Abstract
Background: IL-26 has demonstrated antimicrobial properties, as well as in the degradation of DNA from the Lyme disease spirochete Borrelia burgdorferi (Bb). Additionally, IL-26 can promote macrophage activation and enhance Bb phagocytotic activity. It is unclear if cell-mediated immune responses are modulated through TLR9 signaling when exposed to IL-26 Bb DNA complexes in post-treatment Lyme disease syndrome (PTLDS). Objective: We here aim to explore the effect of IL-26 in human Toll-like receptor (TLR)-9’s activation upon the recognition of Bb DNA. Methods: We utilized a single-receptor cell system, HEK-Dual™ hTLR9 cells, which harbors two reporter plasmids for the NF-κB and IL-8 signaling pathways. Bb DNA was exposed to increasing concentrations of IL-26 in monomeric or dimeric form and then used to stimulate the cells for 4 h. The TLR-9 ligand CpG was used as a control. Results: We observed that NF-κB and IL-8 activation was maximal when the cells were stimulated with Bb DNA that had been treated with 5 µM of IL-26 monomer and 1 µM of IL-26 dimer. The same was observed for IL-8 activation upon CpG stimulation. We observed, however, a decrease in NF-κB activation when treated with either form of IL-26. An NF-κB activation increase did not occur with IL-26-treated TLR9 ligand CpG. Conclusions: Our study shows an enhancement in NF-κB and IL-8 activation upon the recognition of IL-26-treated Bb DNA by TLR9, which suggests an increase in sensing by the TLR9 of Bb DNA when it is in the form of an IL-26-Bb DNA complex. These findings will prompt further studies on the interaction between IL-26 and Bb DNA.
1. Introduction
Caused by the spirochete Borrelia burgdorferi, Lyme disease (LD) continues to be the most commonly transmitted tick-borne infection in the United States [1]. Antibiotic treatment is most effective in the early stages of the disease, and those with good tissue penetration such as ceftriaxone are able to destroy the spirochetes [2]. The concept of post-Lyme disease syndrome comprises rheumatological, neurological, or other late manifestations that remain refractory to prolonged antibiotic therapy [3]. This occurs in a fraction of patients and presents with chronic nonspecific symptoms that do not respond to antibiotics [1].
Various theories try to explain the chronicity of symptomatology in LD patients even after antibiotic therapy. One of these theories is the persistence of B. burgdorferi DNA in tissues [4], which can remain after antibiotic treatment as antibiotics do not target bacterial DNA [2,5]. It appears that only B. burgdorferi genetic material, either DNA or RNA, antigen, or non-culturable spirochetes have been detected following the antibiotic treatment of an established infection [2,6], somewhat proving the effectiveness of antibiotic therapy against the spirochetes. Nevertheless, a report post-mortem has shown that B. burgdorferi DNA can be found in almost every organ, along with biofilm-forming B. burgdorferi [7].
The modes of action of antibiotics are diverse, but none of them can directly target bacterial DNA per se. Antimicrobial peptides (AMPs), on the other hand, exhibit diverse modes of action, which include binding to bacterial nucleic acids [8]. IL-26 is an antimicrobial protein that can bind and form a complex with microbial DNA. Specifically, it has been reported to interact with DNA from Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa [9].
IL-26 is a human antimicrobial peptide which has a proven ability to kill various intracellular [10] and extracellular bacteria [9,11,12]. We previously showed that IL-26 has an antimicrobial effect on the control of B. burgdorferi growth in vitro, as well as being capable of degrading B. burgdorferi DNA following spirochetal membrane degradation [12]. It also has an effect on the immune system of the human host, enhancing the anti-borrelial response of human macrophages.
The innate immune system has pattern recognition receptors (PRRs) that can elicit a rapid response to microbial pathogens. In the case of bacterial infections, bacterial DNA acts as a pathogen-associated molecular pattern (PAMP) and is recognized by endosomal receptor human Toll-like receptor 9 (TLR-9), inducing an inflammatory response [13]. It has been shown that IL-26 combined with bacterial DNA promotes the better sensing and production of Type I IFNs by dendritic cells [9], as Type I IFNs’ secretion can be induced upon the recognition of foreign DNA in the endosome by TLR-9 [14,15]. Given that B. burgdor-feri DNA will remain in tissues, it can be hypothesized that bacterial DNA could be bound by the TLR9 receptor to induce an inflammatory response to the DNA [4].
In this study, we decided to explore the effect of the IL-26 in TLR-9’s activation upon the recognition of B. burgdorferi DNA. We need to better understand the mechanisms of B. burgdorferi DNA sensing, as this could create a basic understanding for the future development of TLR-targeted immune treatments for Lyme disease and patients suffering from chronic inflammatory symptoms.
2. Materials and Methods
Since the PRR for bacterial DNA recognition is human TLR-9, we utilized a single-receptor cell system, HEK-Dual™ hTLR9 cells (Invivogen, San Diego, CA, USA), which harbors two reporter plasmids for the NF-κB and IL-8 signaling pathways. The system relies on the fact that the human embryonic kidney (HEK) cells, being undifferentiated, lack major receptors [16], so they will only express the TLR9 receptor upon transfection.
The B. burgdorferi strain B31, clone 5A2 (Bei Resources, Manassas, VA, USA), was cultured in 0.05× Revised Barbour-Stoenner-Kelly (BSK) medium (Sigma-Aldrich, St. Louis, MO, USA). The spirochetes were initially cultured at room temperature and then shifted to 37 °C to replicate the temperature changes that occur during transmission. Once spirochetes reached a growing plateau, they were centrifuged, and the B. burgdorferi bacterial pellet was used for DNA extraction using the HigherPurity™ Bacterial DNA Isolation Kit (Canvax, Valladolid, Spain). This purified DNA was then exposed to increasing concentrations of IL-26 in monomeric or dimeric forms (R&D Systems, Minneapolis, MN, USA). The B. burgdorferi DNA (250 ng) + IL-26 complex was then used to stimulate the HEK-TLR9 cells for 4 h. The TLR-9 ligand CpG (ODN 2006) (Invivogen), at a concentration of 1 ng/uL, was used as a control.
Upon HEK-TLR9 cell stimulation, the amounts of NF-kB and IL-8 activation were measured using an enzymatic (secretory embryonic alkaline phosphatase) or a luciferase reporter system, respectively. Reactions were conducted according to manufacturer’s instructions.
Statistical Analysis
A response ratio, compared to unstimulated conditions, was calculated and used for activation comparisons. The means of a minimum of three experiments were compared using the t-test, if the data followed a normal distribution, or its equivalent non-parametric test. GraphPad 9 (Prism, Folsom, CA, USA) was used for analysis.
3. Results
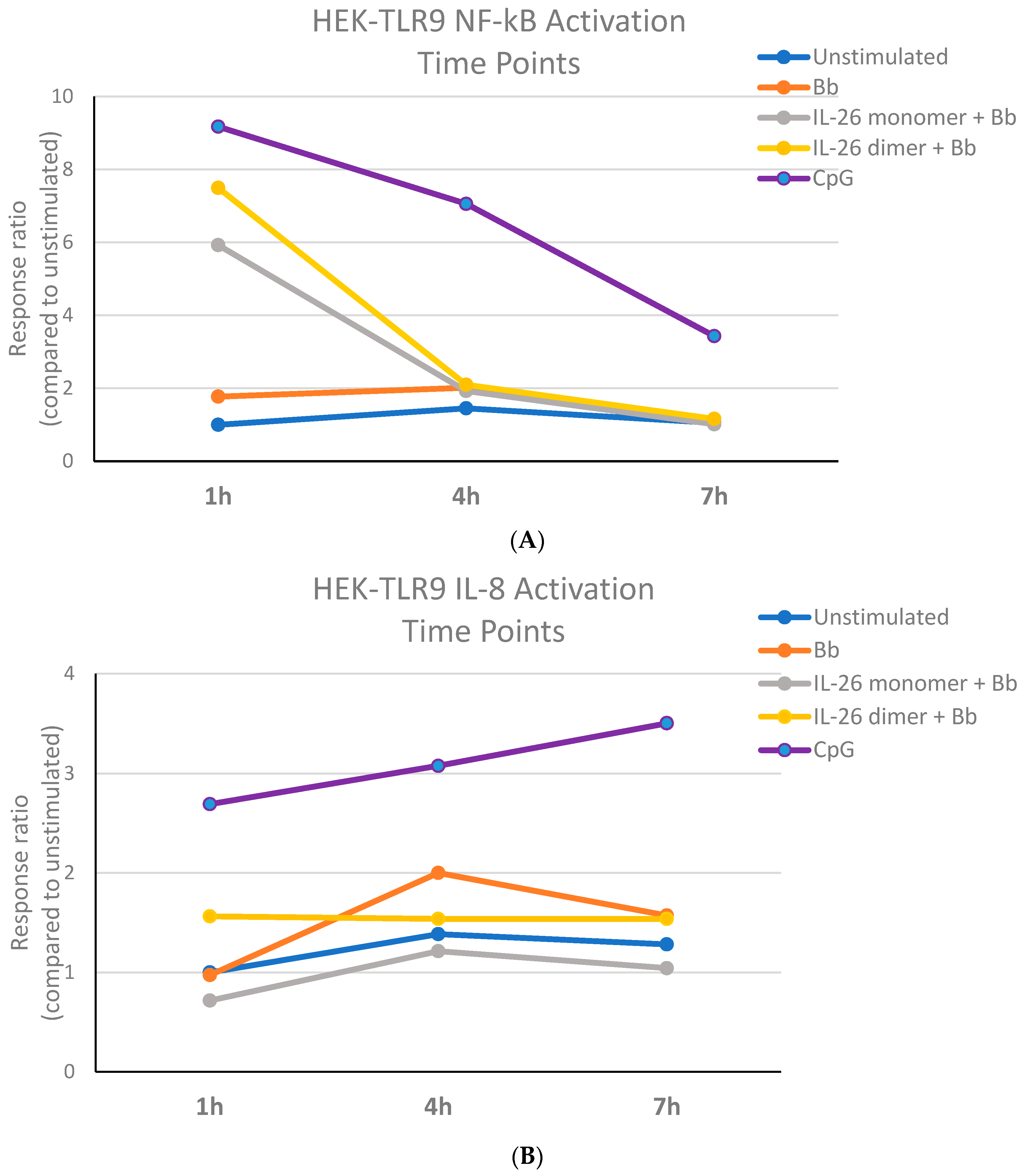
We first aimed to establish the B. burgdorferi DNA stimulation conditions of the HEK-TLR-9 system. Although CpG stimulation led to an increase in IL-8 activation over time, the recognition of B. burgdorferi DNA by TLR-9 caused maximal IL-8 activation at 4 h. At this time point, B. burgdorferi DNA’s stimulation of TLR-9 led to detectable NF-κB activation that was consistent throughout the observed period (Figure 1). We then decided that HEK-TLR-9 cell stimulation with 250 ng of B. burgdorferi DNA for 4 h appeared to be a good time point for the evaluation of NF-kB and IL-8 activation. It is important to note that B. burgdorferi DNA exposed to the IL-26 dimer for one hour increased IL-8 activation to a level higher than the IL-26 monomer or B. burgdorferi DNA alone (Figure 1B). IL-8’s activation by CpG seemed to increase over time, while the opposite occurred with NF-κB’s activation, which appeared to decrease.
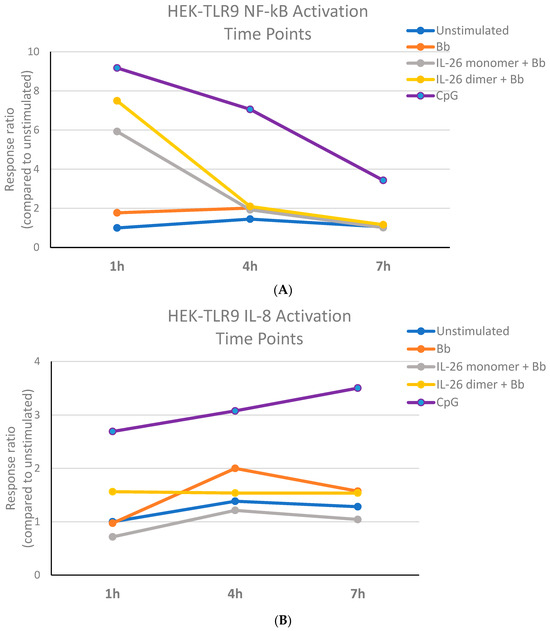
Figure 1.
B. burgdorferi DNA stimulation activates human TLR9. The Bb DNA stimulation of the HEK-TLR9 cell system bearing a plasmid reporter for (A) NF-κB or (B) IL-8.
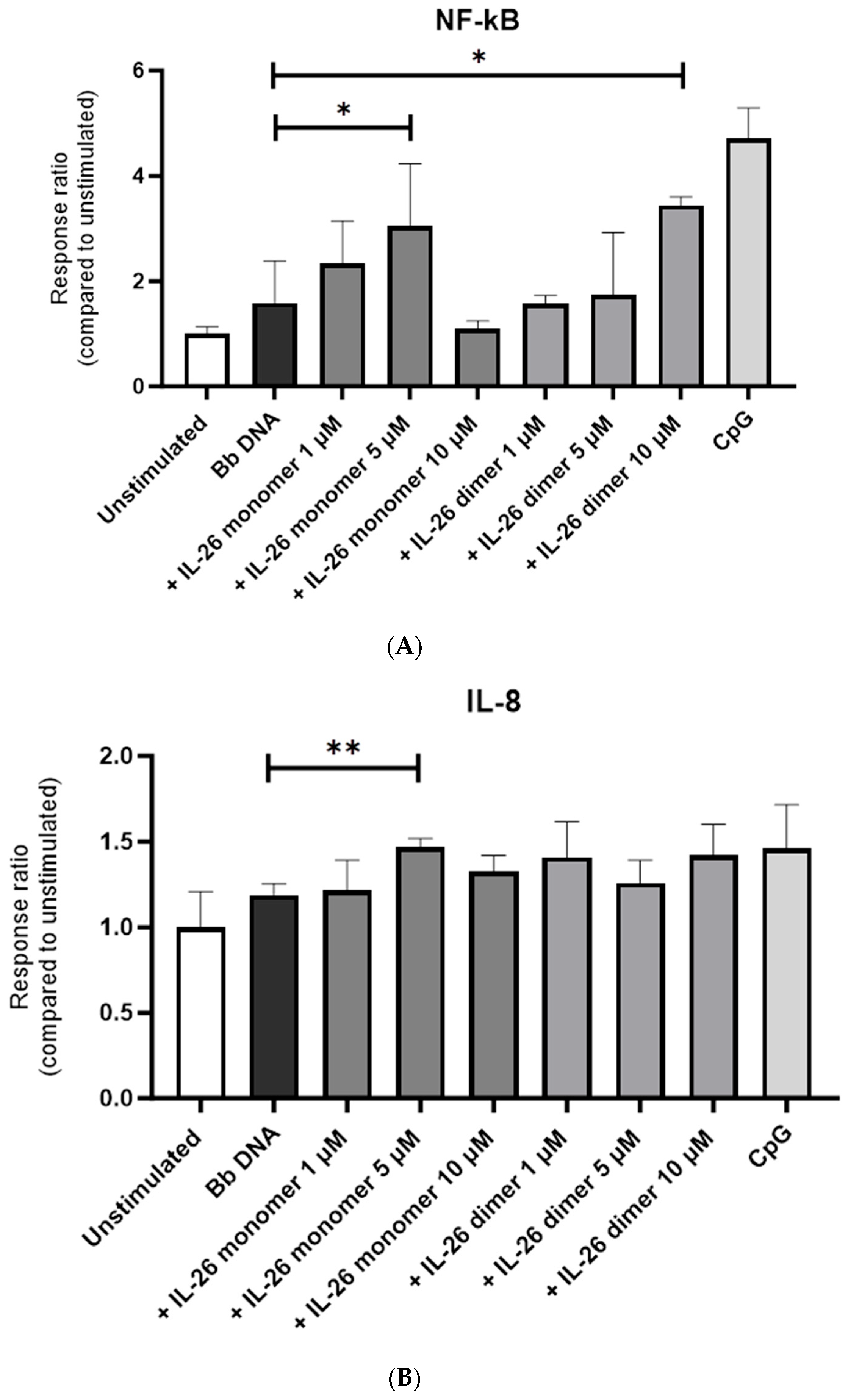
We then treated B. burgdorferi DNA with 1, 5, or 10 µM of either IL-26 monomer or IL-26 dimer for 4 h. We observed that NF-κB’s activation was maximal when the cells were stimulated with B. burgdorferi DNA that had been treated with 5 µM of IL-26 monomer and 1 µM of IL-26 dimer (Figure 2A). The same was observed for IL-8’s activation (Figure 2B).
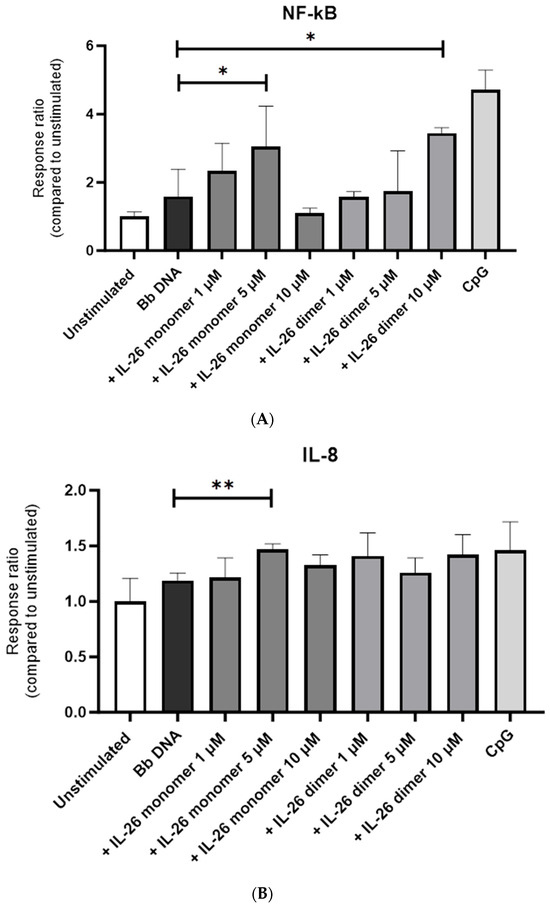
Figure 2.
Effect of IL-26 monomer and dimer on B. burgdorferi DNA recognition by human TLR9. HEK-TLR9 cells were stimulated with 1, 5, or 10 µM of either IL-26 monomer or IL-26 dimer for 4 h. (A) NF-κB and (B) IL-8 response to Bb DNA IL-26 complex upon 4 h stimulation. * p < 0.05 t-test. ** p < 0.005 t-test.
4. Discussion
IL-26 is a secreted protein that may be functional either as a monomer or homodimer [17]. IL-26 monomer and dimer had different optimal concentrations for increasing the cell response upon the recognition of B. burgdorferi DNA by TLR-9. Our findings show an enhancement in NF-κB activation upon the recognition of IL-26-treated B. burgdorferi DNA by TLR-9 as soon as one hour post-stimulation. This was also the case for IL-8 activation when B. burgdorferi DNA was exposed to the IL-26 dimer. Although we considered the possibility that IL-26 could be degrading the B. burgdorferi DNA to an unrecognizable point at higher concentrations, leaving an insufficient amount of DNA to effectively stimulate TLR-9, we dismissed such a scenario as the NF-κB’s activation rising again after using 10 uM of IL-26 dimer.
A lower response upon B. burgdorferi DNA’s recognition by TLR-9, compared to that in the CpG group, could be due to the fact that the B. burgdorferi genome has a relatively low content of cytosine and guanine [18]. Human TLR9’s responsiveness to bacterial DNA relies on specific CpG motif recognition [13]. These findings shall prompt further studies on the interaction between IL-26 and B. burgdorferi DNA.
We previously demonstrated that IL-26 (monomer and dimer) had not only the potential to control B. burgdorferi growth in vitro, but that it also enhanced the anti-borrelial response of human macrophages [12]. Alveolar macrophages can also increase their secretion of IL-8 upon IL-26 treatment [19]. Macrophages, however, do not appear to produce an inflammatory response to B. burgdorferi DNA [12] as they do not express TLR-9 [20]. It will require a cell type with a considerable expression of TLR-9 to mediate this recognition. TLR-9 is a receptor that is highly expressed on antigen-presenting cells such as dendritic cells [20,21]. IL-26-bacterial DNA complexes, in fact, promote better sensing by plasmacytoid dendritic cells, increasing production of Type I interferons. In this case, IL-26–bacterial DNA complexes are endocytosed and bind to endosomal TLR-9 to activate common cell immune response mechanisms [9]. Unlike their role in viral infections, Type I interferons may play a detrimental role in bacterial infections [22,23]. In the case of Lyme disease, a Type I interferon is associated with the early stages of a disseminated B. burgdorferi infection [24]. The ability of dendritic cells to produce Type I interferons is based on their expression of members of the interferon regulatory factor (IRF) family [25]. Although NF-κB can upregulate other transcription factors, including the IRF family, there is cross-regulation between these two main inflammatory pathways [26,27]. NF-κB’s activation may also translate into a decrease in IRF functions [28,29,30,31].
T cell responses can also be modulated by TLR-9 [32,33], which would explain the IL-26 overexpression in activated or transformed T cells [34]. Infiltrating CD3+ T lymphocytes were present next to B. burgdorferi biofilms, along with B. burgdorferi DNA, in post-mortem tissues from a patient with chronic illness [7].
IL-26 can bind DNA released from damaged cells and, as a carrier molecule for extracellular DNA, link DNA to inflammation processes [34]. B. burgdorferi DNA persistent in tissues could be bound by the TLR-9 receptor to induce an inflammatory response even after antibiotic treatment [4]. This activation should occur from the phagosome of the cell in which this TLR-9 recognition occurs. IL-26, for example, can bind directly to intracellular organisms like mycobacteria in the phagosome of macrophages, reducing bacteria’s viability in this organelle [10].
If IL-26 binds B. burgdorferi DNA in the same way it does for that of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa [9], then there may be an interplay between IL-26 and the TLR-9 receptor, opening up a potential pathway towards the development of additional treatments for patients with persistent inflammatory symptoms. IL-26 may act both as a driver and an effector of inflammation, leading to the establishment of a deleterious amplification loop and, ultimately, sustained chronic inflammation.
Furthermore, the persistence of B. burgdorferi DNA may be facilitated by the formation of biofilm, which protects the spirochete both from antibiotics and from the immune system [35]. In fact, biofilms do contain extracellular DNA and may protect bacterial DNA [36]. Recent developments in the identification of Borrelia using phages, part of the spirochete’s own genetic material [37], and in the identification of spirochetal-derived peptides in urine through mass spectrometry [38] in patients with PTLDS [39], will help us in our quest for more precise and selective approaches and targeted treatments. A human antimicrobial peptide able to attach to bacterial DNA and modulate inflammatory responses, such as IL-26, could become a target of therapy that may have an impact on patients suffering from persistent clinical Lyme disease symptoms.
Author Contributions
Conceptualization, J.C.; Methodology, A.T., C.G., K.A., J.B. and J.C.; Formal Analysis, A.T., K.A. and J.C.; Investigation, A.T., C.G. and K.A.; Data Curation, A.T., K.A. and J.C.; Writing—Original Draft Preparation, A.T., K.A. and J.C.; Writing—Review and Editing, A.T., J.B. and J.C.; Supervision, J.C.; Project Administration, J.B. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Skar, G.L.; Simonsen, K.A. Lyme Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bockenstedt, L.K.; Mao, J.; Hodzic, E.; Barthold, S.W.; Fish, D. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J. Infect. Dis. 2002, 186, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, V.K. Lyme Disease: An Overview. Indian Dermatol. Online J. 2023, 14, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, J. Doctor says you are cured, but you still feel the pain. Borrelia DNA persistence in Lyme disease. Microbes Infect. 2017, 19, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Telford, S.R., 3rd; Turk, S.P.; Chung, E.; Williams, C.; Dardick, K.; Krause, P.J.; Brandeburg, C.; Crowder, C.D.; Carolan, H.E.; et al. Xenodiagnosis to detect Borrelia burgdorferi infection: A first-in-human study. Clin. Infect. Dis. 2014, 58, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Bobe, J.R.; Jutras, B.L.; Horn, E.J.; Embers, M.E.; Bailey, A.; Moritz, R.L.; Zhang, Y.; Soloski, M.J.; Ostfeld, R.S.; Marconi, R.T.; et al. Recent Progress in Lyme Disease and Remaining Challenges. Front. Med. 2021, 8, 666554. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Kasliwala, R.S.; Ismail, H.; Torres, J.P.; Oldakowski, M.; Markland, S.; Gaur, G.; Melillo, A.; Eisendle, K.; Liegner, K.B.; et al. The Long-Term Persistence of Borrelia burgdorferi Antigens and DNA in the Tissues of a Patient with Lyme Disease. Antibiotics 2019, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Meller, S.; Di Domizio, J.; Voo, K.S.; Friedrich, H.C.; Chamilos, G.; Ganguly, D.; Conrad, C.; Gregorio, J.; Le Roy, D.; Roger, T.; et al. TH17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nat. Immunol. 2015, 16, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.T.; Teles, R.M.; Weiss, D.I.; Parvatiyar, K.; Sarno, E.N.; Ochoa, M.T.; Cheng, G.; Gilliet, M.; Bloom, B.R.; Modlin, R.L. IL-26 contributes to host defense against intracellular bacteria. J. Clin. Investig. 2019, 129, 1926–1939. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.T.; Maschkowitz, G.; Podschun, R.; Fickenscher, H. The Kinocidin Interleukin-26 Shows Immediate Antimicrobial Effects Even to Multi-resistant Isolates. Front. Microbiol. 2021, 12, 757215. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, J.; Kositangool, P.; Shah, A.; Radwan, Y.; Padilla, D.; Barragan, J.; Cervantes, J. IL-26 mediated human cell activation and antimicrobial activity against Borrelia burgdorferi. Curr. Res. Microb. Sci. 2020, 1, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Kirschning, C.J.; Hacker, H.; Redecke, V.; Hausmann, S.; Akira, S.; Wagner, H.; Lipford, G.B. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 2001, 98, 9237–9242. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Sasai, M.; Iwasaki, A. Toll-like receptor 9 trafficking and signaling for type I interferons requires PIKfyve activity. Int. Immunol. 2015, 27, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Han, J.; Li, H.; Zhang, X.; Liu, L.L.; Chen, F.; Zeng, B. Human Embryonic Kidney 293 Cells: A Vehicle for Biopharmaceutical Manufacturing, Structural Biology, and Electrophysiology. Cells Tissues Organs 2018, 205, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.P.; Sheikh, F.; Dickensheets, H.; Savan, R.; Young, H.A.; Walter, M.R. Interleukin-26: An IL-10-related cytokine produced by Th17 cells. Cytokine Growth Factor Rev. 2010, 21, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Brisson, D.; Drecktrah, D.; Eggers, C.H.; Samuels, D.S. Genetics of Borrelia burgdorferi. Annu. Rev. Genet. 2012, 46, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Che, K.F.; Paulsson, M.; Piersiala, K.; Sax, J.; Mboob, I.; Rahman, M.; Rekha, R.S.; Safholm, J.; Adner, M.; Bergman, P.; et al. Complex Involvement of Interleukin-26 in Bacterial Lung Infection. Front. Immunol. 2021, 12, 761317. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdorfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef] [PubMed]
- Karapetyan, L.; Luke, J.J.; Davar, D. Toll-Like Receptor 9 Agonists in Cancer. Onco Targets Ther. 2020, 13, 10039–10060. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Boxx, G.M.; Cheng, G. The Roles of Type I Interferon in Bacterial Infection. Cell Host Microbe 2016, 19, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Petzke, M.M.; Iyer, R.; Love, A.C.; Spieler, Z.; Brooks, A.; Schwartz, I. Borrelia burgdorferi induces a type I interferon response during early stages of disseminated infection in mice. BMC Microbiol. 2016, 16, 29. [Google Scholar] [CrossRef]
- Lind, N.A.; Rael, V.E.; Pestal, K.; Liu, B.; Barton, G.M. Regulation of the nucleic acid-sensing Toll-like receptors. Nat. Rev. Immunol. 2022, 22, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Iwanaszko, M.; Kimmel, M. NF-kappaB and IRF pathways: Cross-regulation on target genes promoter level. BMC Genom. 2015, 16, 307. [Google Scholar] [CrossRef] [PubMed]
- Kusiak, A.; Brady, G. Bifurcation of signalling in human innate immune pathways to NF-kB and IRF family activation. Biochem. Pharmacol. 2022, 205, 115246. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, L.M. The role of nuclear factor kappaB in the interferon response. J. Interferon Cytokine Res. 2011, 31, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-kappaB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, R.A.; Dorrington, M.G.; Dutta, B.; Krauss, K.S.; Martins, A.J.; Uderhardt, S.; Chan, W.; Tsang, J.S.; Torabi-Parizi, P.; Fraser, I.D.; et al. IFN-mediated negative feedback supports bacteria class-specific macrophage inflammatory responses. eLife 2019, 8, e46836. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.; Lee, S.; Watson, M.W.; Flexman, J.P.; Cheng, W.; Fernandez, S.; Price, P. Toll-like receptor (TLR) expression on CD4+ and CD8+ T-cells in patients chronically infected with hepatitis C virus. Cell Immunol. 2010, 264, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Funderburg, N.; Luciano, A.A.; Jiang, W.; Rodriguez, B.; Sieg, S.F.; Lederman, M.M. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS ONE 2008, 3, e1915. [Google Scholar] [CrossRef] [PubMed]
- Larochette, V.; Miot, C.; Poli, C.; Beaumont, E.; Roingeard, P.; Fickenscher, H.; Jeannin, P.; Delneste, Y. IL-26, a Cytokine With Roles in Extracellular DNA-Induced Inflammation and Microbial Defense. Front. Immunol. 2019, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Bastian, S.L.; Mpoy, C.M.; Scott, S.; Rattelle, A.; Pabbati, N.; Poruri, A.; Burugu, D.; Theophilus, P.A.; Pham, T.V.; et al. Characterization of biofilm formation by Borrelia burgdorferi in vitro. PLoS ONE 2012, 7, e48277. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Toma, L.; Provot, C.; Ascenzioni, F.; Sperduti, I.; Prignano, G.; Gallo, M.T.; Pimpinelli, F.; Bordignon, V.; Bernardi, T.; et al. Development of an in vitro Assay, Based on the BioFilm Ring Test, for Rapid Profiling of Biofilm-Growing Bacteria. Front. Microbiol. 2016, 7, 1429. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Jia, Y.; Mijatovic, T. Use of Specific Borrelia Phages as a New Strategy for Improved Diagnostic Tests. Methods Mol. Biol. 2024, 2742, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Cornero, R.; Irfan, S.S.; Cachaco, S.; Zhou, W.; Byne, A.; Howard, M.; McIntyre, H.; Birkaya, B.; Liotta, L.; Luchini, A. Identification of Unambiguous Borrelia Peptides in Human Urine Using Affinity Capture and Mass Spectrometry. Methods Mol. Biol. 2024, 2742, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Magni, R.; Almofee, R.; Yusuf, S.; Mueller, C.; Vuong, N.; Almosuli, M.; Hoang, M.T.; Meade, K.; Sethi, I.; Mohammed, N.; et al. Evaluation of pathogen specific urinary peptides in tick-borne illnesses. Sci. Rep. 2020, 10, 19340. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).