Pathogenic and Non-Pathogenic Microbes in the Wound Microbiome—How to Flip the Switch
Abstract
:1. Introduction
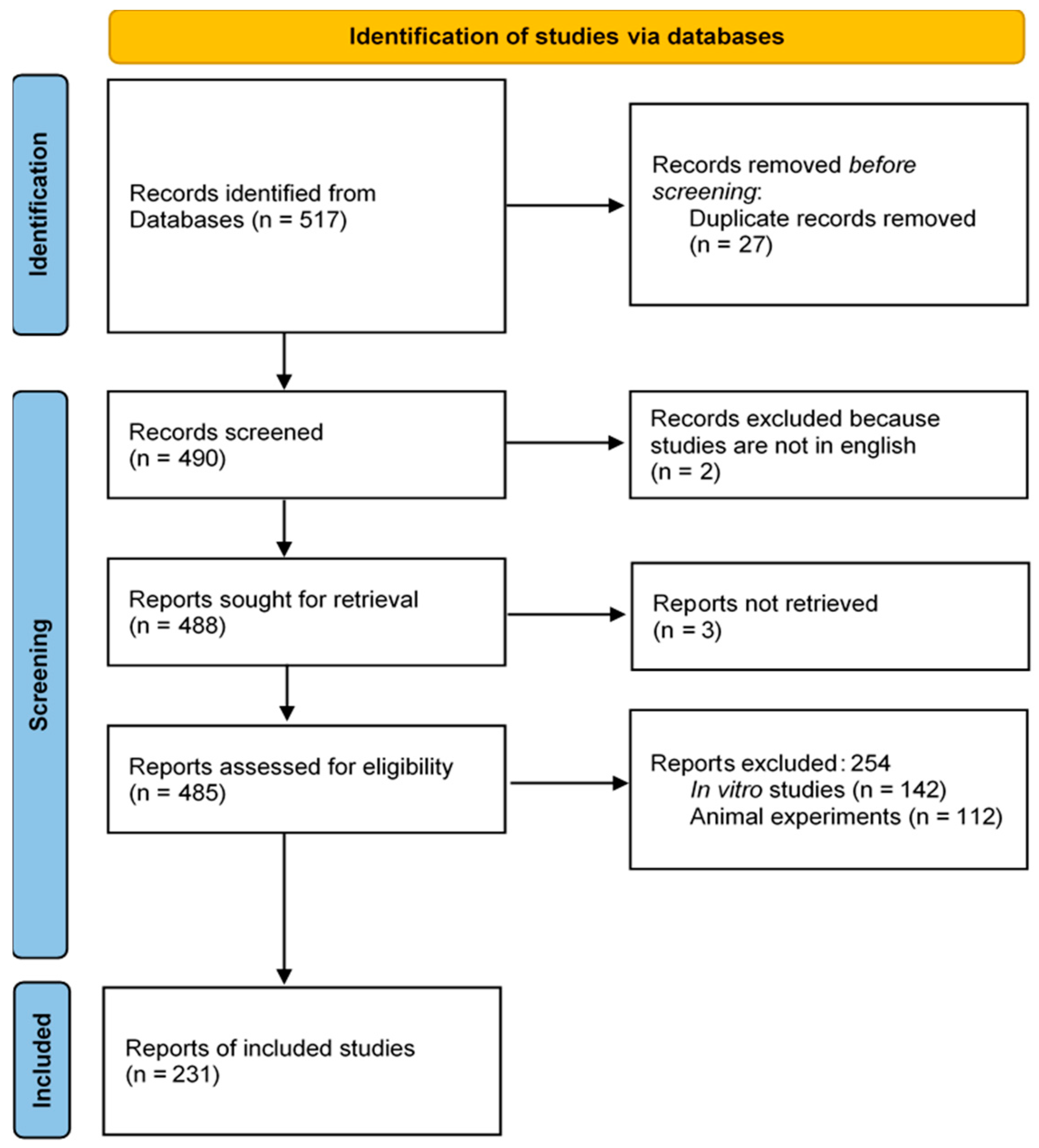
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
3. Results
3.1. Wound Microbiome: An Overview of Wound Entities
| Intact Skin | Acute Wounds | Chronic Wounds |
|---|---|---|
| Staphylococcus epidermidis | Staphylococcus aureus | Staphylococcus aureus |
| Cutibacterium acnes | Streptococcus spp. | Pseudomonas aeruginosa |
| Staphylococcus aureus | Pseudomonas aeruginosa | Enterobacter spp. |
| Corynebacterium spp. | Escherichia coli | Proteus mirabilis |
| Micrococcus spp. | Enterococcus spp. | Corynebacterium spp. |
3.2. Influence of Bacterial Colonization on Wound Healing: The Role of Commensal Bacteria
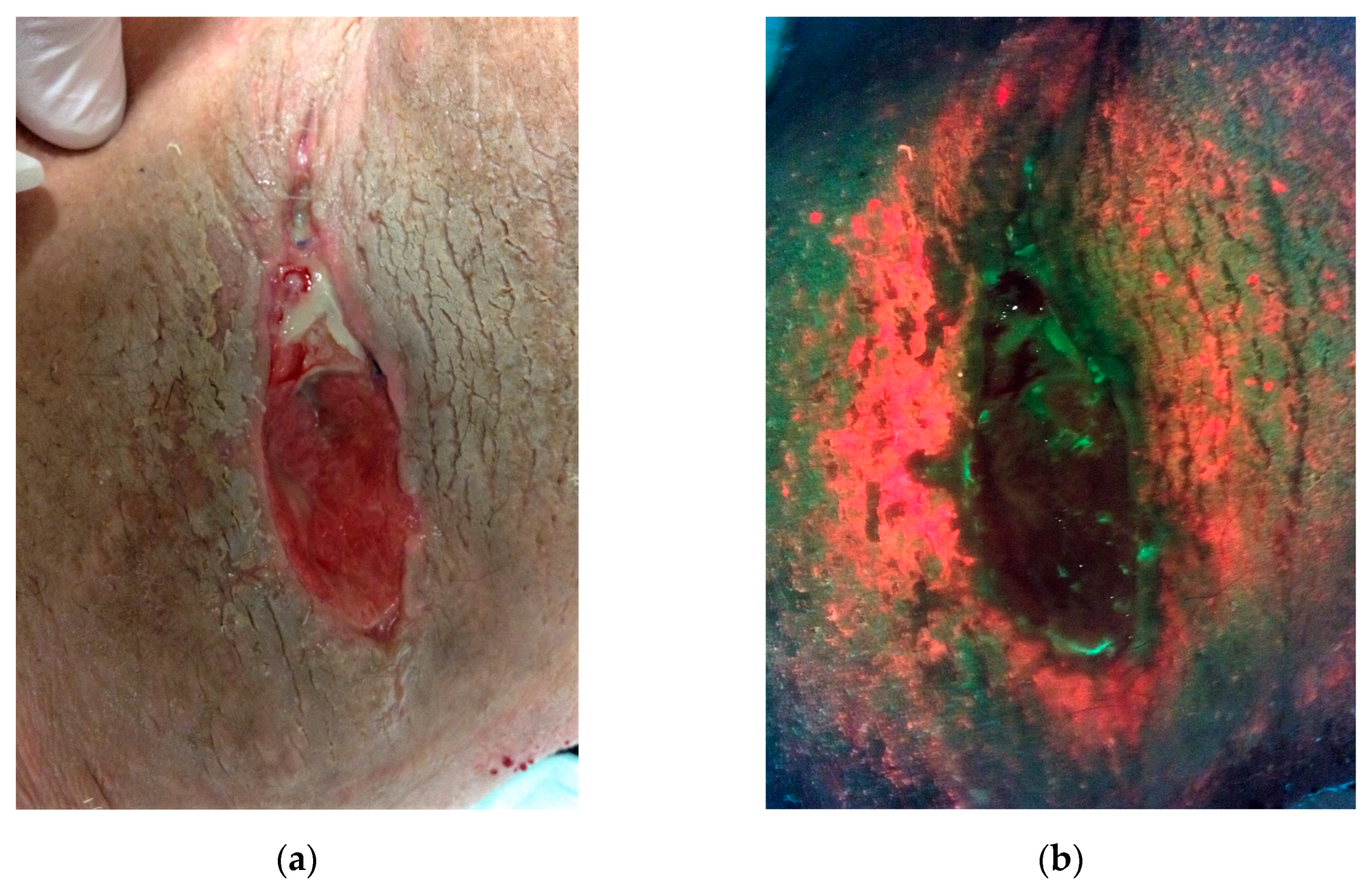
3.3. The Skin Microbiome in Dysbiosis and the Resulting Consequences
3.4. The Switch from Commensal to Pathogenic Bacteria
3.5. The Impact of Antiseptics and Antibiotics
3.6. Probiotics as Allies in the Wound Environment
4. Conclusions for Wound Healing in Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPS | Extracellular polymeric substance |
| HS | Hidradenitis suppurativa |
| LPS | Lipopolysaccharides |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| NGS | Next-generation sequencing |
| PAD | Peripheral arterial disease |
| PAMP | Pathogen-associated molecular pattern |
| PHMB | Polyhexamethylene-biguanide |
| PMN | Polymorphonuclear leukocytes |
| PRR | Pattern recognition receptors |
| RCT | Randomized controlled trials |
| ROS | Reactive oxygen species |
References
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Frykberg, R.G.; Oropallo, A.; Sen, C.K.; Armstrong, D.G.; Nair, H.K.; Serena, T.E. Efficacy of Topical Wound Oxygen Therapy in Healing Chronic Diabetic Foot Ulcers: Systematic Review and Meta-Analysis. Adv. Wound Care 2023, 12, 177–186. [Google Scholar] [CrossRef]
- White, E.K.; Grice, E.A. The Wound Microbiome. Cold Spring Harb. Perspect. Biol. 2023, 15, a041218. [Google Scholar] [CrossRef] [PubMed]
- Canchy, L.; Kerob, D.; Demessant, A.; Amici, J.-M. Wound healing and microbiome, an unexpected relationship. J. Eur. Acad. Dermatol. Venereol. 2023, 37 (Suppl. 3), 7–15. [Google Scholar] [CrossRef]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Schommer, N.N.; Gallo, R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013, 21, 660–668. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Luna, P.C. Skin Microbiome as Years Go By. Am. J. Clin. Dermatol. 2020, 21 (Suppl. 1), 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Wang, S.; Tan, Q.; Maitz, J.; Maitz, P.K.; et al. Challenges and innovations in treating chronic and acute wound in-fections: From basic science to clinical practice. Burns Trauma 2022, 10, tkac014. [Google Scholar] [CrossRef]
- Jockenhöfer, F.; Chapot, V.; Weindorf, M.S.; Körber, A.; Klode, J.; Buer, J.; Küpper, B.; Roesch, A.; Dissemond, J. Bacterial spectrum colonizing chronic leg ulcers: A 10-year comparison from a German wound care center. JDDG J. Dtsch. Dermatol. Ges. 2014, 12, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Morsli, M.; Salipante, F.; Magnan, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.-P. Direct metagenomics investigation of non-surgical hard-to-heal wounds: A review. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 39. [Google Scholar] [CrossRef]
- Verbanic, S.; Shen, Y.; Lee, J.; Deacon, J.M.; Chen, I.A. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microbiomes 2020, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Spanu, T.; Di Leo, M.; Vitiello, R.; Rizzi, A.; Tartaglione, L.; Fiori, B.; Caputo, S.; Tinelli, G.; Zaccardi, F.; et al. Diabetic foot infections: A comprehensive overview. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 26–37. [Google Scholar] [CrossRef]
- Stuermer, E.K.; Bang, C.; Giessler, A.; Smeets, R.; Janke, T.M.; Seki, F.D.; Debus, E.S.; Franke, A.; Augustin, M. Effect of oral multispecies probiotic on wound healing, periodontitis and quality of life on patients with diabetes. J. Wound Care 2024, 33, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Bay, L.; Ring, H.C. Human skin microbiota in health and disease: The cutaneous communities’ interplay in equilibrium and dysbiosis: The cutaneous communities’ interplay in equilibrium and dysbiosis. APMIS 2022, 130, 706–718. [Google Scholar] [CrossRef]
- Pastar, I.; O’neill, K.; Padula, L.; Head, C.R.; Burgess, J.L.; Chen, V.; Garcia, D.; Stojadinovic, O.; Hower, S.; Plano, G.V.; et al. Staphylococcus epidermidis Boosts Innate Immune Response by Activation of Gamma Delta T Cells and Induction of Perforin-2 in Human Skin. Front. Immunol. 2020, 11, 550946. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Gardiner, M.; Vicaretti, M.; Sparks, J.; Bansal, S.; Bush, S.; Liu, M.; Darling, A.; Harry, E.; Burke, C.M. A longitudinal study of the diabetic skin and wound micro-biome. PeerJ 2017, 5, e3543. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Pawłowska, A.; Orzeł, A.; Sulej, L.; Muzyka-Placzyńska, K.; Baran, A.; Filipecka-Tyczka, D.; Pawłowska, P.; Nowińska, A.; Bogusławska, J.; et al. Wound Microbiota and Its Impact on Wound Healing. Int. J. Mol. Sci. 2023, 24, 17318. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Loesche, M.; Gardner, S.E.; Kalan, L.; Horwinski, J.; Zheng, Q.; Hodkinson, B.P.; Tyldsley, A.S.; Franciscus, C.L.; Hillis, S.L.; Mehta, S.; et al. Temporal Stability in Chronic Wound Microbiota Is Associated With Poor Healing. J. Investig. Dermatol. 2017, 137, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Yang, G.; Wei, J.; Liu, P.; Zhang, Q.; Tian, Y.; Hou, G.; Meng, L.; Xin, Y.; Jiang, X. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism 2021, 117, 154712. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Lin, G.; Ferenczi, K. The skin microbiome and the gut-skin axis. Clin. Dermatol. 2021, 39, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef] [PubMed]
- Saarialho-Kere, U. The gut-skin axis. J. Pediatr. Gastroenterol. Nutr. 2004, 39 (Suppl. 3), S734–S735. [Google Scholar] [CrossRef]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. BioEssays 2016, 38, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.L.; Chandra, S.; Shih, D.Q. Skin Manifestations of Inflammatory Bowel Disease. Front. Physiol. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The Skin and Gut Microbiome and Its Role in Common Dermatologic Conditions. Microorganisms 2019, 7, 550. [Google Scholar] [CrossRef]
- Fijan, S.; Frauwallner, A.; Langerholc, T.; Krebs, B.; ter Haar née Younes, J.A.; Heschl, A.; Turk, D.M.; Rogelj, I. Efficacy of Using Probiotics with Antagonistic Activity against Pathogens of Wound Infections: An Integrative Review of Literature. BioMed Res. Int. 2019, 2019, 7585486. [Google Scholar] [CrossRef]
- Dissemond, J.; Witthoff, M.; Brauns, T.C.; Haberer, D.; Goos, M. pH values in chronic wounds. Evaluation during modern wound therapy. Hautarzt 2003, 54, 959–965. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.-M.; Karrer, S.; Heinlin, J.; Landthaler, M.; Babilas, P. The impact of the pH value on skin integrity and cu-taneous wound healing. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Severing, A.-L.; Rembe, J.-D.; Koester, V.; Stuermer, E.K. Safety and efficacy profiles of different commercial sodium hypo-chlorite/hypochlorous acid solutions (NaClO/HClO): Antimicrobial efficacy, cytotoxic impact and physicochemical parameters in vitro. J. Antimicrob. Chemother. 2019, 74, 365–372. [Google Scholar] [CrossRef]
- Sim, P.; Strudwick, X.L.; Song, Y.; Cowin, A.J.; Garg, S. Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment. Int. J. Mol. Sci. 2022, 23, 13655. [Google Scholar] [CrossRef] [PubMed]
- Derwin, R.; Patton, D.; Strapp, H.; Moore, Z. Wound pH and temperature as predictors of healing: An observational study. J. Wound Care 2023, 32, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.J.; Barreto, R.T.; Barrois, B.M.; Gryson, L.G.; Meaume, S.; Monstrey, S.J. Update on the role of antiseptics in the management of chronic wounds with critical colonisation and/or biofilm. Int. Wound J. 2020, 18, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Cwajda-Białasik, J.; Mościcka, P.; Szewczyk, M.T. Antiseptics and antimicrobials for the treatment and management of chronic wounds: A systematic review of clinical trials. Adv. Dermatol. Allergol. 2022, 39, 141–151. [Google Scholar] [CrossRef]
- Nair, H.K.; Mrozikiewicz-Rakowska, B.; Sanches Pinto, D.; Stuermer, E.; Matiasek, J.; Sander, J.; Lázaro-Martínez, J.L.; Ousey, K.; Assadian, O.; Kim, P.J.; et al. Use of Wound Antiseptics in Practice; Wounds Intern: London, UK, 2024. [Google Scholar]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on Wound Antisepsis: Update 2018. Ski. Pharmacol. Physiol. 2017, 31, 28–58. [Google Scholar] [CrossRef] [PubMed]
- Harriott, M.M.; Bhindi, N.; Kassis, S.; Summitt, B.; Perdikis, G.; Wormer, B.A.; Rankin, T.M.; Kaoutzanis, C.; Samaha, M.; Stratton, C.; et al. Comparative Antimicrobial Activity of Commercial Wound Care Solutions on Bacterial and Fungal Biofilms. Ann. Plast. Surg. 2019, 83, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Besser, M.; Dietrich, M.; Weber, L.; Rembe, J.D.; Stuermer, E.K. Efficacy of antiseptics in a novel 3-dimensional human plasma biofilm model (hpBIOM). Sci. Rep. 2020, 10, 4792. [Google Scholar] [CrossRef]
- Roche, E.D.; Woodmansey, E.J.; Yang, Q.; Gibson, D.J.; Zhang, H.; Schultz, G.S. Cadexomer iodine effectively reduces bacterial biofilm in porcine wounds ex vivo and in vivo. Int. Wound J. 2019, 16, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Rembe, J.-D.; Huelsboemer, L.; Plattfaut, I.; Besser, M.; Stuermer, E.K. Antimicrobial Hypochlorous Wound Irrigation Solutions Demonstrate Lower Anti-biofilm Efficacy Against Bacterial Biofilm in a Complex in-vitro Human Plasma Biofilm Model (hpBIOM) Than Common Wound Antimicrobials. Front. Microbiol. 2020, 11, 564513. [Google Scholar] [CrossRef] [PubMed]
- Moelleken, M.; Krimphove, S.H.; Krefting, F.; Benson, S.; Rammos, C.; Cyrek, A.E.; Dissemond, J. How effective is simple mechanical wound debridement in reducing bacterial colonisation? Results of a prospective clinical study. Int. Wound J. 2024, 21, e14824. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.; Tettelbach, W.H.; Ciprandi, G.; Downie, F.; Hampton, J.; Hodgson, H.; Lazaro-Martinez, J.L.; Probst, A.; Schultz, G.; Stürmer, E.K.; et al. Best practice for wound debridement. J. Wound Care 2024, 33, S1–S32. [Google Scholar] [CrossRef]
- Atkin, L.; Bućko, Z.; Montero, E.C.; Cutting, K.; Moffatt, C.; Probst, A.; Romanelli, M.; Schultz, G.S.; Tettelbach, W. Implementing TIMERS: The race against hard-to-heal wounds. J. Wound Care 2019, 28, S1–S50. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H. Gut Microbiota-Mediated Drug-Antibiotic Interactions. Drug Metab. Dispos. 2015, 43, 1581–1589. [Google Scholar] [CrossRef]
- Neut, C.; Mahieux, S.; Dubreuil, L.J. Antibiotic susceptibility of probiotic strains: Is it reasonable to combine probiotics with anti-biotics? Med. Mal. Infect. 2017, 47, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.R.; Gómez, B.I.; McIntyre, M.K.; Dubick, M.A.; Christy, R.J.; Nicholson, S.E.; Burmeister, D.M. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef]
- Bădăluță, V.A.; Curuțiu, C.; Dițu, L.M.; Holban, A.M.; Lazăr, V. Probiotics in Wound Healing. Int. J. Mol. Sci. 2024, 25, 5723. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Y.; Song, J.; Zhou, L.; Wu, K.; Lu, X.; Zhai, X.; Wan, Z.; Gao, J. Highly active probiotic hydrogels matrixed on bacterial EPS accelerate wound healing via maintaining stable skin microbiota and reducing inflammation. Bioact. Mater. 2024, 35, 31–44. [Google Scholar] [CrossRef]
- Soleymanzadeh Moghadam, S.; Momeni, M.; Mazar Atabaki, S.; Mousavi Shabestari, T.; Boustanshenas, M.; Afshar, M.; Roham, M. Topical Treatment of Second-Degree Burn Wounds with Lactobacillus plantarum Supernatant: Phase I Trial. Iran J. Pathol. 2022, 17, 460–468. [Google Scholar] [CrossRef]
- Mohseni, S.; Bayani, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Bayani, M.A.; Jafari, P.; Asemi, Z. The beneficial effects of probiotic admin-istration on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, place-bo-controlled trial. Diabetes Metab. Res. Rev. 2018, 34, e2970. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Roberti, A.; Turrà, F.; Cerulo, M.; Severino, G.; Settimi, A.; Escolino, M. Frequency of Antibiotic-Associated Diarrhea and Related Complications in Pediatric Patients Who Underwent Hypospadias Repair: A Comparative Study Using Probiotics vs. Placebo. Probiotics Antimicrob Proteins 2018, 10, 323–328. [Google Scholar] [CrossRef]
- El-Ghazely, M.H.; Mahmoud, W.H.; A Atia, M.; Eldip, E.M. Effect of probiotic administration in the therapy of pediatric thermal burn. Ann. Burns Fire Disasters 2016, 29, 268–272. [Google Scholar]
- Togo, C.; Zidorio, A.P.; Gonçalves, V.; Botelho, P.; de Carvalho, K.; Dutra, E. Does Probiotic Consumption Enhance Wound Healing? A Systematic Review. Nutrients 2021, 14, 111. [Google Scholar] [CrossRef]
- Nayak, N.; Kapaettu, S.; Sreepathy, M.T. Next-generation sequencing technology for the diagnosis of microbial infections in hard-to-heal wounds. J. Wound Care 2023, 32 (Suppl. 6a), xcvii–cix. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liegenfeld, S.C.; Stenzel, S.; Rembe, J.-D.; Dittmer, M.; Ramos, P.; Stuermer, E.K. Pathogenic and Non-Pathogenic Microbes in the Wound Microbiome—How to Flip the Switch. Microbiol. Res. 2025, 16, 39. https://doi.org/10.3390/microbiolres16020039
Liegenfeld SC, Stenzel S, Rembe J-D, Dittmer M, Ramos P, Stuermer EK. Pathogenic and Non-Pathogenic Microbes in the Wound Microbiome—How to Flip the Switch. Microbiology Research. 2025; 16(2):39. https://doi.org/10.3390/microbiolres16020039
Chicago/Turabian StyleLiegenfeld, Sophie Charlotte, Svenja Stenzel, Julian-Dario Rembe, Mandy Dittmer, Paulo Ramos, and Ewa Klara Stuermer. 2025. "Pathogenic and Non-Pathogenic Microbes in the Wound Microbiome—How to Flip the Switch" Microbiology Research 16, no. 2: 39. https://doi.org/10.3390/microbiolres16020039
APA StyleLiegenfeld, S. C., Stenzel, S., Rembe, J.-D., Dittmer, M., Ramos, P., & Stuermer, E. K. (2025). Pathogenic and Non-Pathogenic Microbes in the Wound Microbiome—How to Flip the Switch. Microbiology Research, 16(2), 39. https://doi.org/10.3390/microbiolres16020039







