Abstract
Urinary tract infections (UTIs) caused by uropathogenic Escherichia coli (UPEC) are a major healthcare challenge, necessitating effective antimicrobial therapy for treatment. However, the prevalence of antimicrobial resistance among UPEC strains is escalating, particularly among patients experiencing recurrent infection. The rise in UPEC strains that exhibit resistance to multiple antimicrobial agents, including the spread of extended-spectrum beta-lactamase (ESBL)-producing UPEC, intensifies the complexity of managing UTIs. Genetic variations within UPEC strains play a major role in their ability to resist antimicrobial agents and adapt to changing environments. Unveiling and understanding the genomic landscape of emerging UPEC strains is essential for comprehending the genetic basis of their resilience. Moreover, monitoring these genetic strains is crucial for identifying patterns of resistance dissemination, guiding infection control measures, and informing the development of targeted therapeutics.
1. Introduction
Urinary tract infections (UTIs) are among the most common and problematic infections and are a major burden for public health, and particularly hospital settings, causing significant discomfort and morbidity and increased antimicrobial resistance risks and economic costs [1,2]. UTIs are categorized into two main groups: symptomatic and asymptomatic cases [3]. Symptomatic UTI cases are mostly classified into three classes depending on the severity: pyelonephritis (referring to upper UTI, with kidney infection), cystitis (referring to lower UTI and bladder infection), and urosepsis [2,4]. In The Kingdom of Saudi Arabia (KSA), UTIs were reported to be responsible for 10 to 14% of all emergency department visits [5,6]. Uropathogenic Escherichia coli (UPEC) is one of the leading causative agents, and often the most common [2,7]. The only treatment for UTI is antimicrobial therapy [2]. However, multidrug-resistant bacteria (MDR) have become a major public health concern, particularly among patients who are affected by recurrent infections [8]. UPEC’s growing antimicrobial resistance poses an even greater threat and raises new challenges in the battle against the growing antimicrobial resistance rates among different pathogens [8].
UPEC strains are able to colonize the urinary tract in an ascending order from the urethra to the urinary bladder, followed by the ureters, ending up in the kidney and, in severe cases, entering the bloodstream [1,2]. This infection is referred to as urosepsis, indicating that the infection originates from the urogenital system, and accounts for approximately 30% of sepsis cases [9]. Urosepsis is a devastating situation for the patient and is linked with a high risk of organ failure, sepsis shock, and even death [9,10]. There are many virulence factors and specific genes present among UPEC strains that facilitate their breaching of the host epithelium in the urinary tract and colonization [11]. UPEC strains are a heterogeneous group of strains and are associated with particular O-serogroups, and their virulence factors are mainly encoded on pathogenicity islands [12]. Some strains of Escherichia coli (E. coli) are responsible for most UTIs and also bloodstream infections, particularly O25b:H4-ST131 E. coli (ST131) [13]. Another threat of E. coli strains is the emergence of extended-spectrum beta-lactamase (ESBL)-producing E. coli. ESBL-E. coli prevalence among UTI patients ranges from 23.1% to 33.49%, and in some studies that reported from hospital settings, the rate was reported to be as high as 51.4% [14,15,16]. Moreover, this rise in the prevalence of ESBL-E. coli is a global healthcare concern. Reports from different countries suggest an increase in the prevalence of ESBL-E. coli and call for a more cautious antibiotic prescription for patients presenting with UTI [17,18,19]. This indicates that a significant proportion of infections are resistant to commonly used antibiotics such as cephalosporins. Treatment of ESBL-E. coli-associated infections is quite challenging as there are limited treatment options, thus forcing the usage of strong antibiotics. Usage of strong antibiotics comes with a lot of drawbacks, including an increased risk of the emergence of antibiotic resistance and side effects.
This review attempts to summarize some of the main molecular characteristics of UPEC as well as its antimicrobial resistance patterns in Saudi Arabia during the past 5 years, as there are limited data on this topic. This review also attempts to address some of the current gaps and aims to contribute to a better understanding of UPEC in the region in order to inform targeted interventions for effective UTI management and prevention.
2. Materials and Methods
2.1. Literature Review and Data Sources
A comprehensive literature search was conducted using different search engines, including the PubMed, Scopus, and Google Scholar databases. Keywords that were used included “Uropathogenic Escherichia coli”, “UPEC”, “Urinary tract infection”, “UTI”, “E. coli”, and “Saudi Arabia”. Studies published between 1 January 2018 and 18 December 2023 were included and further analyzed for appropriate data extraction. Moreover, national journals such as the Saudi Medical Journal and Annals of Saudi Medicine, as well as the reference lists of the included articles, were further screened in order to identify additional relevant published studies.
2.2. Eligibility Criteria
All published studies that reported on (one) urinary tract infections, (two) were conducted in the Kingdom of Saudi Arabia, and (three) reported on UPEC prevalence, (four) its antimicrobial resistance patterns, and (five) molecular characteristics were eligible and included in the review (i.e., inclusion criteria). Additionally, eligible studies had to report on the isolation and identification of UPEC based on standard bacteriological methods following the Clinical Standards Laboratory Institute (CSLI) guidelines. Publications that did not stem from primary research (e.g., opinions and letters to the editor), or were from conference proceedings or abstracts, were not included (i.e., exclusion criteria). The search was limited to English language publications and focused on studies reporting on the prevalence data on UPEC in Saudi Arabia.
2.3. Data Extraction Process and Analysis
Every included study was screened concerning the following data: (one) authors’ names, (two) publication year, (three) study design, (four) sample age and size, (five) study population, (six) UPEC prevalence, (seven) UPEC antimicrobial resistance pattern, (eight) UPEC ESBL production rate, and (nine) UPEC molecular characteristics. The main findings from the screened articles were categorized under broad themes.
2.4. Diagnostic Criteria and Sample Collection
The diagnostic criteria across the studies included the collection of a urine sample in patients with indicative symptoms. Cultures were performed for patients who had bacteriuria. The prescription of broad-spectrum antibiotics started from the time when the culture was ordered. Samples from patients with a urinary catheter or from pediatric patients were obtained by a medical professional under aseptic conditions.
3. Results
3.1. General Description of the Studies
For this review, a total of 20 relevant studies were identified and screened, and data were extracted. Most of the studies reported on patients attending healthcare facilities in Riyadh. The studies were either retrospective or cross-sectional by design and included patients who had a UTI or were suspected of having a UTI. There was a regional disparity among the studies. Most of the studies and publications originated from the capital of the KSA, Riyadh. Riyadh has the highest population in the kingdom and is home to multiple large research centers and tertiary hospitals. Furthermore, the age inclusion across the studies was not limited to a particular age group, with few studies being focused on pediatric patients (Table 1).

Table 1.
General description of the included studies.
3.2. UPEC Prevalence
In our analysis of the prevalence of UPEC across different studies, Alamri et al. reported the lowest prevalence of UPEC among all uropathogenic bacteria, with a UPEC prevalence of 27% [31]. Abalkhail et al. reported a slightly higher prevalence of 33.7%, with slightly higher findings by Alsohaim et al. and Alsubaie et al., reporting 54.5% and 59%, respectively [15,27,34]. Alanazi et al. and Balkhi et al. reported a UPEC prevalence of beyond 60% in their studies, with 62% and 65% [6,20]. Al Qasim et al. reported the highest prevalence of UPEC at 97% and included a fair sample size of 210 patients [21]. There were three studies that included only UPEC, which reported a 100% prevalence [23,26,34]. Across the studies, those with larger sample sizes reported relatively lower prevalences as their sample sizes exceeded 1000 patients [15,28,30]. Emeka et al. conducted a large-scale study and reported a prevalence of 39.7% of UPEC among 49,779 isolated uropathogens over a four-year period [33]. There were no major differences in the UPEC prevalence rates reported between different age groups. A major difference was the inclusion criteria, as some studies included patients suspected of having a UTI, whereas some studies reported only the positive cases.
3.3. ESBL-Producing UPEC Strains
The prevalence of ESBL production among UPEC strains varied greatly across all the studies included in this review. Alamri et al. reported the lowest ESBL production rate of 7.9% [25]. Alanazi et al. reported a slightly higher ESBL production rate among UPEC strains, with a rate of 9% in a retrospective study conducted in Riyadh [6]. Moving upward, Hameed et al. reported a rate of 14.7%, with a similar rate of 15% reported by Alghamdi et al. [29,37]. The highest ESBL production rates were reported by Abalkhail et al. (33%), Alanazi et al. (33.5%), Taher et al. (30%), and Alsubaie et al. (34%), with all studies conducted in Riyadh, reflecting higher levels of antibiotic resistance in this region [15,23,26,34]. It is noteworthy that the Riyadh region was the most represented and that the prevalence rates of ESBL production ranged from low to as high as 34% [23,24,25,26,28,34]. Furthermore, the large-scale studies that included more than 1000 patients reported a relatively high prevalence of ESBL-producing UPEC, such as Balkhi et al. (29%) and Abalkhail et al. (33.5%) [15,22,28,30,33,38]. On the other hand, Alghamdi et al. reported a relatively lower prevalence of 15%. While they reported a lower prevalence of ESBL-producing UPEC in a larger sample size, they reported from a different region, Al Baha [37]. Similar to the UPEC prevalence, some studies only reported on ESBL-producing bacteria, resulting in a prevalence of 100%. Moreover, it is noteworthy that some studies did not provide specific data on ESBL production rates among UPEC strains.
3.4. Antimicrobial Susceptibility
On analyzing the antimicrobial profiles of UPEC across multiple studies, some antimicrobials consistently demonstrated lower resistance rates, while others demonstrated higher levels of resistance. Amikacin is a potent antimicrobial but is associated with several side effects and is not part of the first-line therapies for UTI. However, it exhibited relatively lower resistance rates across multiple studies. For instance, the resistance rates varied from as low as 0.5% to as high as 30%. However, as mentioned earlier, the studies varied greatly in sample size [20,26,27,28,29,35,36]. It is noteworthy that the studies with a large sample size showed a relatively lower resistance rate, particularly Emeka et al., who reported a resistance rate of 13% out of a total of 16,779 isolated UPEC cases [33].
Trimethoprim/sulfamethoxazole (SXT) is among the first-line choices for UTI according to the national guidelines in the KSA [39]. UPEC has shown relatively high and consistent resistance rates against SXT, ranging from 39% in the report of Bazaid et al. (2021) to around 60% in the report of Emeka (2022) et al., who had the largest sample size of 16,779 isolated UPEC cases [30,33]. Nitrofurantoin is used as a second-line treatment after SXT or in cases where there is reported SXT resistance from UPEC. In contrast to SXT, nitrofurantoin has been reported to have a lower resistance rate, ranging between 2% and a maximum of 20%, as reported in different studies. Al Qasim et al. (2018) reported a resistance rate of 14.5%, suggesting safe usage of nitrofurantoin as a first-line treatment [21,22,25,30,33,34,35].
Imipenem is included in the guidelines as a very potent antibiotic and is advised to be used in acute pyelonephritis and among hospitalized patients. Several studies have reported a low resistance rate and suggested effectiveness in severe UTI cases. Al Qasim et al. (2018) [21,22,25,30,33,34,35] reported that imipenem had the lowest antimicrobial resistance rate among all tested antimicrobial drugs (Table 2).

Table 2.
Antimicrobial profiles of UPEC.
3.5. Multidrug Resistance and Biofilms
The antibiotic resistance patterns found in different bacteria can be extended, and they end up developing multidrug resistance (MDR) patterns, which are described as resistance to at least one agent in three or more antimicrobial categories [34]. Alsubaie et al. reported on UPEC isolates’ antibiotic resistance patterns and revealed a high prevalence of MDR, with 67% of isolates exhibiting MDR [34]. They reported the distribution of resistance across various antimicrobial groups, where ESBL-producing UPEC isolates showed a 100% association with MDR. This highlights a significant link between ESBL and MDR.
Albalawi et al. reported that 22.77% of their UPEC isolates exhibited MDR, with varying resistance patterns observed for different types and categories of antibiotics [24]. All of their UPEC isolates that were resistant to augmentin and nitrofurantoin were also MDR. On the other hand, there were reports showing that 73% to as high as 82% of all isolates that were resistant to ciprofloxacin and cefazolin were MDR [24]. MDR was reported to be notably present in intensive care units by Ibrahim et al. [40]. Arafa et al. reported that MDR rates were higher in the KSA as compared to other regional countries [41].
Biofilm formation is a bacterial mechanism that contributes to antibiotic resistance, making infections difficult to treat. Alghoribi et al. tested the ability of UPEC to produce biofilms [42]. Firstly, it was reported that 56% of the UPEC isolates were unable to form biofilms. Moreover, 84% exhibited a weak ability to form biofilms. They concluded that biofilm formation is not a universal trait that is found among all UPEC isolates, and this suggests a significant heterogeneity between UPEC strains in the KSA in their biofilm-forming capabilities. They also attempted to determine whether there is any significant association between MDR and the ability to form biofilms. However, their analysis revealed no significant correlation between MDR and biofilm formation.
3.6. Genomic Characteristics
This comprehensive review highlights some of the molecular characteristics and virulence factors of UPEC identified in different regions of the KSA to provide some insights into the genomic landscape of UPEC. Several studies conducted whole genome sequencing on UPEC isolates. Taher et al. reported diverse phylogroups, with the majority belonging to the B2 phylogenetic group [26]. Sequence types of UPEC varied, with ST131 being the most prevalent across the different studies, such as Taher et al., Alqasim et al., and Hassan et al. [26,43,44]. Serotyping and FimH typing conducted by Taher et al. on different isolates revealed associations between STs and specific serotypes, highlighting the wide diversity of UPEC strains in the Kingdom [26]. Hassan et al. reported that their ST131 isolates were prevalent in clade O25b, and they also identified subclones, further highlighting the genetic diversity within ST131 [44].
Ghany et al. reported the presence of various virulence genes like kpsII, fimH, hly, and uidA among the isolated UPEC strains [45]. Other studies such as Alanazi et al. reported that fimH virulence factors were more prevalent than papE/F [23]. Further genetic testing in the effort to identify antimicrobial resistance genes among the isolates revealed the presence of various resistance genes identified in UPEC strains, including blaNDM, blaCTX-M, and blaTEM [23,45]. It is noteworthy that some of the isolated strains carried multiple carbapenem-hydrolyzing genes which enable UPEC to break down carbapenem antibiotics, a widely used class of antimicrobials in the treatment of UTI. Moreover, fluoroquinolone and sulfonamide resistance genes were identified among several isolates [46]. ESBL production was reported to be relatively high. Further genetic testing revealed that many of the UPEC isolates carried CTX-M genes that encode beta-lactamase enzymes. The presence of CTX-M genes among pathogens, particularly UPEC, poses a significant threat to public health as it is a major limitation factor for the effectiveness of commonly used antibiotics. Bacteria that carry CTX-M genes are often resistant to multiple antibiotics, which results in a more complicated treatment of UTIs caused by these strains.
4. Discussion
The Enterobacteriaceae family continues to be the leading cause of UTIs in all types of patients, with E. coli being the most commonly isolated organism, and in some cases, the only organism detected. These strains exhibited a high incidence of antibiotic resistance; those isolated from inpatients were more likely to exhibit MDR and a high rate of ESBL production [14,29,30,35,44]. Antimicrobial resistance is spreading at an alarming rate. There are multiple factors that influence ESBL production (i.e., older ages, presence of chronic conditions, overuse or misuse of antibiotics, biofilm formation, hygiene, etc.). Some of these factors are not modifiable; however, more efforts should be directed toward reducing antibiotic misuse and overuse and increasing the awareness among patients. Furthermore, continued, more extensive and inclusive investigation is required to understand all of the antimicrobial sensitivity patterns in the KSA, both locally and nationally, and to establish an updated treatment strategy.
UPEC poses a significant public health concern. The rise in community- and hospital-acquired UTIs is alarming. The increasing antibiotic resistance among UPEC strains is part of the risk factors contributing to such high prevalences. Several reports indicate alarmingly high resistance rates to many of the commonly used antibiotics as the first line of treatment, including antibiotics like nitrofurantoin and cephalexin, leaving healthcare professionals with limited treatment options. As mentioned earlier, another concern is that the MDR rates have continued to grow, and their genetic mutations are associated with this widely spread resistance. The continuous emergence of different and dangerous mutations among UPEC strains circulating within the KSA enhances the virulence factors among UPEC strains, allowing them to evade the immune system and resist different antibiotics and colonize the urinary tract more efficiently. This is associated with the increased difficulty in effectively treating infections. It also raises the risk of complications like pyelonephritis, a potentially life-threatening kidney infection.
Since the breakthrough discovery of penicillin in 1945, a major healthcare concern has been solved, allowing infectious diseases to be cured [47,48]. However, a new and more dangerous threat has emerged: bacterial resistance against antibiotics [48,49]. Since this issue emerged, the resistance rates have continued to increase, even with the discovery of new antibiotics, with major efforts being taken to reduce this growing concern, especially the latest G20 countries’ collaboration beginning in 2017 to increase the efforts in combating the increase in antibiotic resistance rates [49]. Even though there have been around eight new approved antibiotics, this is not the main way of solving the growing resistance rates. The core issue stands with the misuse of antibiotics that leads to the development of resistant strains, which is a global issue [49,50,51,52]. There are multiple strategies that can be used to reduce antibiotic misuse (i.e., antibiotic stewardship, improving infection and disease control measures, avoiding unnecessary prescriptions, and patient education on antibiotic use and hygiene) (Figure 1).
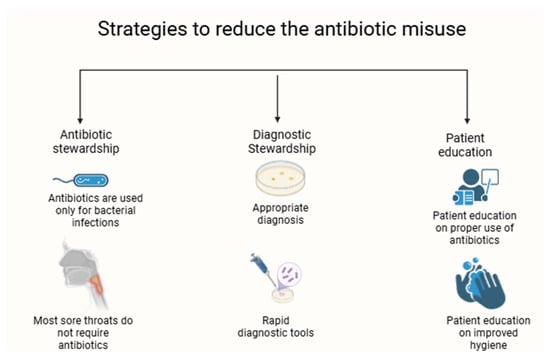
Figure 1.
Strategies to reduce the antibiotic misuse.
Studies in Saudi Arabia report a high prevalence of certain behaviors that lead to higher antibiotic resistance rates and complications: not completing the antibiotic course, using leftover antibiotics, reusing the same antibiotics for another infection, self-prescribing antibiotics, and using antibiotics to cure viral infections [51,53,54,55]. Antibiotic misuse and overprescribing are other factors which are very important in the increased resistance rates [56,57]. Reports from the KSA suggested a high prevalence of the overuse of broad-spectrum antibiotics, with Alanazi et al. (2022) reporting that in some emergency departments, nearly half of all drug prescriptions were antibiotics [56]. These findings were reported in 2019, before the COVID-19 pandemic. Reports suggest an increased awareness of the phenomenon of increased antibiotic prescriptions during the pandemic, especially in the early days of the outbreak where there were no clear guidelines, and according to reports, the vast majority of COVID-19 patients received antibiotics as part of their treatment regimen [58,59,60].
Apart from improving antibiotic prescription and usage, continuous efforts in identifying and discovering new treatment strategies are crucial in order to combat infectious diseases [61]. Metallophores represent a new field of research and are being studied for their potential in combatting the rise in antibiotic resistance [62]. In recent years, these strategies have garnered considerable attention for their potential as antimicrobial agents [63]. Metal ions are essential for bacteria and also other living organisms; the catalysis of metal ion-containing enzymes is essential in many biochemical reactions, but the metal levels and presence can also become toxic [63,64]. In common practice, metal ions are used for their antibiotic properties; for example, silver ion-coated urinary catheters are commonly used to treat UTI infections, and currently, studies and trials are underway to identify potential routes for delivering metal ions inside the bacterium [32,63]. However, it is worth mentioning that metal ion incorporation in the treatment regimen is still in its infancy, and studies are currently in the early phases.
Most investigations reporting on the resistance patterns of UPEC are retrospective in nature, and they do not include patient follow-up until the completion of treatment and full recovery from the infection. As a result, evidence on the efficacy of therapies is scarce, and there is a gap in the available literature about the effectiveness of the therapies. Future prospective studies will improve our understanding of the course of the infection and should follow the patients from diagnosis to full recovery. A follow-up of the patients will help in preventing untreated infections or improper usage of antimicrobial drugs and relapses of complications of the infection that result in increased antimicrobial resistance rates. Furthermore, no information on the prescribed empirical therapy and its suitability for microbiological reports has been provided. Future research should focus on the assessment of the appropriateness of empirical therapies by continuously assessing and evaluating the empirical therapy choices based on continuous microbiology reports. Last but not least, patient education is crucial in increasing their awareness and understanding of the importance of completing their prescribed treatment regimens and the risks associated with improper antibiotic use.
5. Conclusions
Urinary tract infections are a very common infection and remain a major challenge for the healthcare system. E. coli remains the major causative pathogen and is continuously developing new resistance rates. Continuous surveillance is crucial for combatting this rising antimicrobial resistance among UPEC strains in the Kingdom of Saudi Arabia and worldwide. Future research should prioritize prospective studies that track patients from diagnosis to recovery, assessing the appropriateness of empirical therapies and their alignment with microbiological reports. Additionally, patient education programs are essential for promoting adherence to prescribed treatments and reducing the risk of antimicrobial resistance.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to thank Sulaiman Al Rajhi University for its support.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
| ESBL | Extended-spectrum beta-lactamase |
| KSA | Kingdom of Saudi Arabia |
| MDR | Multidrug resistance |
| SXT | Trimethoprim/sulfamethoxazole |
| UTI | Urinary tract infection |
| UPEC | Uropathogenic Escherichia coli |
References
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Halaji, M.; Fayyazi, A.; Rajabnia, M.; Zare, D.; Pournajaf, A.; Ranjbar, R. Phylogenetic Group Distribution of Uropathogenic Escherichia coli and Related Antimicrobial Resistance Pattern: A Meta-Analysis and Systematic Review. Front. Cell. Infect. Microbiol. 2022, 12, 790184. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Smelov, V.; Naber, K.; Bjerklund Johansen, T.E. Improved Classification of Urinary Tract Infection: Future Considerations. Eur. Urol. Suppl. 2016, 15, 71–80. [Google Scholar] [CrossRef]
- Alrashid, S.; Ashoor, R.; Alruhaimi, S.; Hamed, A.; Alzahrani, S.; Al Sayyari, A. Urinary Tract Infection as the Diagnosis for Admission Through the Emergency Department: Its Prevalence, Seasonality, Diagnostic Methods, and Diagnostic Decisions. Cureus 2022, 14, e27808. [Google Scholar] [CrossRef]
- Alanazi, M.Q. An evaluation of community-acquired urinary tract infection and appropriateness of treatment in an emergency department in Saudi Arabia. Ther. Clin. Risk Manag. 2018, 14, 2363–2373. [Google Scholar] [CrossRef]
- Sula, I.; Alreshidi, M.A.; Alnasr, N.; Hassaneen, A.M.; Saquib, N. Urinary Tract Infections in the Kingdom of Saudi Arabia, a Review. Microorganisms 2023, 11, 952. [Google Scholar] [CrossRef]
- Halaji, M.; Feizi, A.; Mirzaei, A.; Sedigh Ebrahim-Saraie, H.; Fayyazi, A.; Ashraf, A.; Havaei, S.A. The Global Prevalence of Class 1 Integron and Associated Antibiotic Resistance in Escherichia coli from Patients with Urinary Tract Infections, a Systematic Review and Meta-Analysis. Microb. Drug Resist. 2020, 26, 1208–1218. [Google Scholar] [CrossRef]
- Tocut, M.; Zohar, I.; Schwartz, O.; Yossepowitch, O.; Maor, Y. Short- and long-term mortality in patients with urosepsis caused by Escherichia coli susceptible and resistant to 3rd generation cephalosporins. BMC Infect. Dis. 2022, 22, 571. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.; Lichtenstern, C.; Rolfes, C.; Mayer, K.; Uhle, F.; Weidner, W.; Weigand, M.A. Diagnosis and management for urosepsis. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2013, 20, 963–970. [Google Scholar] [CrossRef]
- Sadeghi, A.; Halaji, M.; Fayyazi, A.; Havaei, S.A. Characterization of Plasmid-Mediated Quinolone Resistance and Serogroup Distributions of Uropathogenic Escherichia coli Among Iranian Kidney Transplant Patients. BioMed Res. Int. 2020, 2020, 2850183. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.L.; Rasko, D.A.; Mobley, H.L.T. Defining genomic islands and uropathogen-specific genes in uropathogenic Escherichia coli. J. Bacteriol. 2007, 189, 3532–3546. [Google Scholar] [CrossRef] [PubMed]
- Petty, N.K.; Ben Zakour, N.L.; Stanton-Cook, M.; Skippington, E.; Totsika, M.; Forde, B.M.; Phan, M.-D.; Gomes Moriel, D.; Peters, K.M.; Davies, M.; et al. Global dissemination of a multidrug resistant Escherichia coli clone. Proc. Natl. Acad. Sci. USA 2014, 111, 5694–5699. [Google Scholar] [CrossRef] [PubMed]
- El-Masry, E.A.; Alruwaili, F.M.; Taha, A.E.; Saad, A.E.; Taher, I.A. Prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae among clinical isolates in Turaif general hospital, northern borders- Saudi Arabia. J. Infect. Dev. Ctries. 2023, 17, 477–484. [Google Scholar] [CrossRef]
- Abalkhail, A.; AlYami, A.S.; Alrashedi, S.F.; Almushayqih, K.M.; Alslamah, T.; Alsalamah, Y.A.; Elbehiry, A. The Prevalence of Multidrug-Resistant Escherichia coli Producing ESBL Among Male and Female Patients with Urinary Tract Infections in Riyadh Region, Saudi Arabia. Healthcare 2022, 10, 1778. [Google Scholar] [CrossRef]
- Mashwal, F.A.; El Safi, S.H.; George, S.K.; Adam, A.A.; Jebakumar, A.Z. Incidence and molecular characterization of the extended spectrum beta lactamase-producing Escherichia coli isolated from urinary tract infections in Eastern Saudi Arabia. Saudi Med. J. 2018, 38, 811–815. [Google Scholar] [CrossRef]
- Jia, P.; Zhu, Y.; Li, X.; Kudinha, T.; Yang, Y.; Zhang, G.; Zhang, J.; Xu, Y.; Yang, Q. High Prevalence of Extended-Spectrum Beta-Lactamases in Escherichia coli Strains Collected from Strictly Defined Community-Acquired Urinary Tract Infections in Adults in China: A Multicenter Prospective Clinical Microbiological and Molecular Study. Front. Microbiol. 2021, 12, 663033. [Google Scholar] [CrossRef]
- Bou Chebl, R.; Assaf, M.; Kattouf, N.; Abou Arbid, S.; Haidar, S.; Geha, M.; Makki, M.; Tamim, H.; Abou Dagher, G. The prevalence and predictors of extended spectrum B-lactamase urinary tract infections among emergency department patients: A retrospective chart review. Am. J. Emerg. Med. 2021, 49, 304–309. [Google Scholar] [CrossRef]
- Sokhn, E.S.; Salami, A.; El Roz, A.; Salloum, L.; Bahmad, H.F.; Ghssein, G. Antimicrobial Susceptibilities and Laboratory Profiles of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis Isolates as Agents of Urinary Tract Infection in Lebanon: Paving the Way for Better Diagnostics. Med. Sci. 2020, 8, 32. [Google Scholar] [CrossRef]
- Balkhi, B.; Mansy, W.; AlGhadeer, S.; Alnuaim, A.; Alshehri, A.; Somily, A. Antimicrobial susceptibility of microorganisms causing Urinary Tract Infections in Saudi Arabia. J. Infect. Dev. Ctries. 2018, 12, 220–227. [Google Scholar] [CrossRef]
- Alqasim, A.; Abu Jaffal, A.; Alyousef, A.A. Prevalence of Multidrug Resistance and Extended-Spectrum β-Lactamase Carriage of Clinical Uropathogenic Escherichia coli Isolates in Riyadh, Saudi Arabia. Int. J. Microbiol. 2018, 2018, 3026851. [Google Scholar] [CrossRef] [PubMed]
- Al Wutayd, O.; Nafeesah, A.A.; Adam, I.; Babikir, I. The antibiotic susceptibility patterns of uropathogens isolated in Qassim, Saudi Arabia. J. Infect. Dev. Ctries. 2018, 12, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, M.Q.; Alqahtani, F.Y.; Aleanizy, F.S. An evaluation of E. coli in urinary tract infection in emergency department at KAMC in Riyadh, Saudi Arabia: Retrospective study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, S.; Albalawi, B.; Shwameen, M.; Alharbi, M. Bacterial Susceptibility to Antibiotics in Urinary Tract Infections in Children, KSAFH, Saudi Arabia, Tabuk. Egypt. J. Hosp. Med. 2018, 73, 6952–6954. [Google Scholar] [CrossRef]
- Alamri, A.; Hamid, M.E.; Abid, M.; Alwahhabi, A.M.; Alqahtani, K.M.; Alqarni, M.S.; Abomughaid, M. Trend analysis of bacterial uropathogens and their susceptibility pattern: A 4-year (2013–2016) study from Aseer region, Saudi Arabia. Urol Ann. 2018, 10, 41–46. [Google Scholar] [CrossRef]
- Taher, I.; Almaeen, A.; Aljourfi, H.; Bohassan, E.; Helmy, A.; El-Masry, E.; Saleh, B.; Aljaber, N. Surveillance of antibiotic resistance among uropathogens in Aljouf region northern Saudi Arabia. Iran. J. Microbiol. 2019, 11, 468. [Google Scholar] [CrossRef]
- Alsohaim, S.I.A.; Bawadikji, A.A.; Elkalmi, R.; Mahmud, M.I.A.M.; Hassali, M.A. Relationship Between Antimicrobial Prescribing and Antimicrobial Resistance Among UTI Patients at Buraidah Central Hospital, Saudi Arabia. J. Pharm. Bioallied Sci. 2019, 11, 162–169. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Shariq, A.; Alsalloom, A.A.; Babikir, I.H.; Alhomoud, B.N. Uropathogens and their antimicrobial resistance patterns: Relationship with urinary tract infections. Int. J. Health Sci. 2019, 13, 48–55. [Google Scholar]
- Hameed, T.; Al Nafeesah, A.; Chishti, S.; Al Shaalan, M.; Al Fakeeh, K. Community-acquired urinary tract infections in children: Resistance patterns of uropathogens in a tertiary care center in Saudi Arabia. Int. J. Pediatr. Adolesc. Med. 2019, 6, 51–54. [Google Scholar] [CrossRef]
- Bazaid, A.S.; Saeed, A.; Alrashidi, A.; Alrashidi, A.; Alshaghdali, K.; Hammam, S.A.; Alreshidi, T.; Alshammary, M.; Alarfaj, A.; Thallab, R.; et al. Antimicrobial Surveillance for Bacterial Uropathogens in Ha’il, Saudi Arabia: A Five-Year Multicenter Retrospective Study. Infect. Drug Resist. 2021, 14, 1455–1465. [Google Scholar] [CrossRef]
- Alamri, A.; Hassan, B.; Hamid, M.E. Susceptibility of hospital-acquired uropathogens to first-line antimicrobial agents at a tertiary health-care hospital, Saudi Arabia. Urol. Ann. 2021, 13, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, M.A.; Sadoma, H.H.M.; Mathew, S.; Alghamdi, S.; Malik, J.A.; Anwar, S. Retrospective Analysis of Antimicrobial Susceptibility of Uropathogens Isolated from Pediatric Patients in Tertiary Hospital at Al-Baha Region, Saudi Arabia. Healthcare 2021, 9, 1564. [Google Scholar] [CrossRef] [PubMed]
- Badger-Emeka, L.I.; Kausar, N.; Estrella, E.; Angeles, G.B. A Three-Year Look at the Phylogenetic Profile, Antimicrobial Resistance, and Associated Virulence Genes of Uropathogenic Escherichia coli. Pathogens 2022, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Alsubaie, M.A.; Alsuheili, A.Z.; Aljehani, M.N.; Alothman, A.A.; Alzahrani, A.S.; Mohammedfadel, H.A.; Alshehry, M.A.; Alnajjar, A.A. Antibiotic resistance patterns of pediatric community-acquired urinary tract infections in a tertiary care center in Jeddah, Saudi Arabia. J. Infect. Dev. Ctries. 2023, 17, 1430–1435. [Google Scholar] [CrossRef]
- Aljohani, R.H.; ElFeky, D.S.; Alswaji, A.A.; Alrashidi, E.; Okdah, L.; Alalwan, B.; Aljohani, S.M.; Balkhy, H.H.; Redhwan, A.; Alghoribi, M.F. Genomic Characterization of Uropathogenic Escherichia coli Isolates from Tertiary Hospitals in Riyadh, Saudi Arabia. Int. J. Mol. Sci. 2023, 24, 7582. [Google Scholar] [CrossRef]
- Altamimi, I.; Almazyed, A.; Alshammary, S.; Altamimi, A.; Alhumimidi, A.; Alnutaifi, R.; Malhis, M.; Altamimi, A. Bacterial Pathogens and Antimicrobial Susceptibility Patterns of Urinary Tract Infections in Children during COVID-19 2019–2020: A Large Tertiary Care Center in Saudi Arabia. Children 2023, 10, 971. [Google Scholar] [CrossRef]
- Alghamdi, S.A.A.; Mir, S.S.; Alghamdi, F.S.; Al Banghali, M.A.M.M.A.; Almalki, S.S.R. Evaluation of Extended-Spectrum Beta-Lactamase Resistance in Uropathogenic Escherichia coli Isolates from Urinary Tract Infection Patients in Al-Baha, Saudi Arabia. Microorganisms 2023, 11, 2820. [Google Scholar] [CrossRef]
- Antimicrobial Stewardship Subcommittee of the National Antimicrobial Resistance Committee and the General Administration of Pharmaceutical Care at Ministry of Health, Saudi Arabia. National Antimicrobial Therapy Guidelines for Community and Hospital Acquired Infections in Adults. 2018. Available online: https://www.moh.gov.sa/en/CCC/healthp/regulations/Documents/National%20Antimicrobial%20%20Guidelines.pdf (accessed on 15 December 2023).
- Azim, N.; Al-Harbi, M.; Al-Zaban, M.; Nofal, M. Prevalence and Antibiotic Susceptibility among Gram Negative Bacteria Isolated from Intensive Care Units at a Tertiary Care Hospital in Riyadh, Saudi Arabia. J. Pure Appl. Microbiol. 2019, 13, 201–208. [Google Scholar] [CrossRef]
- Ibrahim, M.; Bilal, N.; Hamid, M. Comparison of Phenotypic Characteristics and Antimicrobial Resistance Patterns of Clinical Escherichia coli Collected from Two Unrelated Geographical Areas. Glob. J. Health Sci. 2014, 6, 126–135. [Google Scholar] [CrossRef]
- Arafa, S.H.; Alshehri, W.A.; Organji, S.R.; Elbanna, K.; Obaid, N.A.; Aldosari, M.S.; Asiri, F.H.; Ahmad, I.; Abulreesh, H.H. Antimicrobial Resistance, Virulence Factor-Encoding Genes, and Biofilm-Forming Ability of Community-Associated Uropathogenic Escherichia coli in Western Saudi Arabia. Pol. J. Microbiol. 2022, 71, 325–339. [Google Scholar] [CrossRef]
- Alghoribi, M.F.; Doumith, M.; Upton, M.; Al Johani, S.M.; Alzayer, M.; Woodford, N.; Ellington, M.J.; Balkhy, H.H. Complete Genome Sequence of a Colistin-Resistant Uropathogenic Escherichia coli Sequence Type 131 fimH22 Strain Harboring mcr-1 on an IncHI2 Plasmid, Isolated in Riyadh, Saudi Arabia. Microbiol. Resour. Announc. 2019, 8, e00104-19. [Google Scholar] [CrossRef] [PubMed]
- Alqasim, A.; Abu Jaffal, A.; Alyousef, A.A. Prevalence and molecular characteristics of sequence type 131 clone among clinical uropathogenic Escherichia coli isolates in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Gaber, A.; Alsanie, W.F.; El-Hallous, E.I.; Mohamed, A.A.; Alharthi, A.A.; Ibrahim, A.M. Phylogeny and Detection of blaTEM, blaSHV, blaCTX-M Genes in Escherichia coli Isolates from Patients with Urinary Tract Infections in Taif Hospitals, Saudi Arabia. Annu. Res. Rev. Biol. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Ghany, M.A.E.; Sharaf, H.; Al-agamy, M.H.; Shibl, A.; Hill-Cawthorne, G.A.; Hong, P.-Y. Genomic characterization of NDM-1 and 5, and OXA-181 carbapenemases in uropathogenic Escherichia coli isolates from Riyadh, Saudi Arabia. PLoS ONE 2018, 13, e0201613. [Google Scholar] [CrossRef]
- Chhabra, S.; Taksande, A.B.; Munjewar, P. The Penicillin Pioneer: Alexander Fleming’s Journey to a Medical Breakthrough. Cureus 2024, 16, e65179. [Google Scholar] [CrossRef]
- Salzberger, B.; Fätkenheuer, G. A brief history of infectious diseases—The last 150 years. Dtsch. Med. Wochenschr. 1946 2024, 149, 1507–1513. [Google Scholar] [CrossRef]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef]
- Ahmed, D.S.; AboElela, A.M.; Ismail, S.S.; Hammour, Z.E.; Fawaz, R.A.; Abdelmoniem, M.E. Pattern of antibiotic use among children caregivers: A cross-sectional study. J. Egypt. Public Health Assoc. 2024, 99, 33. [Google Scholar] [CrossRef]
- Al-Mehmadi, B.; Alsubaie, S.; Al-Morikhi, O.; Alqahtani, F.; Almutairi, W.; Al-Mutairi, M.; Alotaibi, M.; Alenazi, S.; Alanazi, K. Knowledge and Attitude of self-medication with leftover antibiotics in Saudi Arabia: A cross-sectional study. F1000Research 2023, 12, 304. [Google Scholar] [CrossRef]
- Nguyen, S.H.; Tran, M.T. Enzyme-free biosensor utilizing chitosan-capped ZnS doped by Mn nanomaterials for tetracycline hydrochloride detection. Heliyon 2024, 10, e40340. [Google Scholar] [CrossRef]
- Bin Abdulhak, A.A.; Altannir, M.A.; Almansor, M.A.; Almohaya, M.S.; Onazi, A.S.; Marei, M.A.; Aldossary, O.F.; Obeidat, S.A.; Obeidat, M.A.; Riaz, M.S.; et al. Non prescribed sale of antibiotics in Riyadh, Saudi Arabia: A Cross Sectional Study. BMC Public Health 2011, 11, 538. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.L.; Navarro, R.; Watanabe, T.; Morán, F.; Balmaceda, M.P.; Reateguí, A.; Elias, R.; Bardellini, M.; Ochoa, T.J. Knowledge, attitudes and practices of parents towards antibiotic use in rural communities in Peru: A cross-sectional multicentre study. BMC Public Health 2022, 22, 459. [Google Scholar] [CrossRef] [PubMed]
- Akbar, Z.; Alquwez, N.; Alsolais, A.; Thazha, S.K.; Ahmad, M.D.; Cruz, J.P. Knowledge about antibiotics and antibiotic resistance among health-related students in a Saudi University. J. Infect. Dev. Ctries. 2021, 15, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, M.Q.; Salam, M.; Alqahtani, F.Y.; Ahmed, A.E.; Alenaze, A.Q.; Al-Jeraisy, M.; Al Salamah, M.; Aleanizy, F.S.; Al Daham, D.; Al Obaidy, S.; et al. An Evaluation of Antibiotics Prescribing Patterns in the Emergency Department of a Tertiary Care Hospital in Saudi Arabia. Infect. Drug Resist. 2019, 12, 3241–3247. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, M.Q.; AlQahtani, H.; Almangour, T.A.; Aleanizy, F.S.; Alqahtani, F.Y. Evaluation of the Clinical Outcome and Cost Analysis of Antibiotics in the Treatment of Acute Respiratory Tract Infections in the Emergency Department in Saudi Arabia. Antibiotics 2022, 11, 1478. [Google Scholar] [CrossRef]
- Chaaban, T.; Ezzeddine, Z.; Ghssein, G. Antibiotic Misuse During the COVID-19 Pandemic in Lebanon: A Cross-Sectional Study. COVID 2024, 4, 921–929. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Ghssein, G. Towards new antibiotics classes targeting bacterial metallophores. Microb. Pathog. 2023, 182, 106221. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Chandrangsu, P.; Rensing, C.; Helmann, J.D. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 2017, 15, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Vaitkus, S.; Simoes-Torigoe, R.; Wong, N.; Morris, K.; Spada, F.E.; Alagiri, M.; Talke, F.E. A comparative study of experimental urinary catheters containing silver and zinc for biofilm inhibition. J. Biomater. Appl. 2021, 35, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).