The Genus Clonostachys (Bionectria) as a Potential Tool Against Agricultural Pest and Other Biotechnological Applications: A Review
Abstract
1. Introduction
1.1. Public Health Risks of Using Chemical Pesticides
1.2. Environmental Risks of Using Chemical Pesticides
1.3. Effects of Pesticides on Pollinating Organisms
1.4. Pesticide Resistance in Plague Organisms
1.5. Side Effects on the Environment
Side Effects and Public Health Risks of Using Chemical Pesticides
1.6. Sustainable Alternative Methods of Control of Plague of Importance in Agriculture
Clonostachys spp. as an Alternative to the Use of Chemically Synthesized Pesticides
1.7. Genus Clonostachys
1.7.1. General Aspects
1.7.2. General Morphological Aspects of Clonostachys Genus
1.8. Uses and Other Applications of Clonostachys Species
1.9. Clonostachys as a Biological Control Agent of Pests of Importance in Agriculture
1.9.1. Entomopathogenic Activity
1.9.2. Mycoparasitic Activity
1.9.3. Nematocidal Activity
1.9.4. Clonostachys as a Biological Control Agent of Pests of Importance in the Livestock Industry
1.10. Other Biotechnological Applications of Species of Clonostachys Genus
1.10.1. Endophytic and Anticancer Properties of Clonostachys
1.10.2. Biological Activities of Secondary Metabolites Identified in Clonostachys Genus
1.11. Undesirable Effects in the Use of Some Clonostachys Strains
1.12. Limitations in the Use of Clonostachys Strains
1.13. Future Perspectives and Challenges
2. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. News Article: Climate Change Fans Spread of Pests and Threatens Plants and Crops, New FAO Study. Available online: https://www.fao.org/newsroom/detail/Climate-change-fans-spread-of-pests-and-threatens-plants-and-crops-new-FAO-study/en (accessed on 28 November 2024).
- Rani, A.K.P.; Gowrishankar, S. Pathogen-based classification of plant diseases: A deep transfer learning approach for intelligent support systems. IEEE Acces 2023, 11, 64476–64493. [Google Scholar] [CrossRef]
- Jankielsohn, A. The importance of insects in agricultural ecosystems. Adv. Entomol. 2018, 6, 62–73. [Google Scholar] [CrossRef]
- Anggraini, E.; Anisa, W.N.; Herlinda, S.; Irsan, C.; Suparman, S.; Suwandi, S.; Harum, M.U.; Gunawan, B. Phytophagous insects and predatory arthropods in soybean and zinnia. Biodiversitas J. Biol. Div. 2021, 22, 1405–1414. [Google Scholar] [CrossRef]
- Allali, A.; Rezouki, S.; Lougraimzi, H.; Touati, E.; Eloutassi, N.; Fadli, M. Agricultural traditional practices and risks of using insecticides during seed storage in Morocco. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 29–37. [Google Scholar]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public. Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, Y.E.; Kambal, N.; et al. Pesticides impacts on human health and the environment with their mechanisms of action and possible countermeasures. Heliyon 2024, 10, e29129. [Google Scholar] [CrossRef] [PubMed]
- Cioato, F.M.; Stedile, N.L.R.; Lucas, J.I.P. The use of pesticides and the development of cancer in farmers: A scoping review. Saúde Debate 2025, 49, e9298. [Google Scholar] [CrossRef]
- Leyte, Y.L.; Duque, T.S.; dos Santos, J.B.; dos Santos, E.A. Potential Residual Pesticide Consumption: A Stratified Analysis of Brazilian Families. JoX 2025, 15, 37. [Google Scholar] [CrossRef]
- Beyuo, J.; Sackey, L.N.; Yeboah, C.; Kayoung, P.Y.; Koudadje, D. The implications of pesticide residue in food crops on human health: A critical review. Discov. Agric. 2024, 2, 123. [Google Scholar] [CrossRef]
- Feng, X.; Liu, Z.; Duy, S.V.; Parent, L.; Barbeau, B.; Sauvé, S. Temporal trends of 46 pesticides and 8 transformation products in surface and drinking water in Québec, Canada (2021–2023): Potential higher health risks of transformation products than parent pesticides. Water Res. 2025, 277, 123339. [Google Scholar] [CrossRef]
- MAFF. Pesticide Toxicity. Ministry of Agriculture, Food and Fisheries. British Columbia. 2022. Available online: https://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/agriculture-and-seafood/animal-and-crops/plant-health/pesticide-toxicity-hazard.pdf (accessed on 24 March 2025).
- Yang, M.; Wang, Y.; Yang, G.; Wang, Y.; Liu, F.; Chen, C. A review of cumulative risk assessment of multiple pesticide residues in food: Current status, approaches and future perspectives. Trends Food Sci. Technol. 2024, 144, 104340. [Google Scholar] [CrossRef]
- Alum, E.U. The role of toxicology in climate change: Understanding the risks of novel environmental toxins. Sustain. Environ. 2025, 11, 2467485. [Google Scholar] [CrossRef]
- Ansari, I.; El-Kady, M.M.; Mahmoud, A.E.D.; Arora, C.; Verma, A.; Rajarathinam, R.K.; Singh, P.; Verma, D.K.; Mittal, J. Persistent pesticides: Accumulation, health risk assessment, management and remediation: An overview. Desalin Water Treat. 2024, 317, 100274. [Google Scholar] [CrossRef]
- Nsaif, A.A.A.; Aljawhar, N.M.; Jassim, S.; Nazar, Z. Pesticides and Their Effects on Gut Microbiota: A Comprehensive Review of Environmental and Health Consequences. Med. Sci. J. Adv. Res. 2024, 5, 1–13. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, K.O.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, S.T.; Moussa, M.A.; Gomaa, S.I.; Qiu, C.L.; Wang, M.Q. Agrochemicals: Safety evaluation and characterization for humans and biodiversity. In Sustainable Development and Biodiversity; Ogwu, M.C., Chibuese, I.S., Eds.; Springer: Singapore, 2023; Volume 34, pp. 3–51. [Google Scholar] [CrossRef]
- Xie, P.P.; Zong, Z.Q.; Qiao, J.C.; Li, Z.Y.; Hu, C.Y. Exposure to pesticides and risk of colorectal cancer: A systematic review and meta-analysis. Environ. Pollut. 2024, 345, 123530. [Google Scholar] [CrossRef]
- Kushwaha, A.; Gabre, R.M.; Srivastava, S.; íguez-Morales, A.J.; Mohanty, A.; Sharma, S.; Sah, S.; Apostolopoulos, V.; Ulloque-Badaraco, R.; Rodríguez-Molrales, A.J. The Revolving Outbreaks of Japanese Encephalitis in Nepal. Curr. Trop. Med. Rep. 2025, 12, 8. [Google Scholar] [CrossRef]
- AbdAllah, O.R.; Gabre, R.M.; Mohammed, S.A.; Korayem, A.M.; Hussein, A.E.; Ahmad, A.A. Evaluating the role of synanthropic filth flies in the transmission of zoonotic parasites: Field and laboratory evidence from different animal rearing sites in upper Egypt with focus on Cryptosporidium spp. BMC Vet. Res. 2025, 21, 188. [Google Scholar] [CrossRef]
- Cardinal, M.V.; Enriquez, G.F.; Gaspe, M.S.; Fernández, M.D.P.; Capello, V.; Gürtler, R.E. Estimation of Trypanosoma cruzi infection in the main vector Triatoma infestans: Accounting for imperfect detection using site-occupancy models. Parasit. Vectors 2025, 18, 58. [Google Scholar] [CrossRef]
- Lorenz, E.S. Potential health effects of pesticides. Ag. Commun. Mark. 2009, 1. Available online: https://extension.psu.edu/potential-health-effects-of-pesticides (accessed on 27 March 2025).
- Bailey, H.D.; Infante-Rivard, C.; Metayer, C.; Clavel, J.; Lightfoot, T.; Kaatsch, P.; Roman, E.; Magnani, C.; Spector, L.G.; Th Petridou, E.; et al. Home pesticide exposures and risk of childhood leukemia: Findings from the childhood leukemia international consortium. Int. J. Cancer 2015, 137, 2644–2663. [Google Scholar] [CrossRef]
- PAN-UK. Impacts of Pesticide on the Environment. Pesticide Action Network, UK. 2017. Available online: https://www.pan-uk.org/our-environment/ (accessed on 27 March 2025).
- Triana-Velásquez, T.M.; Bernal-Bautista, M.H. Acute toxicity of the insecticide Imidacloprid and the herbicide 2,4-D in two species of tropical anurans. Ecotoxicology 2025, 34, 392–400. [Google Scholar] [CrossRef]
- Minutoli, R.; Fazio, F.; Granata, A.; Aragona, F.; Parrino, V. Pesticide and hydrocarbon toxicity in fish: Effects on Chelon labrosus (Risso, 1827) along the northeastern Sicilian coast (Italy) evaluated by enzymatic biomarkers. J. Environ. Sci. Health B 2025, 60, 139–147. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Highly hazardous Pesticides (HHPs). UN Environment Programme. 2025. Available online: https://www.unep.org/topics/chemicals-management/pollution-and-health/highly-hazardous-pesticides-hhps (accessed on 27 March 2025).
- Daisley, B.A.; Chernyshova, A.M.; Thompson, G.J.; Allen-Vercoe, E. Deteriorating microbiomes in agriculture-the unintended effects of pesticides on microbial life. Microbiome Res. Rep. 2022, 1, 6. [Google Scholar] [CrossRef]
- Onwudiegwu, C.; Nabebe, G.; Izah, S.C. Environmental and Public Health Implications of Pesticide Residues: From Soil Contamination to Policy Interventions. Greener J. Biol. Sci. 2025, 15, 1–12. [Google Scholar] [CrossRef]
- Wan, N.F.; Fu, L.; Dainese, M.; Kiær, L.P.; Hu, Y.Q.; Xin, F.; Goulson, D.; Woodcock, A.A.; Vanvergen, A.J.; Spurgeon, D.J.; et al. Pesticides have negative effects on non-target organisms. Nat. Com. 2025, 16, 1360. [Google Scholar] [CrossRef]
- Ali, I.M. Harmful effects of pesticides on the Environment and human health: A review. DASJ 2016, 15, 114–126. [Google Scholar] [CrossRef]
- Tandon, V.; Kumar, A.; Rana, C.; Rana, A. Pollination—Its type, threats and role in environment conservation. Int. J. Curr. Res. 2016, 8, 35744–35751. [Google Scholar]
- Ollerton, J. Pollinator diversity: Distribution, ecological function, and conservation. Ann. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Klatt, B.K.; Holzschuh, A.; Westphal, C.; Clough, Y.; Smit, I.; Pawelsik, E.; Tscharntke, T. Bee pollination improves crop quality, shelf life and commercial value. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132440. [Google Scholar] [CrossRef]
- Vanbergen, A. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef]
- Gebhardt, S.; van Dijk, J.; Lof, M.E.; Wassen, M.J.; Baker, M. Understanding interactive effects between habitat configuration and pesticide use for pollination: Towards better informed landscape management. Ecol. Proces. 2025, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Chreil, R.; Maggi, C. Pesticides and pollinators. Pollinators 2023, 6, 115–124. [Google Scholar]
- Kumari, P.; Lohar, S.; Godara, V.; Prajapati, M.K.; Nagar, V.; Singhal, A.; Mavry, B.; Pandit, O.O.; Sharma, A.; Rai, A.R.; et al. Exploring the Adverse Impact of Pesticides in Honey Bees and Their Virulence. Lett. Appl. Nano BioSicence 2024, 13, 68. [Google Scholar] [CrossRef]
- Albeltagy, A.M. Pesticide Resistance: The global problem of pest management. In Proceedings of the Conference: Entomology Society of America, Knoxville, TN, USA, 11–14 November 2012. [Google Scholar]
- Malook, U.S.; Arora, A.K.; Wong, A.C.N. The role of microbiomes in shaping insecticide resistance: Current insights and emerging paradigms. Curr. Opin. Insect Sci. 2025, 26, 101346. [Google Scholar] [CrossRef]
- Liang, A.; Zhang, Y.; Xu, X.; Wang, H.; Gong, C.; Hu, J.; Li, X.; Yang, J.; Peng, A.; Wang, X. Eco-friendly chitosan base chlorantraniliprole nano-pesticides for effective control of Chilo suppressalis (walker) through bidirectional transport. Environ. Sci. Nano 2025, 12, 1214–1229. [Google Scholar] [CrossRef]
- Khan, S.; Uddin, M.N.; Rizwan, M.; Khan, W.; Farooq, M.; Shah, A.S.; Subhan, F.; Aziz, F.; Rahman, K.U.; Khan, A.; et al. Mechanism of Insecticide Resistance in Insects/Pests. Pol. J. Environ. Stud. 2020, 29, 2023–2030. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Yuan, J.; Wang, S.; Xu, B.; Wang, S.; Zhang, Y.; Wu, Q. Insecticide Resistance Monitoring of the Diamondback Moth (Lepidoptera: Plutellidae) Populations in China. J. Econ. Entomol. 2021, 114, 1282–1290. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, C.; Wang, R. Insecticide resistance and its management in two invasive cryptic species of Bemisia tabaci in China. Int. J. Mol. Sci. 2023, 24, 6048. [Google Scholar] [CrossRef]
- Shahi, G.; Kumar, M.; Skwarecki, A.S.; Edmondson, M.; Banerjee, A.; Usher, J.; Gow, N.A.R.; Milewski, S.; Prasad, R. Fluconazole resistant Candida auris clinical isolates have increased levels of cell wall chitin and increased susceptibility to a glucosamine-6-phosphate synthase inhibitor. Cell Surf. 2022, 25, 100076. [Google Scholar] [CrossRef] [PubMed]
- Mammoo, J.; Bruntha, P.M.; Saha, A. Detecting Pesticide Contamination in Fruits and Vegetables with Deep Learning Models. In Proceedings of the 2025 6th International Conference on Mobile Computing and Sustainable Informatics (ICMCSI), Villach, Austria, 7–8 January 2025; pp. 1084–1090. [Google Scholar]
- Alzohairy, S.A.; Gillett, J.; Saito, S.; Naegele, R.N.; Xiao, C.L.; Miles, T.D. Fungicide resistance profiles of Botrytis cinerea isolates from Michigan vineyards and development of a TaqMan assay for detection of fenhexamid resistance. Plant Dis. 2021, 105, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torres, P. Molecular mechanisms underlying fungicide resistance in citrus postharvest green mold. JoF 2021, 7, 783. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mao, Y.; Li, S.; Li, T.; Wang, J.; Zhou, M.; Duan, Y. Molecular mechanism of Sclerotinia sclerotiorum resistance to succinate dehydrogenase inhibitor fungicides. J. Agric. Food Chem. 2022, 70, 7039–7048. [Google Scholar] [CrossRef]
- Wang, R.; Chen, B.; Yue, M.; Ding, W.; Li, Y. Multi-resistance of Botrytis cinerea isolates from ginseng growing regions in China to carbendazim, iprodione and pyrimethanil. Crop Prot. 2022, 156, 105929. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, Y.; Zhang, Q.; Zhao, L.; Zhu, Y.; Wu, Y.; Li, Z.; Yang, W. Detection of fungicide resistance to fludioxonil and tebuconazole in Fusarium pseudograminearum, the causal agent of Fusarium crown rot in wheat. PeerJ 2023, 11, e14705. [Google Scholar] [CrossRef]
- Beg, M.A.; Aktaruzzaman, M.; Lewis, K.J.; Oliver, J.E. Fungicide resistance profiles of Alternaria spp. associated with fruit rot of blueberry in Georgia, USA. Front. Plant Sci. 2025, 16, 1524586. [Google Scholar] [CrossRef] [PubMed]
- Meher, H.C.; Gajbhiye, V.T.; Chawla, G.; Singh, G. Virulence development and genetic polymorphism in Meloidogyne incognita (Kofoid & White) Chitwood after prolonged exposure to sublethal concentrations of nematicides and continuous growing of resistant tomato cultivars. Pest. Manag. Sci. 2009, 65, 1201–1207. [Google Scholar] [CrossRef]
- Adamou, H.; Mamadou, A.; Adamou, B.; Ali, D.; Toudou, A. On-farm testing of Savanem 20 EC (Ethoprophos) for control of plant parasitic nematodes associated with pepper (Capsicum annuum) in Tillaberi (Niger). Asian J. Agricl Sci. 2013, 5, 83–87. [Google Scholar] [CrossRef]
- Saraiva, R.M.; Borges, A.V.; Borel, F.C.; Maffia, L.A. Compounds produced by Clonostachys rosea deletereous to Botrytis cinerea. Braz. J. Agric. 2020, 695, 34–37. [Google Scholar] [CrossRef]
- Sharma, R.; Walia, A.; Putatunda, C.; Solanki, P. Impact of pesticides on microbial diversity. In Current Developments in Biotechnology and Bioengineering; Singh, J., Pandey, A., Singh, S., Kumar, G.V., Praveen, R., Eds.; Elsevier: Nueva Delhi, India, 2023; pp. 427–458. [Google Scholar]
- Mendoza-de Gives, P. Soil-Borne nematodes: Impact in agriculture and livestock and sustainable strategies of prevention and control with special reference to the use of nematode natural enemies. Pathogens 2022, 11, 640. [Google Scholar] [CrossRef]
- Sikora, R.A.; Helder, J.; Molendijk, L.P.; Desaeger, J.; Eves-van den Akker, S.; Mahlein, A.K. Integrated nematode management in a world in transition: Constraints, policy, processes, and technologies for the future. Ann. Rev. Phytopathol. 2023, 61, 209–230. [Google Scholar] [CrossRef]
- Sarri, K.; Mourouzidou, S.; Ntalli, N.; Monokrousos, N. Recent advances and developments in the nematicidal activity of essential oils and their components against root-knot nematodes. Agronomy 2024, 14, 213. [Google Scholar] [CrossRef]
- Shahid, M.; Gowen, S.R.; Burhan, M.; Niaz, Z.; Haq, A.U. Studies on the efficacy of heterogeneously produced Pasteuria penetrans (PP3) isolate over individual Pasteuria isolates in the spore attachment, and pathogenic potential on three Meloidogyne species. Plant Prot. 2023, 7, 9–16. [Google Scholar] [CrossRef]
- Ayaz, M.; Zhao, J.T.; Zhao, W.; Chi, Y.K.; Ali, Q.; Ali, F.; Khan, A.R.; Yu, Q.; Yu, J.W.; Wu, W.C.; et al. Biocontrol of plant parasitic nematodes by bacteria and fungi: A multi-omics approach for the exploration of novel nematicides in sustainable agriculture. Front. Microbiol. 2024, 15, 1433716. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Bioch Mol. Biol. 2011, 41, 23–431. [Google Scholar] [CrossRef]
- Liang, Z.; Ali, Q.; Wang, Y.; Mu, G.; Kan, X.; Ren, Y.; Manghwar, H.; Gu, Q.; Wu, H.; Gao, X. Toxicity of Bacillus thuringiensis strains derived from the novel crystal protein Cry31Aa with high nematicidal activity against rice parasitic nematode Aphelenchoides besseyi. Int. J. Mol. Sci. 2022, 23, 8189. [Google Scholar] [CrossRef]
- da Silva, M.E.D.; Uriostegui, M.A.M.; Millán-Orozco, J.; Mendoza-de Gives, P.; Hernández, E.L.; Braga, F.R.; Araújo, J.V.D. Predatory activity of Butlerius nematodes and nematophagous fungi against Haemonchus contortus infective larvae. Braz. J. Vet. Parasitol. 2017, 26, 92–95. [Google Scholar] [CrossRef]
- Manochaya, S.; Udikeri, S.; Srinath, B.S.; Sairam, M.; Bandlamori, S.V.; Ramakrishna, K. In vivo culturing of entomopathogenic nematodes for biological control of insect pests: A review. J. Nat. Pest. Res. 2022, 1, 100005. [Google Scholar] [CrossRef]
- Yüksel, E.; Ormanoğlu, N.; Imren, M.; Canhilal, R. Assessment of biocontrol potential of different Steinernema species and their bacterial symbionts, Xenorhabdus species against larvae of almond moth, Ephestia cautella (Walker). J. Stored Prod. Res. 2023, 101, 102082. [Google Scholar] [CrossRef]
- Aguilar-Marcelino, L.; Quintero-Martínez, M.T.; Mendoza-de Gives, P.; López-Arellano, M.E.; Liébano-Hernández, E.; Torres-Hernández, G.; Del Prado, I.C. Evaluation of predation of the mite Lasioseius penicilliger (Aracnida: Mesostigmata) on Haemonchus contortus and bacteria-feeding nematodes. J. Helminthol. 2014, 88, 20–23. [Google Scholar] [CrossRef]
- Rodríguez-Esquivel, D.L.; Ocampo-Gutiérrez, A.Y.; Olmedo-Juárez, A.; López-Arellano, M.E.; Hernández-Romano, J.; Aguilar-Marcelino, L.; Mendoza-de Gives, P. Using Arthrobotrys oligospora (Orbiliales) spores mixed with sterile sheep faeces for disinfesting soil micro-plots infested with Nacobbus aberrans (Nematoda: Pratylenchidae). Biocon Sci. Tech. 2023, 34, 96–110. [Google Scholar] [CrossRef]
- Nagaraj, G.; Kannan, R.; Raguchander, T.; Narayanan, S.; Saravanakumar, D. Nematicidal action of Clonostachys rosea against Meloidogyne incognita: In-vitro and in-silico analyses. J. Taibah Univ. Sci. 2024, 18. [Google Scholar] [CrossRef]
- Sun, Z.B.; Li, S.D.; Ren, Q.; Xu, J.L.; Lu, X.; Sun, M.H. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020, 129, 486–495. [Google Scholar] [CrossRef]
- Costa, D.A.; Williams, T.C.; do Vale, L.H.F.; Edivaldo Filho, X.F. Characterization of mannanases from Clonostachys byssicola involved in the breakdown of lignocellulosic substrates. Biocatal. Agric. Biotechnol. 2023, 50, 102680. [Google Scholar] [CrossRef]
- Schroers, H.-F.; Samuels, G.J.; Seifert, K.A. Classification of the mycoparasite Gliocladium roseum in Clonostachys as C. rosea its relationship to Bionectria ochroleuca, and notes on other Gliocladium-like fungi. Mycologia 1999, 91, 365–385. [Google Scholar] [CrossRef]
- Sutton, J.C.; Liu, W.; Ma, J.; Brown, W.G.; Stewart, J.F.; Walker, G.D. Evaluation of the fungal endophyte Clonostachys rosea as an inoculant to enhance growth, fitness, and productivity of crop plants. Acta Hortic. 2006, 782, 279–286. [Google Scholar] [CrossRef]
- Kapeua-Ndacnou, M.; de Abreu, L.M.; de Macedo, D.M.; da Nóbrega, T.F.; Pereira, C.M.; Evans, H.C.; Barreto, R.W. Assessing the biocontrol potential of Clonostachys species isolated as endophytes from Coffea species and as mycoparasites of Hemileia rusts of coffee in Africa. JoF 2023, 9, 248. [Google Scholar] [CrossRef]
- Funck-Jensen, D.; Dubey, M.; Jensen, B.; Karlsson, M. Clonostachys rosea to control plant diseases. In Microbial Bioprotectants for Plant Disease Management; Funck-Jensen, D., Dubey, M., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; Volume 3, pp. 429–472. [Google Scholar]
- Nobre, S.A.; Maffia, L.A.; Mizubuti, E.S.; Cota, L.V.; Dias, A.P.S. Selection of Clonostachys rosea isolates from Brazilian ecosystems effective in controlling Botrytis cinerea. Biol. Control 2005, 34, 132–143. [Google Scholar] [CrossRef]
- Mueller, J.D.; Sinclair, J.B. Occurrence and role of Gliocladium roseum in field-grown soybeans in Illinois. Trans. Brit. Mycol. Soc. 1986, 86, 677–680. [Google Scholar] [CrossRef]
- Index-Fungorum. Available online: https://www.indexfungorum.org/names/names.asp (accessed on 28 December 2024).
- Mahmoudi, H.; Amini, A.; Mirzaee, M.R.; Sadeghi, H.; Tavakkoli, G.R. Clonostachys rosea, a new and promising entomopathogenic fungus infecting pupa of jujube fruit fly, Carpomya vesuviana. Mycol. Iran. 2018, 5, 43–49. [Google Scholar] [CrossRef]
- Waheed, A.W.; Ali, S.; Nawaz, K.; Iftikhar, S.; Javed, M.A.; Hashem, A.; Akhter, A. Entomopathogenic fungus Clonostachys rosea as a biocontrol agent against whitefly (Bemisia tabaci). Biocontrol Sci. Technol. 2018, 28, 750–760. [Google Scholar] [CrossRef]
- da Silva, M.E.; Braga, F.R.; Mendoza-de Gives, P.; Uriostegui, M.A.; Reyes, M.; Soares, F.E.F.; De Carvalho, L.M.; Rodrigues, F.B.; Araújo, J.V.D. Efficacy of Clonostachys rosea and Duddingtonia flagrans in reducing the Haemonchus contortus infective larvae. BioMed Res. Int. 2015. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.; Mendoza-de-Gives, P.; Aguilar-Marcelino, L.; López-Arellano, M.E.; Gamboa-Angulo, M.; Hanako Rosas-Saito, G.; Guadalupe García-Rubio, V. In vitro lethal activity of the nematophagous fungus Clonostachys rosea (Ascomycota: Hypocreales) against nematodes of five different taxa. BioMed Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nygren, K.; Dubey, M.; Zapparata, A.; Iqbal, M.; Tzelepis, G.D.; Durling, M.B.; Jensen, D.F.; Karlsson, M. The mycoparasitic fungus Clonostachys rosea responds with both common and specific gene expression during interspecific interactions with fungal prey. Evol. Appl. 2018, 11, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Salamone, A.L.; Gundersen, B.; Inglis, D.A. Clonostachys rosea, a potential biological control agent for Rhizoctonia solani AG-3 causing black scurf on potato. Biocon Sci. Tech. 2018, 28, 895–900. [Google Scholar] [CrossRef]
- Gowrisri, N.; Elango, K. Unveiling the Antimicrobial and Biocontrol Potential of the Ascomycete Fungus, Clonostachys rosea: A Review. Microbe 2024, 6. [Google Scholar] [CrossRef]
- Schroers, H.J. A monograph of Bionectria (Ascomycota, Hypocreales, Bionectriaceae) and its Clonostachys anamorphs. Stud. Mycol. 2001, 46, 1–211. [Google Scholar]
- Forin, N.; Vizzini, A.; Nigris, S.; Ercole, E.; Voyron, S.; Girlanda, M.; Baldan, B. Illuminating type collections of nectriaceous fungi in Saccardo’s fungarium. Persoonia 2020, 45, 221–249. [Google Scholar] [CrossRef]
- Zhao, L.; Groenewald, J.Z.; Hernández-Restrepo, M.; Schroers, H.J.; Crous, P.W. Revising Clonostachys and allied genera in Bionectriaceae. Stud. Mycol. 2023, 105, 205–266. [Google Scholar] [CrossRef]
- Chen, W.H.; Han, Y.F.; Liang, J.D.; Zou, X.; Liang, Z.Q.; Jin, D.C. A new araneogenous fungus of the genus Clonostachys. Mycosystema 2016, 35, 1061–1069. [Google Scholar]
- Dumont, K.P.; Carpenter, S.E.; Sherwood, M.A.; Buriticá, P. Clonostachys araucaria Corda; The Botanical New York Garden: New York, NY, USA, 1976; Available online: https://www.gbif.org/occurrence/1928958668 (accessed on 1 March 2025).
- Ayers, S.; Zink, D.L.; Mohn, K.; Powell, J.S.; Brown, C.M.; Bills, G.; Singh, S.B. Anthelmintic constituents of Clonostachys candelabrum. J. Antibiot. 2010, 63, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Madrassi, L.M.; González, R.D.; Mónaco, C.I.; Zapata, P.D.; Alvarenga, A.E. Clonostachys chloroleuca: A novel pathogen causing cassava root rot disease in Misiones Province, Argentina. New Diss. Rep. 2023, 48, e12229. [Google Scholar] [CrossRef]
- Zeng, Z.Q.; Zhuang, W.Y. Three New Species of Clonostachys (Hypocreales, Ascomycota) from China. JoF 2022, 8, 1027. [Google Scholar] [CrossRef]
- Phaund, W.; Somaly, U.; Das, K.; Lee, S.Y.; Jung, H.Y. Clonostachys divergens and Chrysosporium merdarium: Two New Records from Soil in Korea. Kor J. Mycol. 2023, 51, 91–100. [Google Scholar]
- Dugan, F.M.; Lupien, S.L.; Chen, W. Clonostachys rhizophaga and other fungi from chickpea debris in the Palouse region of the Pacific Northwest, USA. N Am Fungi 2012, 7, 1–11. [Google Scholar] [CrossRef][Green Version]
- Farhaoui, A.; Tahiri, A.; Radouane, N.; Khadiri, M.; Amiri, S.; El Alami, N.; Lahlali, R. First report of Clonostachys rosea causing root rot of Beta vulgaris in Morocco. New Dis. Rep. 2023, 48, e12235. [Google Scholar] [CrossRef]
- Lechat, C.; Fournier, J.; Chaduli, D.; Lesage-Meessen, L.; Favel, A. Clonostachys saulensis (Bionectriaceae, Hypocreales), a new species from French Guiana. Ascomycete Org 2019, 11, 65–68. [Google Scholar]
- Bao, D.-F.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Perera, R.H.; Thiyagaraja, V.; Hongsanan, S.; Wanasinghe, D.N.; Shen, H.W.; Tian, X.G.; Yang, L.Q.; et al. Taxonomy, phylogeny and evolution of freshwater Hypocreomycetidae (Sordariomycetes). Fungal Div. 2023, 121, 1–94. [Google Scholar] [CrossRef]
- Raymundo, T.; Escudero-Leyva, E.; Soto-Agudelo, R.; García-Jiménez, J.; Romero-Bautista, L.; Valenzuela, R. New records of Hypocreales (Sordariomycetes, Ascomycota) of the cloud forest from the Sierra Alta Hidalguense in Mexico. Acta Bot. Mex. 2017, 120, 39–57. [Google Scholar] [CrossRef]
- Zhao, J.L.; Zou, L.; Zhong, L.Y.; Peng, L.X.; Ying, P.L.; Tan, M.L.; Zhao, G. Effects of polysaccharide elicitors from endophytic Bionectria pityrodes Fat6 on the growth and flavonoid production in tartary buckwheat sprout cultures. Cereal Res. Commun. 2015, 43, 661–671. [Google Scholar] [CrossRef]
- Türkölmez, Ş.; Özer, G.; Derviş, S. Clonostachys rosea Strain ST1140: An endophytic plant-growth-promoting fungus, and its potential use in seedbeds with wheat-grain substrate. Curr. Microbiol. 2023, 80. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ghanizadeh, H.; Zhang, H.; Li, X.; Li, T.; Wang, Q.; Wang, A. Clonostachys rosea promotes root growth in tomato by secreting auxin produced through the tryptamine pathway. JoF 2022, 8, 1166. [Google Scholar] [CrossRef] [PubMed]
- Gambarini, V.; Pavlov, N.; Young, P.; Dawes, S.; Auffret, A.; Kingsbury, J.M.; Lear, G. Molecular mechanisms of plastic biodegradation by the fungus Clonostachys rosea. BioRxiv 2025. [Google Scholar] [CrossRef]
- Marín, F.; Navarrete, H.; Narvaez-Trujillo, A. Total petroleum hydrocarbon degradation by endophytic fungi from the Ecuadorian Amazon. Adv. Microbiol. 2018, 8, 1029–1053. [Google Scholar] [CrossRef]
- Barres, B.A.M.; Corio-Costet, M.F.; Debieu, D.; Fillinger, S.; Walker, A.S.; Délye, C.; Grosman, J.; Siegwart, M. Trends and challenges in pesticide resistance detection. Trends Plant Sci. 2016, 21, 834–853. [Google Scholar] [CrossRef]
- Funck-Jensen, D.; Knudsen, I.M.; Lü Beck, M.; Mamarabadi, M.; Hockenhull, J.; Jensen, B. Development of a biocontrol agent for plant disease control with special emphasis on the near commercial fungal antagonist Clonostachys rosea strain ‘IK726’. Australas. Plant Pathol. 2007, 36, 95–101. [Google Scholar] [CrossRef]
- Bihal, R.; Al-Khayri, J.M.; Banu, A.N.; Kudesia, N.; Ahmed, F.K.; Sarkar, R.; Abd-Elsalam, K.A. Entomopathogenic fungi: An eco-friendly synthesis of sustainable nanoparticles and their nanopesticide properties. Microorganisms 2023, 11, 1617. [Google Scholar] [CrossRef]
- Razinger, J.; Lutz, M.; Schroers, H.J.; Urek, G.; Grunder, J. Evaluation of insect associated and plant growth promoting fungi in the control of cabbage root flies. J. Econ. Entomol. 2014, 107, 1348–1354. [Google Scholar] [CrossRef]
- Han, P.; Zhang, X.; Xu, D.; Zhang, B.; Lai, D.; Zhou, L. Metabolites from Clonostachys fungi and their biological activities. JoF 2020, 6, 229. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Bendebbah, R.; Benssaci, B.; Toudji, F.; Tafifet, L.; Krimi, Z. Entomopathogenic efficacy of the endophytic fungi: Clonostachys sp. and Beauveria bassiana on Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) larvae under laboratory and greenhouse conditions. Egypt. J. Biol. Pest. Control 2021, 31. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Younus, A.S.; Ali, A.N. Efficacy of Clonostachys rosea, as a promising entomopathogenic fungus, against coleopteran stored product insect pests under laboratory conditions. Egypt. J. Biol. Pest. Control 2021, 31, 55. [Google Scholar] [CrossRef]
- Tamta, A.K.; Pandey, R.; Sharma, J.R.; Rai, R.; Barman, M.; Deeksha, M.G.; Mitra, D.; Das Mohapatra, P.K.; Sami, R.; Al-Mushhin, A.A.M.; et al. First Record of Clonostachys rosea (Ascomycota: Hypocreales) Entomopathogenic Fungus in the Mango Hopper Amritodus atkinsoni (Hemiptera: Cicadellidae). Pathogens 2022, 11, 1447. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.M.; Muthusamy, K.; Iruthayasamy, J.; Prithiviraj, B.; Kumaresan, P.V.; Lakshmanan, P.; Perianadar, I.V. First report of Clonostachys rosea as a Mycoparasite on Sclerotinia sclerotiorum causing head rot of cabbage in India. Plants 2023, 12, 199. [Google Scholar] [CrossRef]
- Piombo, E.; Guaschino, M.; Jensen, D.F.; Karlsson, M.; Dubey, M. Insights into the ecological generalist lifestyle of Clonostachys fungi through analysis of their predicted secretomes. Front. Microbiol. 2023, 14, 1112673. [Google Scholar] [CrossRef]
- Karlsson, M.; Durling, M.B.; Choi, J.; Kosawang, C.; Lackner, G.; Tzelepis, G.D.; Nygen, K.; Dubey, M.K.; Kamou, N.; Levasseur, A.; et al. Insights on the evolution of mycoparasitism from the genome of Clonostachys rosea. GBE 2015, 7, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Demissie, Z.A.; Witte, T.; Robinson, K.A.; Sproule, A.; Foote, S.J.; Johnston, A.; Harris, L.J.; Ovey, D.P.; Loewen, M.C. Transcriptomic and exometabolomic profiling reveals antagonistic and defensive modes of Clonostachys rosea action against Fusarium graminearum. Mol. Plant Microbe Interac. 2020, 33, 842–858. [Google Scholar] [CrossRef]
- Sun, Z.B.; Yu, S.F.; Sun, M.H.; Li, S.D.; Hu, Y.F.; Song, H.J. Transcriptomic Response of Clonostachys rosea Mycoparasitizing Rhizoctonia solani. J. Fungi 2023, 9, 818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Górzyńska, K.; Ślachetka, M.; Ryszka, P.; Turnau, K.; Płachno, B.J.; Lembicz, M. Incidence, identification, and mycoparasitic activity of Clonostachys epichloë, a hyperparasite of the fungal endophyte Epichloë typhina. Plant Dis. 2018, 102, 1973–1980. [Google Scholar] [CrossRef]
- Geiger, A.; Karácsony, Z.; Geml, J.; Váczy, K.Z. Mycoparasitism capability and growth inhibition activity of Clonostachys rosea isolates against fungal pathogens of grapevine trunk diseases suggest potential for biocontrol. PLoS ONE 2022, 17, e0273985. [Google Scholar] [CrossRef]
- Khairullina, A.; Micic, N.; Jørgensen, H.J.L.; Bjarnholt, N.; Bülow, L.; Collinge, D.B.; Jensen, B. Biocontrol Effect of Clonostachys rosea on Fusarium graminearum Infection and Mycotoxin Detoxification in Oat (Avena sativa). Plants 2023, 12, 500. [Google Scholar] [CrossRef]
- Stucky, T.; Sy, E.T.; Egger, J.; Mathlouthi, E.; Krauss, J.; De Gianni, L.; Ruthes, A.C.; Dahlin, P. Control of the plant-parasitic nematode Meloidogyne incognita in soil and on tomato roots by Clonostachys rosea. J. Appl. Microbiol. 2024, 135, lxae111. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal-Alejo, J.; Diaz-Braga, A.; Herrera-Parra, E.; Heredia, G.; Medina-Baizabal, I.L.; Canto-Canche, B.; Gamboa-Angulo, M. Clonostachys rosea selected by nematicidal screening and its efficacy against Meloidogyne incognita in a greenhouse. Biocon Sci. Tech. 2021, 31, 1283–1297. [Google Scholar] [CrossRef]
- Iqbal, M.; Dubey, M.; McEwan, K.; Menzel, U.; Franko, M.A.; Viketoft, M.; Karlsson, M. Evaluation of Clonostachys rosea for control of plant-parasitic nematodes in soil and in roots of carrot and wheat. Phytopathology 2018, 108, 52–59. [Google Scholar] [CrossRef]
- Zou, C.-G.; Tao, N.; Lin, W.-J.; Yang, J.-K.; Huang, X.-W.; Liu, X.-Y.; Tu, H.-H.; Gan, Z.-W.; Zhang, K.-Q. Regulation of subtilisin-like protease prC expression by nematode cuticle in the nematophagous fungus Clonostachys rosea. Environ. Microbiol. 2010, 12, 3243–3252. [Google Scholar] [CrossRef]
- Shravani, V.; Nallusamy, S.; Govindasamy, J.; Eswaran, K.; Iruthayasamy, J.; Annaiyan, S. Unravelling the potent nematotoxic compounds from Clonostachys rosea effective against root-knot nematode, Meloidogyne incognita-an in-vitro and in-silico approach. Physiol. Mol. Plant Pathol. 2024, 131, 102279. [Google Scholar] [CrossRef]
- Márquez-Dávila, K.; Arévalo-López, L.; Gonzáles, R.; Vega, L.; Meza, M. Trichoderma and Clonostachys as biocontrol agents against Meloidogyne incognita in sacha inchi. Pesq. Agropec Trop. 2020, 50, e60890. [Google Scholar] [CrossRef]
- Ahmed, M.; Laing, M.D.; Nsahlai, I.V. A new control strategy for nematodes of sheep using chlamydospores of a fungus, Clonostachys rosea f. rosea, and an ethanolic extract of a plant, Ananas comosus. Biocontrol. Sci. Tech. 2014, 24, 860–871. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Chandrika, M.; Sruthi, D.; Yashavantha Rao, H.C.; Jayabaskaran, C. Clonostachys rosea, a marine algal endophyte, as an alternative source of chrysin and its anticancer effect. Arch. Microbiol. 2023, 205, 275. [Google Scholar] [CrossRef]
- Ouchi, T.; Watanabe, Y.; Nonaka, K.; Muramatsu, R.; Noguchi, C.; Tozawa, M.; Hokari, R.; Ishiyama, A.; Koike, R.; Matsui, H.; et al. Clonocoprogens A, B and C, new antimalarial coprogens from the Okinawan fungus Clonostachys compactiuscula FKR-0021. J. Antibiot. 2020, 73, 365–371. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Li, H.; Pan, Y.; Liu, X.; Che, Y.; Liu, G. The disruption of verM activates the production of gliocladiosin A and B in Clonostachys rogersoniana. Org. Biomol. Chem. 2019, 17, 6782–6785. [Google Scholar] [CrossRef]
- Rodrigues, J.; Rocha, L.F.; Martinez, J.M.; Montalva, C.; Humber, R.A.; Luz, C. Clonostachys spp., natural mosquito antagonists, and their prospects for biological control of Aedes aegypti. Parasitol. Res. 2022, 121, 2979–2984. [Google Scholar] [CrossRef]
- Fatema, U.; Broberg, A.; Jensen, D.F.; Karlsson, M.; Dubey, M. Functional analysis of polyketide synthase genes in the biocontrol fungus Clonostachys rosea. Sci. Rep. 2018, 8, 15009. [Google Scholar] [CrossRef] [PubMed]
- Ghoran, H.S.; Taktaz, F.; Sousa, E.; Fernandes, C.; Kijjoa, A. Peptides from Marine-Derived Fungi: Chemistry and Biological Activities. Mar. Drugs 2023, 21, 510. [Google Scholar] [CrossRef]
- Qi, H.; Duan, X.; Xu, W.; Zhou, Y.; Ma, H.; Ma, W.; Ma, G. First report disease of Clonostachys rosea causing root rot on Astragalus membranaceus in China. Plant Dis. 2022, 106, 1752. [Google Scholar] [CrossRef] [PubMed]
- Díaz, R.; Chávez, E.C.; Delgado-Ortiz, J.C.; Velazquez Guerrero, J.J.; Roque, A.; Ochoa, Y.M. First report of Clonostachys rosea causing root rot on garlic in Mexico. Plant Dis. 2022, 106, 3000. [Google Scholar] [CrossRef] [PubMed]
- Coyotl-Pérez, W.A.; Romero-Arenas, O.; Mosso-González, C.; Pacheco-Hernández, Y.; Rivera-Tapia, J.; Villa-Ruano, N. First report of Clonostachys rosea associated with avocado fruit rot in Puebla, Mexico. Rev. Mex. Fitopat 2022, 40, 298–307. [Google Scholar] [CrossRef]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and perspectives in the use of biocontrol agents against fungal plant diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
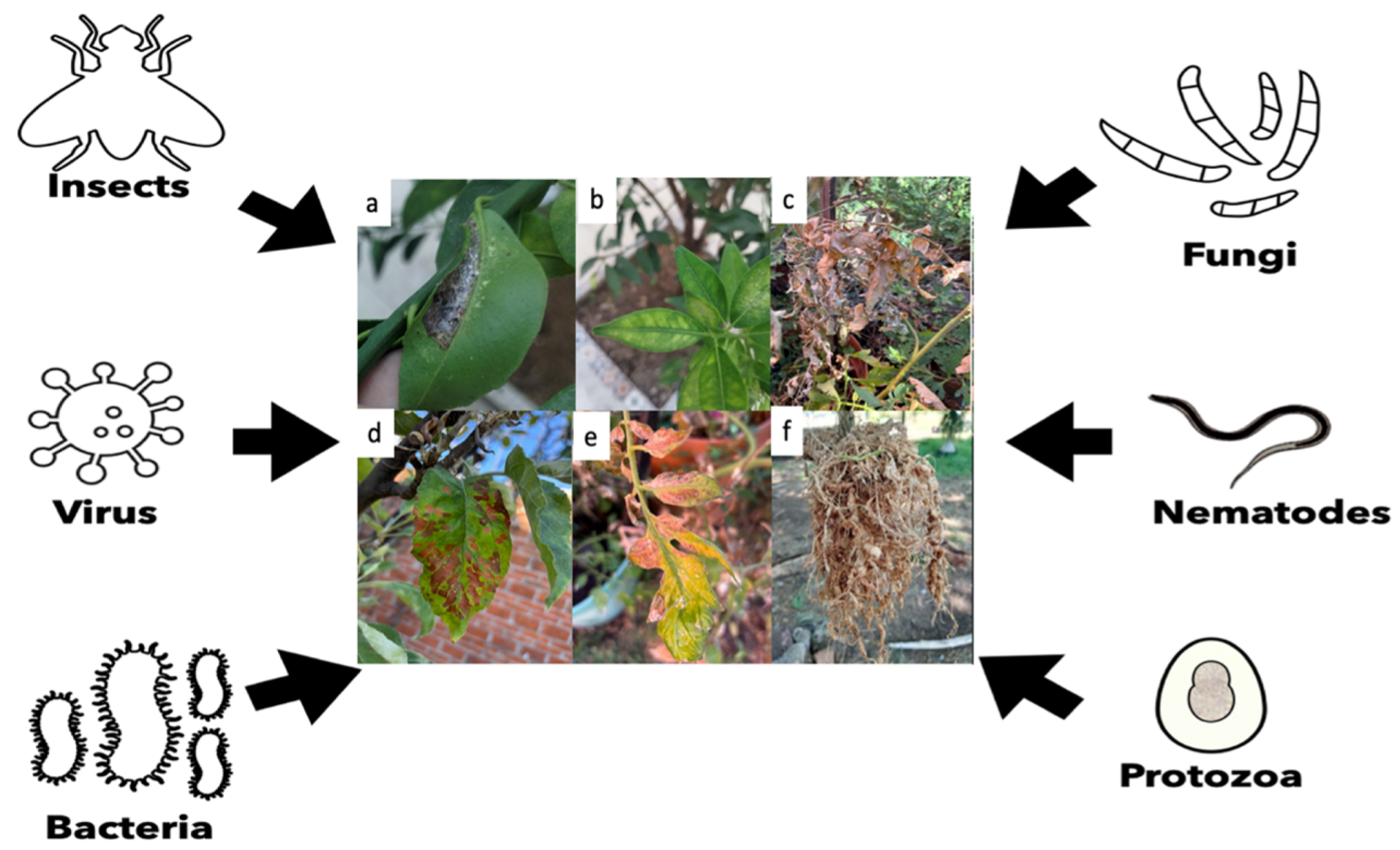


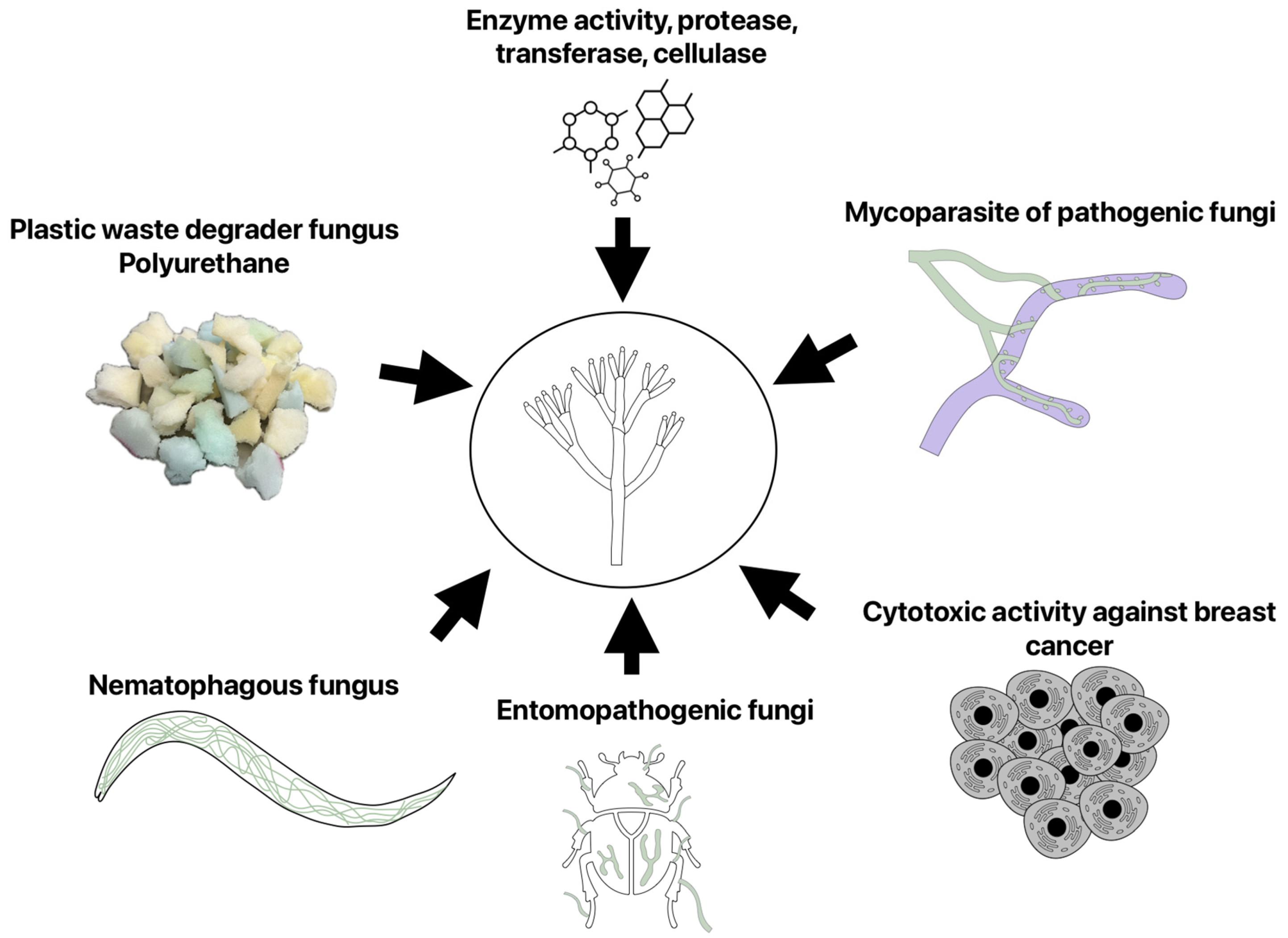
| Pathogen/Host Crop | Pesticide | Results | Author |
|---|---|---|---|
| Phytopathogenic fungi | |||
| Botrytis cinerea (Pers. 1797)/fruit and vegetable pre- and post-harvesting | Fenhexamid | Multi-resistance | [48] |
| Penicillium digitatum (Pers.) Sacc. 1881/citrus | Methyl benzimidazoles Succinate deshydrogenase inhibitors | High resistance | [49] |
| Sclerotinia sclerotiorum (Lib.) de Bary 1884 | Boscalid 2-chloro-N-(4’-chloro diphenyl-2-yl) Nicotinamide | High resistance | [50] |
| Botrytis cinerea/Ginseng | Carbendazim, prodione, and pyrimethanil | Multi-resistance | [51] |
| Fusarium seudograminearum O’Donnell & T. Aoki 1999/wheat | Tebuconazole | Low resistance | [52] |
| Alternaria spp./blueberries | Fludioxonil, Fluazinam, Metconazole, and Cyprodinil | Multi-resistance | [53] |
| Phytoparasitic nematodes | |||
| Meloidogyne incognita (Kofoid & Ehite, 1919) Chitwood 1949 | Carbofuran, carbosulfan, cadusafos, triazophos | Development of virulent populations of the parasite | [54] |
| Meloidogyne spp. | Furadan | Low efficacy | [55] |
| M. incognita | Fosthiazate (Organophospate) | High resistance | [56] |
| Species | Isolation Source | Country | Author |
|---|---|---|---|
| Clonostachys agrawalii | Decomposing buffalo horn from animal house floor sweepings | India | [87] |
| Clonostachys ambigua | On bark | Indonesia | [88] |
| Clonostachys apociny | Dead steam of Apocynum cannabium L. 1753 | USA | [89] |
| Clonostachys aquatica | Submerged decaying wood | China | [89] |
| Clonostachys aranearum | From a spider (Araneae) Landfill/Australia | China | [90] |
| Clonostachys araucaria | From an Araucaria tree twig | Peru | [91] |
| Clonostachys aureofulvella | Bark | Australia | [87] |
| Clonostachys byssicola | Wood and falling leaves From strawberry fields Fragaria ananassa (Duchesne ex Weston) Duchesne ex Rozier, 1785 From fruits of Annona squamosa L. 1753, A. x atemoya From different sources: barks, wood, Bryophyte, litter, Pipper nigrum L. 1753, Hemelia sp., Coffea arabica, L. 1753 endophyte stems, wild C. arabica | Venezuela Brazil Brazil Brazil, Venezuela, and Ethiopia | [75] |
| Clonostachys candelabrum | Soil | Netherlands | [92] |
| Clonostachys chlorina | Soil | Brazil | [87] |
| Clonostachys chloroleuca | Different sources: Bryophyte, native soil, soil under soybean field, soil under cotton field | Brazil | [93] |
| Clonostachys chonggingensis | Decaying rotten from a mountain | China | [94] |
| Clonostachys compactiuscula | Soil/Germany On bark of Prunus laurocerasus L. 1753 | France | [88] |
| Clonostachys divergens | Soil Soil | Germany Korea | [87] [95] |
| Clonostachys granuligera | Orchid bark | Sweden | [88] |
| Clonostachys intermedia | Soil | Netherlands | [87] |
| Clonostachys leptoderma | Alnus sp. (tree/bush); rotten bark | China | [94] |
| Clonostachys miodochialis | Soil | Netherlands | [87] |
| Clonostachys oligospora | Decaying rotten twig | China | [94] |
| Clonostachys pallens | On bark | Indonesia | [88] |
| Clonostachys phyllophila | Leaves of mistletoe (Viscum album L. 1753) Another isolate (Unknown source) | France Cuba | [87] |
| Clonostachys pseudocrholeuca | Bark | French Guiana | [87] |
| Clonostachys rhizophaga | Chickpea debries Culture contaminant As mycoparasitic fungi from species of a rust Hemileia vastatrix Berck & Broome. As a mycoparasite in Fusarum oxysporum Schltdl. 1824, native soil and on H. vastatrix, Coffea canephora Culture contaminant | USA Chile Switzerland Africa Cameroon Ethiopia Switzerland | [96] [75] |
| Clonostachys rogersoniana, Clonostachys rosea | Soil | Brazil | [87] |
| C. rosea | Dead rhizome of the perennial plant Hedychium coronarium J. Köning On bamboo (Phyllostachys bambusoides Sieb. et Zucc. var. aurea (Carr. ex Riv.) Makino (Xie) (=P. reticulata (Rupr.) K. Koch) Root rot of beet root (Beta vulgaris L. 1753) Arctic soil | Italy Japan USA Norway | [87] [97] |
| Clonostachys rosea f. catenulata | Soil Soil Soil Soil Soil | Ukraine USA Germany USA Ukraine | [87] [75] |
| Clonostachys saulensis, | Dead bark on Bauhinia (Fabaceae) | French Guiana | [98] |
| Clonostachys setosa | Trophis racemose L. (Urb.) (Moraceae) | Cuba | [87] |
| Clonostachys solani f. nigrovirens | On egg of Arion ater (Mollusca) and soil On tuber of potato (Solanum tuberosum L. 1753) and soil | Germany Netherlands | [87] |
| Clonostachys solani f. solani | On tuber of Solanum tuberosum Bark Rotten fruit of chestnut (Aesculus hippocastrum L. 1753) Wood From an unknown source | Netherlands Germany France Canada USA | [87] |
| Clonostachys squamulligera | On branch bark of willow (Salix babylonica L. 1753) On bark of soja (Glycine sinensis Sweet 1826) | Italy Portugal | [88] |
| Species | Target Pest | Hosts | Experimental Conditions/Results | Authors |
|---|---|---|---|---|
| Clonostachys rosea | Carpomya vesuviana A. Costa, 1854 (jujuve fruit fly) | Jujube fruit | Larval mortality of pupa = 46% using 1010 spore/mL | [80] |
| C. rosea | Bemisia tabaci (Whitefly) | Tomato | 50% mortality of nymphs after 6 days using 4 × 106 spores | [45] |
| C. rosea | Trogoderma granarium Everst, 1898 (Coleoptera: Dermestidae), Tribolium castaneum Herbst, 1797 (Coleoptera: Tenebrionidae) and Callosobruchus maculatus Fabricius, 1775 (Coleoptera: Chrysomelidae) | Stored grains of a number of crops: wheat, oats, barley, corn, rice | Range of mortality 70.7–75.7% Under in vitro conditions | [112] |
| C. rosea | The mango Hopper Amritodus atkunsoni Lethierry (Hemiptera) | Mango | 3 × 108 spores Caused 96.67% mortality | [113] |
| Clonostachys | Target Pest/Disease | Experimental Conditions/Efficacy | Authors |
|---|---|---|---|
| Clonostachys epichloë | Mycoparasite of Epichloë typhina (Pers.) Brockm. 1863 chole disease in grass species | Clo G 68.33–85.28 vs. 3 E. typhina and Clo j 75–100% in a pre-colonization experiment | [119] |
| Clonostachys rosea | Eutypa lata (Pers.) Tul. & C. Tul. 1863 Phaemoniella chlamydospora (W. Gams, Crous, M.J. Wingf. & Mugnai) Crous & W. Gams 2000 Botryosphaeria dothidea (Moug.) Ces. & De Not. 1863, Diaporthe spp. Grape vine trunk | In vitro and in vivo, growth inhibition 1.9–54% Phaeomoniella chlamydospora (Antagonism) (mycoparasitism) (diseases) | [120] |
| C. rosea | Sclerotinia sclerotiorum mycoparasite | Root of cabbage 79.63% Crude extract 97.17% inhibition | [114] |
| C. rosea | Botrytis cinerea tomato plants | Efficiency > 90% | [71] |
| C. rosea | Fusarium graminearum/oat | Inhibits infection effectively | [121] |
| Clonostachys | Blank Nematode/Host | Host | Experimental Conditions/Efficacy | Authors |
|---|---|---|---|---|
| C. rosea | Pratylenchus spp. Heterodera spp. Tylenchorrhynchus spp. Helicotylenchus spp. Roptylenchys spp. | Carrots and wheat | Nematocidal activities: Pratylenchus spp. = 38% Heterodera spp. = 4% Tylenchorhynchus spp. = 6% Pratylenchys spp. = 5% Helycotylenchys spp. = 5% Rotylenchys spp. = 3% | [124] |
| C. rosea | M. incognita | Sacha inchi | Significantly reduced the number of root goals (around 75%) | [127] |
| C. rosea | M. incognita 2nd state juveniles | Tomato | LD50 = 375 μg mL−1 | [123] |
| C. rosea | M. incognita | Tomato | Liquid culture filtrates of C. rosea caused 69.38% parasitized eggs | [126] |
| Clonostachys Species | Compound | Biological Activity | Author |
|---|---|---|---|
| Clonostachys compactiuscula | Clonocoprogens A, B and C | Anti-malaria | [130] |
| Clonostachys rogersoniana | Gliocladiosin A and B | Anti-bacterial | [131] |
| Clonosytachys byssicola | Holocellulolicic enzymes | Production of prebiotic manno-oligosacharides | [72] |
| Clonostachys eriocamporessi C. byssicola | Unidentified compounds | Anti-mosquito Aedes aegypti | [132] |
| Clonostachys rosea | Polyketids | Anti-pathogenic fungi | [133] |
| C. rosea | Di-peptides Glycol(-Gly-Phe) | Anticancer | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Estebanez, M.; Mendoza-de Gives, P. The Genus Clonostachys (Bionectria) as a Potential Tool Against Agricultural Pest and Other Biotechnological Applications: A Review. Microbiol. Res. 2025, 16, 86. https://doi.org/10.3390/microbiolres16040086
Reyes-Estebanez M, Mendoza-de Gives P. The Genus Clonostachys (Bionectria) as a Potential Tool Against Agricultural Pest and Other Biotechnological Applications: A Review. Microbiology Research. 2025; 16(4):86. https://doi.org/10.3390/microbiolres16040086
Chicago/Turabian StyleReyes-Estebanez, Manuela, and Pedro Mendoza-de Gives. 2025. "The Genus Clonostachys (Bionectria) as a Potential Tool Against Agricultural Pest and Other Biotechnological Applications: A Review" Microbiology Research 16, no. 4: 86. https://doi.org/10.3390/microbiolres16040086
APA StyleReyes-Estebanez, M., & Mendoza-de Gives, P. (2025). The Genus Clonostachys (Bionectria) as a Potential Tool Against Agricultural Pest and Other Biotechnological Applications: A Review. Microbiology Research, 16(4), 86. https://doi.org/10.3390/microbiolres16040086






