: Antimicrobial consumption does not follow antimicrobial resistance. Clinics 2023, 78, 100231. [Google Scholar] [CrossRef]
- Pérez-Granda, M.; Carrillo, C.; Rabadán, P.; Valerio, M.; Olmedo, M.; Muñoz, P.; Bouza, E. Increase in the frequency of catheter-related bloodstream infections during the COVID-19 pandemic: A plea for control. J. Hosp. Infect. 2022, 119, 149–154. [Google Scholar] [CrossRef]
- Erbay, K.; Ozger, H.S.; Tunccan, O.G.; Gaygısız, Ü.; Buyukkoruk, M.; Sultanova, F.; Yıldız, M.; Dündar, N.B.; Aydoğdu, M.; Bozdayi, G.; et al. Evaluation of prevalance and risk factors for bloodstream infection in severe coronavirus disease 2019 (COVID-19) patients. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e30. [Google Scholar] [CrossRef]
- Cava, E.; Neri, B.; Carbonelli, M.G.; Riso, S.; Carbone, S. Obesity pandemic during COVID-19 outbreak: Narrative review and future considerations. Clin. Nutr. 2021, 40, 1637–1643. [Google Scholar] [CrossRef]
- Fakih, M.G.; Bufalino, A.; Sturm, L.; Huang, R.-H.; Ottenbacher, A.; Saake, K.; Winegar, A.; Fogel, R.; Cacchione, J. Coronavirus disease 2019 (COVID-19) pandemic, central-line–associated bloodstream infection (CLABSI), and catheter-associated urinary tract infection (CAUTI): The urgent need to refocus on hardwiring prevention efforts. Infect. Control Hosp. Epidemiol. 2021, 43, 26–31. [Google Scholar] [CrossRef]
- Massart, N.; Maxime, V.; Fillatre, P.; Razazi, K.; Moine, P.; Legay, F.; Voiriot, G.; Amara, M.; Santi, F.; Nseir, S.; et al. Characteristics and prognosis of bloodstream infection in patients with COVID-19 admitted in the ICU: An ancillary study of the COVID-ICU study. Ann. Intensive Care 2021, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Li, G.M.; Xu, F.M.; Yin, X.; Wu, N.; Li, Y.M.; Zhang, T.M.; Chen, D.; Liu, K.; Qiu, Q. Lactic Dehydrogenase-Lymphocyte Ratio for Predicting Prognosis of Severe COVID-19. Medicine 2021, 100, e24441. [Google Scholar] [CrossRef]
- La Torre, F.P.F.; Baldanzi, G.; Troster, E.J. Risk Factors for Vascular Catheter-Related Bloodstream Infections in Pediatric Intensive Care Units. Rev. Bras. Ter. Intensive 2018, 30, 436–442. [Google Scholar] [CrossRef]
- Desiderio, M.d.M.; Neto, J.d.R.B.J.; Reis, F.B.; Romero, M.G.d.V.; Aguiar, M.F.d.C.; Pimentel, I.D.S.; Filho, D.F.d.F.; Cavalcante, A.C.O.; Cavalcante, G.O.; Santos, F.; et al. O Impacto da Pandemia por COVID-19 na Resistência Antimicrobiana Para os Gram Negativos em Ambiente Hospitalar. Braz. J. Infect. Dis. 2022, 26, 102257. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Germinario, B.N.; Ferrante, M.; Frangi, C.; Voti, R.L.; Muccini, C.; Ripa, M.; Canetti, D.; Castiglioni, B.; Oltolini, C.; et al. Candidemia in Coronavirus Disease 2019 (COVID-19) Patients: Incidence and Characteristics in a Prospective Cohort Compared With Historical Non–COVID-19 Controls. Clin. Infect. Dis. 2020, 73, e2838–e2839. [Google Scholar] [CrossRef]
- Cruz, A.B.; LeRose, J.; Evans, K.J.; Meyer, M.; Chopra, T. 291. Epidemiology of Candidemia Rates during COVID-19 and Comparison of Outcomes in Candidemia Between COVID-19 and Non-COVID-19 Patients. Open Forum Infect. Dis. 2021, 8, S253–S254. [Google Scholar] [CrossRef]
- WHO. World Health Organization. Corticosteroids for COVID-19: Living Guidance, 2 September 2020. iris.who.int. 2020. Available online: https://iris.who.int/handle/10665/334125 (accessed on 10 March 2024).
- Patel, A.; Emerick, M.; Cabunoc, M.K.; Williams, M.H.; Preas, M.A.; Schrank, G.; Rabinowitz, R.; Luethy, P.; Johnson, J.K.; Leekha, S. Rapid Spread and Control of Multidrug-Resistant Gram-Negative Bacteria in COVID-19 Patient Care Units. Emerg. Infect. Dis. 2021, 27, 1234–1237. [Google Scholar] [CrossRef]
- Nori, P.; Cowman, K.; Chen, V.; Bartash, R.; Szymczak, W.; Madaline, T.; Katiyar, C.P.; Jain, R.; Aldrich, M.; Weston, G.; et al. Bacterial and Fungal Coinfections in COVID-19 Patients Hospitalized during the New York City Pandemic Surge. Infect. Control Hosp. Epidemiol. 2020, 42, 84–88. [Google Scholar] [CrossRef]
- Hill, J.T.; Tran, K.-D.T.; Barton, K.L.; Labreche, M.J.; Sharp, S.E. Evaluation of the Nanosphere Verigene BC-GN Assay for Direct Identification of Gram-Negative Bacilli and Antibiotic Resistance Markers from Positive Blood Cultures and Potential Impact for More-Rapid Antibiotic Interventions. J. Clin. Microbiol. 2014, 52, 3805–3807. [Google Scholar] [CrossRef][Green Version]
- Osme, S.F.; Souza, J.; Osme, I.T.; Almeida, A.P.S.; Arantes, A.; Mendes-Rodrigues, C.; Filho, P.P.G.; Ribas, R.M. Financial impact of Healthcare-associated infections (HAI) on Intensive Care Units (ICUs) estimated for fifty Brazilian University Hospitals, affiliated to the Unified Health System (SUS). J. Hosp. Infect. 2021, 117, 96–102. [Google Scholar] [CrossRef]
- Szabó, B.G.; Czél, E.; Nagy, I.; Korózs, D.; Petrik, B.; Marosi, B.; Gáspár, Z.; Rajmon, M.; Di Giovanni, M.; Vályi-Nagy, I.; et al. Clinical and microbiological outcomes and follow-up of secondary bacterial and fungal infections among critically ill COVID-19 adult patients treated with and without immunomodulation: A prospective cohort study. Antibiotics 2023, 12, 1196. [Google Scholar] [CrossRef]
| Variables | N of Yes (% of Yes) [95% Confidence Interval] | p-Value | |
|---|---|---|---|
| CABSI Presence (n = 104) | CABSI Absence (n = 309) | ||
| Admitted from another service | 69 (66.35) [57.26–5.43] | 200 (64.72) [59.4–70.5] | 0.764 |
| Obesity presence | 45 (43.27) [33.75–57.79] | 99 (32.04) [26.84–37.24] | 0.040 |
| Systemic arterial hypertension presence | 60 (57.69) [48.2–67.19] | 155 (50.16) [44.59–55.74] | 0.183 |
| Diabetes mellitus presence | 35 (33.65) [24.57–42.74] | 89 (28.8) [23.75–33.85] | 0.354 |
| Cardiovascular disease presence | 14 (13.46) [6.9–20.02] | 34 (11) [7.51–14.49] | 0.505 |
| Chronic obstructive pulmonary disease presence | 10 (9.62) [3.95–15.28] | 32 (10.36) [6.96–13.75] | 0.828 |
| Chronic kidney disease presence | 11 (10.58) [4.67–16.49] | 24 (7.77) [4.78–10.75] | 0.384 |
| Etilism habit presence | 7 (6.73) [1.92–11.55] | 25 (8.09) [5.05–11.13] | 0.649 |
| Smoking habit presence | 22 (21.15) [13.3–29] | 65 (21.04) [16.49–25.58] | 0.980 |
| COVID-19 vaccine prior to hospital admission | 26 (25) [16.68–33.32] | 50 (16.18) [12.07–20.29] | 0.050 |
| Renal replacement therapy prior to CABSI | 40 (38.46) [29.11–47.81] | 142 (45.95) [40.4–51.51] | 0.181 |
| Hydrocortisone use prior to CABSI | 50 (48.08) [38.47–57.68] | 185 (59.87) [54.41–65.34] | 0.036 |
| Antibiotic use prior to CABSI | 83 (79.81) [72.09–87.52] | 253 (81.88) [77.58–86.17] | 0.642 |
| 3 or more antibiotics use prior to CABSI | 26 (25) [16.68–33.32] | 122 (39.48) [34.03–44.93] | 0.007 |
| Use of cephalosporin prior to CABSI | 19 (18.27) [10.84–25.7] | 56 (18.12) [13.83–22.42] | 0.973 |
| Use of carbapenem prior to CABSI | 41 (39.42) [30.03–48.82] | 153 (49.51) [43.94–55.09] | 0.073 |
| Use of antifungal prior to CABSI | 11 (10.58) [4.67–16.49] | 65 (21.04) [16.49–25.58] | 0.013 |
| Variable | Median (Quartile 1–Quartile 3) [n] | p-Value | |
|---|---|---|---|
| CABSI Presence | CABSI Absence | ||
| Age in years | 60 (45.75–68) [104] | 61 (49–71) [309] | 0.396 |
| Weight in Kg | 80 (71–95) [94] | 75.5 (68–87.2) [248] | 0.007 |
| Height in m | 1.68 (1.64–1.73) [101] | 1.67 (1.6–1.72) [256] | 0.782 |
| Body Mass Index in Kg/m2 | 28.03 (25.01–33.3) [93] | 26.99 (24.69–31.24) [235] | 0.008 |
| Total number of comorbidities | 1 (1–3) [104] | 1 (0–2) [309] | 0.070 |
| Simplified Acute Physiology Score | 57 (44.75–70) [104] | 59 (46–69) [309] | 0.417 |
| Simplified Acute Physiology Score prognosis in % | 32 (11.75–57.25) [104] | 34 (13–56.5) [309] | 0.362 |
| Days of central venous catheter use until diagnosis | 9 (6–14) [99] | 11 (6–20) [294] | 0.043 |
| Creatine in mg/dL | 1.06 (0.76–1.75) [104] | 1.1 (0.78–1.9) [308] | 0.945 |
| Albumin in mg/dL | 3.15 (2.73–3.44) [248] | 3.22 (2.63–3.52) [93] | 0.362 |
| Glutamic-Oxaloacetic Transaminase in U/L | 49.65 (39–71.85) [100] | 50.75 (33–86.48) [276] | 0.852 |
| Pyruvic Glutamic Transaminase in U/L | 38.9 (29.8–53.9) [99] | 39 (23–66.4) [277] | 0.819 |
| Lactate Dehydrogenase in U/L | 636 (519–850) [75] | 563 (423–800) [241] | 0.042 |
| Polymerase Chain Reaction in mg/dL | 13.61 (7.83–20.28) [101] | 12.77 (6.92–20.86) [293] | 0.654 |
| D-Dimer in mg/dL | 2149 (595.5–5845) [87] | 1928 (775.14–5897) [257] | 0.537 |
| Interleukin-6 in pg/dL | 74.55 (23.77–124.38) [72] | 86.22 (34.64–186) [217] | 0.114 |
| Prothrombin Activity Time in % | 100 (83.75–100) [98] | 100 (77–100) [295] | 0.262 |
| International Standardized Prothrombin Ratio | 1 (1–1.08) [98] | 1 (1–1.1) [293] | 0.334 |
| Significant Variables in the Association Tests | Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|---|
| Simple Model | Multiple Model | |||
| p-Value | Unadjusted | p-Value | Adjusted | |
| Obesity presence | 0.038 | 1.62 (1.03–2.55) | 0.003 | 2.39 (1.36–4.22) |
| COVID-19 vaccination prior to hospital admission | 0.046 | 1.73 (1.01–2.96) | 0.232 | 1.50 (0.77–2.91) |
| Hydrocortisone use prior to CABSI | 0.036 | 0.62 (0.40–0.97) | 0.585 | 0.85 (0.48–1.51) |
| 3 or more antibiotics use prior to CABSI | 0.008 | 0.51 (0.31–0.88) | 0.597 | 1.21 (0.60–2.42) |
| Use of antifungal prior to CABSI | 0.020 | 0.44 (0.22–0.88) | 0.242 | 0.56 (0.22–1.47) |
| Lactate Dehydrogenase in U/L | 0.940 | 1.00 (1.00–1.00) | 0.647 | 0.99 (0.9996–1.0003) |
| Days of central venous catheter use until diagnosis | 0.002 | 0.96 (0.94–0.99) | 0.019 | 0.95 (0.91–0.99) |
| Variable | Level | % (n) [95% Confidence Interval] |
|---|---|---|
| Isolated microorganism | Staphylococcus aureus | 13.76 (15) [7.29–20.23] |
| Enterococcus faecium | 3.67 (4) [0.14–7.2] | |
| Proteus mirabilis | 0.92 (1) [0–2.71] | |
| Acinetobacter baumanni | 15.6 (17) [8.78–22.41] | |
| Enterecoccus faecalis | 12.84 (14) [6.56–19.13] | |
| Klebisiela pneumoniae | 17.43 (19) [10.31–24.55] | |
| Pseudomonas aeruginosa | 5.5 (6) [1.22–9.79] | |
| Stenotropomonas maltophilia | 3.67 (4) [0.14–7.2] | |
| Burkholderia cepacia | 0.92 (1) [0–2.71] | |
| Serratia mascescens | 0.92 (1) [0–2.71] | |
| Escherichia coli | 0.92 (1) [0–2.71] | |
| Pasteurella sp. | 0.92 (1) [0–2.71] | |
| Klebisiella oxytoca | 0.92 (1) [0–2.71] | |
| Streptococcus viridans | 0.92 (1) [0–2.71] | |
| Candida peliculosa | 0.92 (1) [0–2.71] | |
| Candida tropicalis | 0.92 (1) [0–2.71] | |
| Candida albicans | 11.01 (12) [5.13–16.89] | |
| Candida utilis | 0.92 (1) [0–2.71] | |
| Candida glabrata | 0.92 (1) [0–2.71] | |
| Aspergillu sp. | 1.83 (2) [0–4.35] | |
| Geotrichum candidum | 0.92 (1) [0–2.71] | |
| Agent classification | Gram positive bacteria | 27.52 (30) [19.14–35.91] |
| Gram negative bacteria | 55.05 (60) [45.71–64.38] | |
| Fungi | 17.43 (19) [10.31–24.55] | |
| ESBL resistance mechanism | No | 96.63 (86) [92.88–100.38] |
| Yes | 3.37 (3) [0–7.12] | |
| Resistant to 3 or more antibiotics | No | 55.96 (61) [46.64–65.28] |
| Yes | 44.04 (48) [34.72–53.36] |
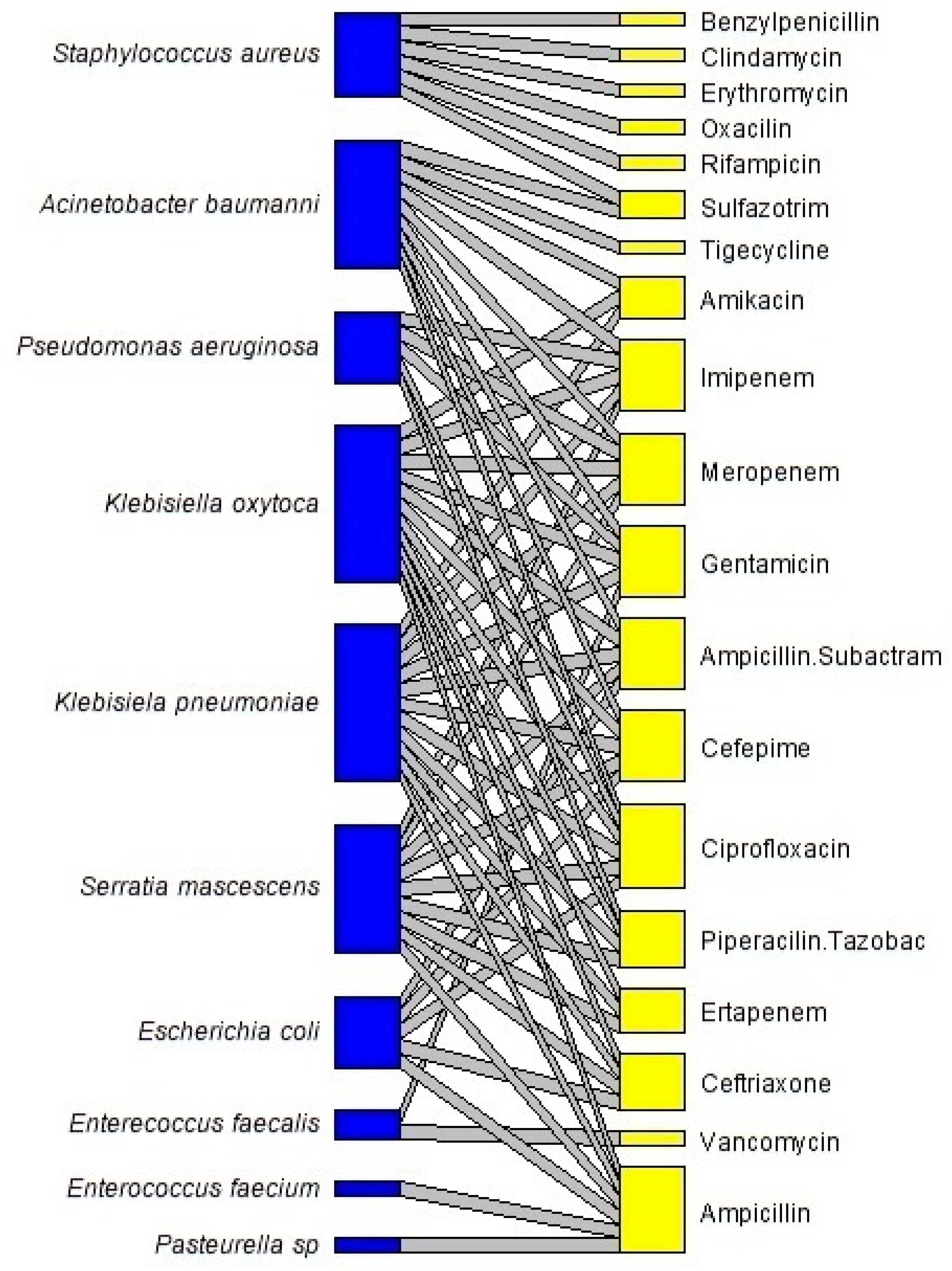
| Antibiotics | Microorganisms 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | |
| Amikacin | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Ampicillin | 0 | 0 | 4 | 1 | 18 | 1 | 1 | 0 | 3 | 0 |
| Ampicilin-Sulbactam | 14 | 0 | 0 | 1 | 16 | 1 | 0 | 0 | 3 | 0 |
| Benziylpenicillin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 |
| Cefepime | 14 | 0 | 0 | 1 | 15 | 1 | 0 | 0 | 2 | 0 |
| Ceftriaxone | 0 | 0 | 0 | 1 | 17 | 1 | 0 | 0 | 2 | 0 |
| Ciprofloxacin | 14 | 0 | 0 | 1 | 15 | 1 | 0 | 1 | 1 | 0 |
| Clindamycin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| Erythomicin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| Ertapenem | 0 | 0 | 0 | 0 | 14 | 1 | 0 | 0 | 1 | 0 |
| Gentamicin | 4 | 6 | 0 | 0 | 12 | 1 | 0 | 1 | 0 | 0 |
| Imipenem | 13 | 0 | 0 | 0 | 14 | 1 | 0 | 1 | 1 | 0 |
| Meropenem | 13 | 0 | 0 | 0 | 14 | 1 | 0 | 1 | 1 | 0 |
| Oxacilin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Piperacilin-Tazobac | 0 | 0 | 0 | 0 | 13 | 1 | 0 | 3 | 1 | 0 |
| Rifampicin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Sulfazotrim | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Tigecycline | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vancomycin | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, A.L.d.S.; Campos, T.; Mendes-Rodrigues, C.; Pedroso, R.d.S.; Röder, D.V.D.d.B. Factors Influencing Central Venous Catheter-Associated Bloodstream Infections in COVID-19 Patients. Microbiol. Res. 2024, 15, 1134-1143. https://doi.org/10.3390/microbiolres15030076
Neto ALdS, Campos T, Mendes-Rodrigues C, Pedroso RdS, Röder DVDdB. Factors Influencing Central Venous Catheter-Associated Bloodstream Infections in COVID-19 Patients. Microbiology Research. 2024; 15(3):1134-1143. https://doi.org/10.3390/microbiolres15030076
Chicago/Turabian StyleNeto, Adriana Lemos de Sousa, Thalita Campos, Clesnan Mendes-Rodrigues, Reginaldo dos Santos Pedroso, and Denise Von Dolinger de Brito Röder. 2024. "Factors Influencing Central Venous Catheter-Associated Bloodstream Infections in COVID-19 Patients" Microbiology Research 15, no. 3: 1134-1143. https://doi.org/10.3390/microbiolres15030076
APA StyleNeto, A. L. d. S., Campos, T., Mendes-Rodrigues, C., Pedroso, R. d. S., & Röder, D. V. D. d. B. (2024). Factors Influencing Central Venous Catheter-Associated Bloodstream Infections in COVID-19 Patients. Microbiology Research, 15(3), 1134-1143. https://doi.org/10.3390/microbiolres15030076






