Tick Dispersal and Borrelia Species in Ticks from Migratory Birds: Insights from the Asinara National Park, Sardinia, Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
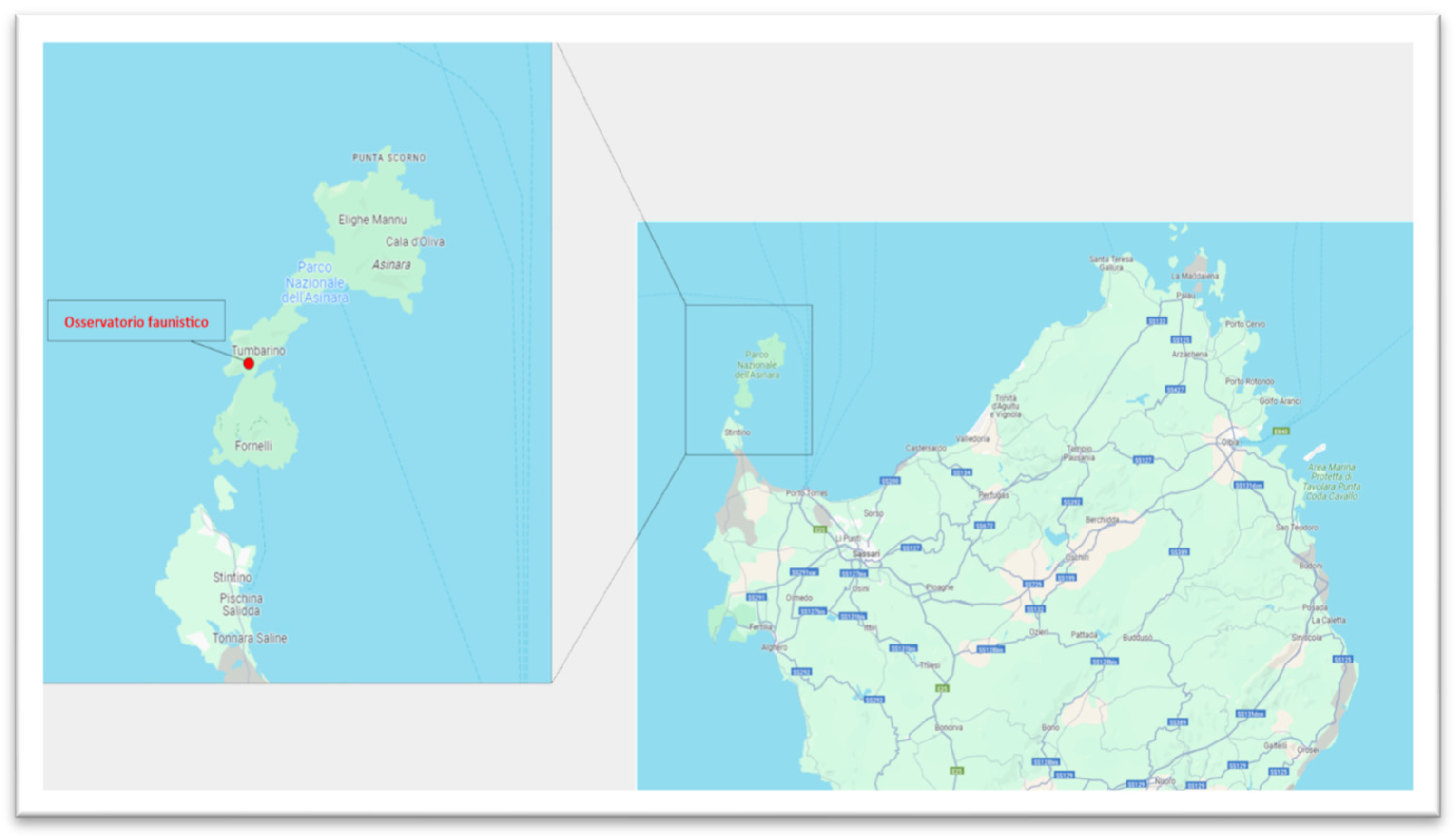
2.2. Bird Capture and Study Area
2.3. Ectoparasite Sampling
2.4. DNA Extraction
2.5. Tick Molecular Identification
2.6. Molecular Detection of Borrelia spp. and Sequencing
2.7. Sequence Accession Numbers
3. Results
3.1. Analysis of Bird Species and Ixodida Distribution
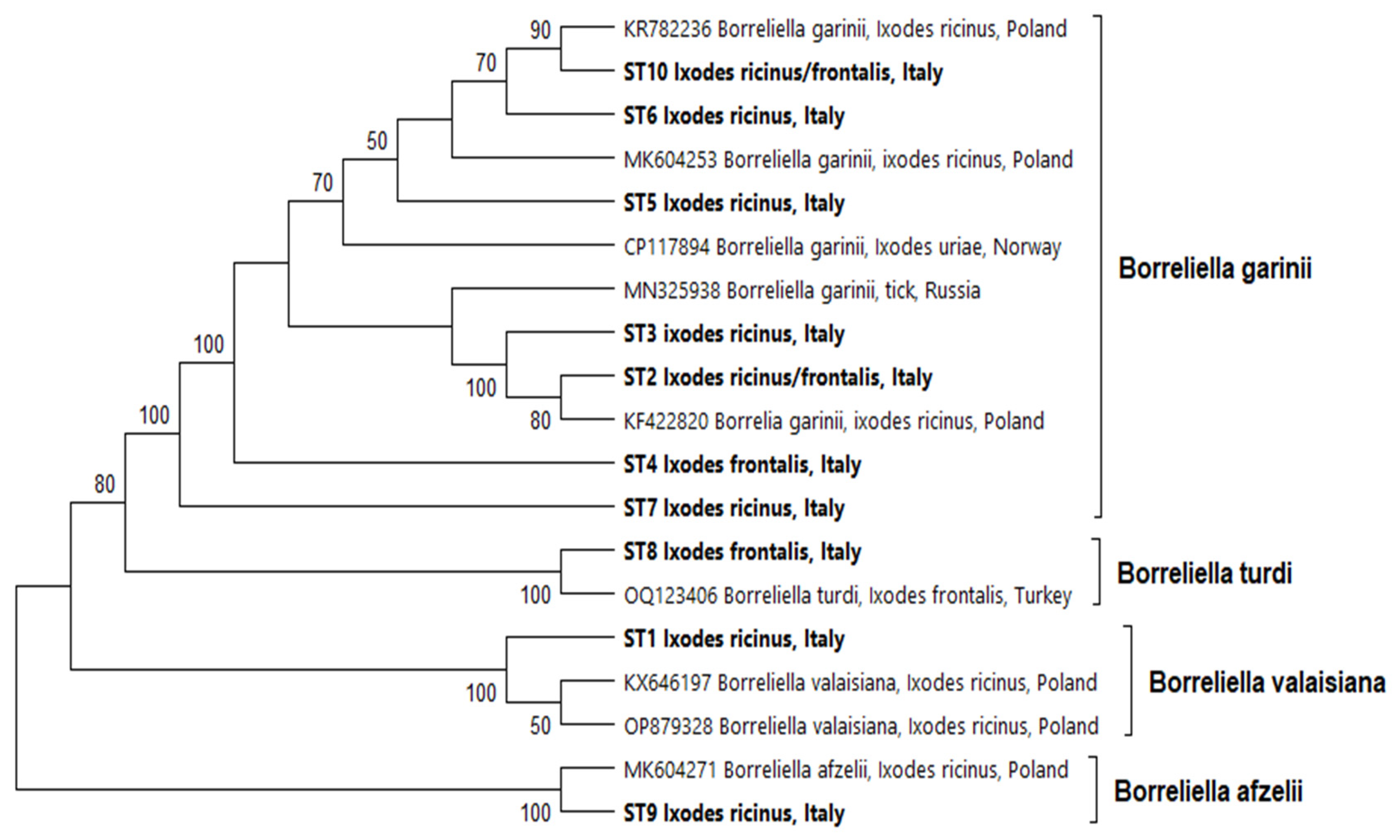
3.2. Molecular Analysis for Detection of Borrelia spp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Socha, W.; Kwasnik, M.; Larska, M.; Rola, J.; Rozek, W. Vector-Borne Viral Diseases as a Current Threat for Human and Animal Health—One Health Perspective. J. Clin. Med. 2022, 11, 3026. [Google Scholar] [CrossRef] [PubMed]
- Chala, B.; Hamde, F. Emerging and Re-Emerging Vector-Borne Infectious Diseases and the Challenges for Control: A Review. Front. Public Health 2021, 9, 715759. [Google Scholar] [CrossRef]
- Kumar, L.; Chhogyel, N.; Gopalakrishnan, T.; Hasan, M.K.; Jayasinghe, S.L.; Kariyawasam, C.S.; Kogo, B.K.; Ratnayake, S. Chapter 4—Climate Change and Future of Agri-Food Production. In Future Foods; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 49–79. ISBN 978-0-323-91001-9. [Google Scholar]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The Importance of Vector Control for the Control and Elimination of Vector-Borne Diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, G.; Fiore, V.; Mancini, F.; Caddeo, A.; Ciervo, A.; Babudieri, S.; Masala, G.; Bagella, P.; Nunnari, G.; Rezza, G.; et al. Mediterranean Spotted Fever-like Illness in Sardinia, Italy: A Clinical and Microbiological Study. Infection 2016, 44, 733–738. [Google Scholar] [CrossRef]
- Chisu, V.; Loi, F.; Foxi, C.; Chessa, G.; Masu, G.; Rolesu, S.; Masala, G. Coexistence of Tick-Borne Pathogens in Ticks Collected from Their Hosts in Sardinia: An Update. Acta Parasitol. 2020, 65, 999–1004. [Google Scholar] [CrossRef]
- Foti, M.; Rinaldo, D.; Guercio, A.; Giacopello, C.; Aleo, A.; De Leo, F.; Fisichella, V.; Mammina, C. Pathogenic Microorganisms Carried by Migratory Birds Passing through the Territory of the Island of Ustica, Sicily (Italy). Avian Pathol. 2011, 40, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Sándor, A.D.; Mărcuţan, D.I.; D’Amico, G.; Gherman, C.M.; Dumitrache, M.O.; Mihalca, A.D. Do the Ticks of Birds at an Important Migratory Hotspot Reflect the Seasonal Dynamics of Ixodes Ricinus at the Migration Initiation Site? A Case Study in the Danube Delta. PLoS ONE 2014, 9, e89378. [Google Scholar] [CrossRef]
- Hoffman, T.; Olsen, B.; Lundkvist, Å. The Biological and Ecological Features of Northbound Migratory Birds, Ticks, and Tick-Borne Microorganisms in the African–Western Palearctic. Microorganisms 2023, 11, 158. [Google Scholar] [CrossRef]
- Loureiro, F.; Mesquita, J.R.; Cardoso, L.; Santos-Silva, S.; Moreira, G.; Bento, J.T.; Soeiro, V.; Gonçalves, A.; Silva, F.; Barradas, P.F.; et al. Screening Wild Birds for Tick-Borne Zoonotic Pathogens in Portugal. Pathogens 2025, 14, 75. [Google Scholar] [CrossRef]
- Defaye, B.; Moutailler, S.; Vollot, B.; Galon, C.; Gonzalez, G.; Moraes, R.A.; Leoncini, A.-S.; Rataud, A.; Le Guillou, G.; Pasqualini, V.; et al. Detection of Pathogens and Ticks on Sedentary and Migratory Birds in Two Corsican Wetlands (France, Mediterranean Area). Microorganisms 2023, 11, 869. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Kleinerman, G.; Baneth, G. Genus Argas Latreille, 1795. In Ticks of Europe and North Africa: A Guide to Species Identification; Estrada-Peña, A., Mihalca, A.D., Petney, T.N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 13–14. ISBN 978-3-319-63760-0. [Google Scholar]
- Black, W.C.; Piesman, J. Phylogeny of Hard- and Soft-Tick Taxa (Acari: Ixodida) Based on Mitochondrial 16S rDNA Sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034–10038. [Google Scholar] [CrossRef] [PubMed]
- Dahmana, H.; Amanzougaghene, N.; Davoust, B.; Normand, T.; Carette, O.; Demoncheaux, J.-P.; Mulot, B.; Fabrizy, B.; Scandola, P.; Chik, M.; et al. Great Diversity of Piroplasmida in Equidae in Africa and Europe, Including Potential New Species. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100332. [Google Scholar] [CrossRef] [PubMed]
- Skotarczak, B.; Wodecka, B.; Cichocka, A. Coexistence DNA of Borrelia Burgdorferi Sensu Lato and Babesia Microti in Ixodes Ricinus Ticks from North-Western Poland. Ann. Agric. Environ. Med. 2002, 9, 25–28. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Hall, T. Bioedit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/ NT. Available online: https://www.semanticscholar.org/paper/BIOEDIT%3A-A-USER-FRIENDLY-BIOLOGICAL-SEQUENCE-EDITOR-Hall/0ae262d9cf78536754bc064e07113ab5e978f208 (accessed on 12 February 2025).
- Kimura, M. A Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. CONFIDENCE LIMITS ON PHYLOGENIES: AN APPROACH USING THE BOOTSTRAP. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Springer, A.; Schütte, K.; Brandes, F.; Reuschel, M.; Fehr, M.; Dobler, G.; Margos, G.; Fingerle, V.; Sprong, H.; Strube, C. Potential Drivers of Vector-Borne Pathogens in Urban Environments: European Hedgehogs (Erinaceus europaeus) in the Spotlight. One Health 2024, 18, 100764. [Google Scholar] [CrossRef]
- Veiga, J.; Baltà, O.; Figuerola, J. Does Bird Life-History Influence the Prevalence of Ticks? A Citizen Science Study in North East Spain. One Health 2024, 18, 100718. [Google Scholar] [CrossRef]
- Buczek, A.M.; Buczek, W.; Buczek, A.; Bartosik, K. The Potential Role of Migratory Birds in the Rapid Spread of Ticks and Tick-Borne Pathogens in the Changing Climatic and Environmental Conditions in Europe. Int. J. Environ. Res. Public Health 2020, 17, 2117. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, P.; Jaenson, T.G.T.; Olsen, B.; Waldenström, J.; Lindgren, P.-E. Migratory Birds as Disseminators of Ticks and the Tick-Borne Pathogens Borrelia Bacteria and Tick-Borne Encephalitis (TBE) Virus: A Seasonal Study at Ottenby Bird Observatory in South-Eastern Sweden. Parasites Vectors 2020, 13, 607. [Google Scholar] [CrossRef]
- Dubska, L.; Literak, I.; Kocianova, E.; Taragelova, V.; Sverakova, V.; Sychra, O.; Hromadko, M. Synanthropic Birds Influence the Distribution of Borrelia Species: Analysis of Ixodes Ricinus Ticks Feeding on Passerine Birds. Appl. Environ. Microbiol. 2011, 77, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Taragel’ová, V.; Koči, J.; Hanincová, K.; Kurtenbach, K.; Derdáková, M.; Ogden, N.H.; Literák, I.; Kocianová, E.; Labuda, M. Blackbirds and Song Thrushes Constitute a Key Reservoir of Borrelia Garinii, the Causative Agent of Borreliosis in Central Europe. Appl. Environ. Microbiol. 2008, 74, 1289–1293. [Google Scholar] [CrossRef]
- Kahl, O.; Gray, J.S. The Biology of Ixodes Ricinus with Emphasis on Its Ecology. Ticks Tick-Borne Diseases 2023, 14, 102114. [Google Scholar] [CrossRef]
- Bertola, M.; Montarsi, F.; Obber, F.; Da Rold, G.; Carlin, S.; Toniolo, F.; Porcellato, E.; Falcaro, C.; Mondardini, V.; Ormelli, S.; et al. Occurrence and Identification of Ixodes Ricinus Borne Pathogens in Northeastern Italy. Pathogens 2021, 10, 1181. [Google Scholar] [CrossRef]
- Olsén, B.; Jaenson, T.G.; Bergström, S. Prevalence of Borrelia Burgdorferi Sensu Lato-Infected Ticks on Migrating Birds. Appl. Environ. Microbiol. 1995, 61, 3082–3087. [Google Scholar] [CrossRef]
- Ford, L.; Tufts, D.M. Lyme Neuroborreliosis: Mechanisms of B. Burgdorferi Infection of the Nervous System. Brain Sci. 2021, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Radolf, J.D.; Caimano, M.J.; Stevenson, B.; Hu, L.T. Of Ticks, Mice and Men: Understanding the Dual-Host Lifestyle of Lyme Disease Spirochaetes. Nat. Rev. Microbiol. 2012, 10, 87–99. [Google Scholar] [CrossRef]
- Hanincová, K.; Taragelová, V.; Koci, J.; Schäfer, S.M.; Hails, R.; Ullmann, A.J.; Piesman, J.; Labuda, M.; Kurtenbach, K. Association of Borrelia Garinii and B. Valaisiana with Songbirds in Slovakia. Appl. Environ. Microbiol. 2003, 69, 2825–2830. [Google Scholar] [CrossRef]
- Norte, A.C.; Ramos, J.A.; Gern, L.; Núncio, M.S.; Lopes de Carvalho, I. Birds as Reservoirs for Borrelia Burgdorferi s.l. in Western Europe: Circulation of B. Turdi and Other Genospecies in Bird-Tick Cycles in Portugal. Environ. Microbiol. 2013, 15, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Literak, I.; Norte, A.C.; Núncio, M.S.; de Carvalho, I.L.; Ogrzewalska, M.; Nováková, M.; Martins, T.F.; Sychra, O.; Resendes, R.; Rodrígues, P. Ticks on Passerines from the Archipelago of the Azores as Hosts of Borreliae and Rickettsiae. Ticks Tick. Borne Dis. 2015, 6, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Chamorro, A.; Battilotti, F.; Cayol, C.; Mappes, T.; Koskela, E.; Boulanger, N.; Genné, D.; Sarr, A.; Voordouw, M.J. Susceptibility to Infection with Borrelia Afzelii and TLR2 Polymorphism in a Wild Reservoir Host. Sci. Rep. 2019, 9, 6711. [Google Scholar] [CrossRef] [PubMed]
- Lindsø, L.K.; Viljugrein, H.; Mysterud, A. Vector Competence of Ixodes Ricinus Instars for the Transmission of Borrelia Burgdorferi Sensu Lato in Different Small Mammalian Hosts. Parasit. Vectors 2024, 17, 23. [Google Scholar] [CrossRef]
- Franke, J.; Moldenhauer, A.; Hildebrandt, A.; Dorn, W. Are Birds Reservoir Hosts for Borrelia Afzelii? Ticks Tick-Borne Dis. 2010, 1, 109–112. [Google Scholar] [CrossRef]
| Pathogen | Gene Target | PCR Assay | Primer/Probe Sequence | Reference |
|---|---|---|---|---|
| Borrelia spp. | 16S rRNA | PCR | 16S+1_F 5′-CTGCTCAATGATTTTTTAAATTGCTGTGG-3′ | [13] |
| 16S+1_R 5′-CCGGTCTGAACTCAGATCAAGT-3′ | ||||
| 16S rRNA | rt-PCR | 16S For 5′-GGATATAGTTAGAGATAATTATCCCCGTTTG-3′ | [14] | |
| 16S Rev 5′-CATTACATGCTGGTAACAGATAACAAGG-3′ | ||||
| 16S Borr Probe 6FAM-CAGGTGCTGCATGGT-BHQ1 | ||||
| FLA | PCR | FLA1 F 5′-AGAGCAACTTACAGACGAAATTAAT-3′ | [15] | |
| FLA2 R 5′-CAAGTCTATTTTGGAAAGCACCTAA-3′ |
| Bird Species (Scientific Name) | Migration Type | Breeding Range | Wintering Range | Migration Timing |
|---|---|---|---|---|
| European Robin (Erithacus rubecula) | Short to medium distance | Continental Europe, east to Ural Mountains, northwestern Africa | Western and southern Europe, northern Africa | Southward in autumn, northward in spring |
| Common Blackbird (Turdus merula) | Short distance/partial migrant | Europe, North Africa, western Asia | Some populations winter in southern Europe and North Africa | Partial migration; northern populations migrate south in autumn |
| Song Thrush (Turdus philomelos) | Medium distance | Europe, western Siberia | Southern Europe, North Africa, Middle East | Migrates south in autumn, returns in spring |
| Woodlark (Lullula arborea) | Short distance/resident | Europe, North Africa | Southern Europe, North Africa | Some move south in autumn, others remain resident |
| Blackcap (Sylvia atricapilla) | Medium distance | Europe, western Asia | Southern Europe, North Africa | Migrates south in autumn, returns in spring |
| Garden Warbler (Sylvia borin) | Long distance | Europe, western Asia | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Common Redstart (Phoenicurus phoenicurus) | Long distance | Europe, western Asia | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Little Owl (Athene noctua) | Resident | Europe, North Africa, Asia | Resident | Non-migratory |
| Willow Warbler (Phylloscopus sibilatrix) | Long distance | Europe, western Siberia | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Pied Flycatcher (Ficedula hypoleuca) | Long distance | Europe | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Common Whitethroat (Sylvia communis) | Long distance | Europe, western Asia | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Chiffchaff (Phylloscopus collybita) | Medium to long distance | Europe, western Asia | Southern Europe, North Africa | Migrates south in autumn, returns in spring |
| Nightingale (Luscinia megarhynchos) | Long distance | Europe, western Asia | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Redstart (Saxicola rubetra) | Long distance | Europe, western Asia | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Little Owl (Athene noctua) | Resident | Europe, North Africa, Asia | Resident | Non-migratory |
| Willow Warbler (Phylloscopus sibilatrix) | Long distance | Europe, western Siberia | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Pied Flycatcher (Ficedula hypoleuca) | Long distance | Europe | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Common Whitethroat (Sylvia communis) | Long distance | Europe, western Asia | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Chiffchaff (Phylloscopus collybita) | Medium to long distance | Europe, western Asia | Southern Europe, North Africa | Migrates south in autumn, returns in spring |
| Nightingale (Luscinia megarhynchos) | Long distance | Europe, western Asia | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Redstart (Saxicola rubetra) | Long distance | Europe, western Asia | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Tyrrhenian Spotted Flycatcher (Muscicapa striata tyrrhenica) | Short to medium distance | Western Mediterranean islands | Southern Europe, North Africa | Migrates south in autumn, returns in spring |
| Magpie (Pica pica) | Resident | Europe, Asia, North Africa | Resident | Non-migratory |
| Southern Grey Shrike (Lanius senator) | Long distance | Southern Europe, North Africa | Sub-Saharan Africa | Migrates south in autumn, returns in spring |
| Season | Bird Species (Scientific Name) | Number of Birds Captured (n°/%) | Number of Ticks (n°/%) |
|---|---|---|---|
| Fall | European Robin (Erithacus rubecula) | 301 (74.3%) | 579 (67%) |
| Common Blackbird (Turdus merula) | 54 (13.3%) | 188 (21.8%) | |
| Song Thrush (Turdus philomelos) | 34 (8.4%) | 68 (8%) | |
| Woodlark (Lullula arborea) | 7 (1.73%) | 17 (2%) | |
| Blackcap (Sylvia atricapilla) | 4 (1%) | 4 (0.5%) | |
| Garden Warbler (Sylvia borin) | 2 (0.5%) | 2 (0.2%) | |
| Common Redstart (Phoenicurus phoenicurus) | 2 (0.5%) | 3 (0.35%) | |
| Little Owl (Athene noctua) | 1 (0.3%) | 1 (0.15%) | |
| Total Fall | 405 | 862 | |
| Spring | Common Redstart (Phoenicurus phoenicurus) | 25 (44.6%) | 54 (55%) |
| Willow Warbler (Phylloscopus sibilatrix) | 7 (12.5%) | 11 (11%) | |
| Pied Flycatcher (Ficedula hypoleuca) | 7 (12.5%) | 8 (8%) | |
| European Robin (Erithacus rubecula) | 5 (9%) | 10 (10%) | |
| Common Whitethroat (Sylvia communis) | 3 (5.4%) | 3 (3%) | |
| Chiffchaff (Phylloscopus trochilus) | 2 (3.5%) | 2 (2%) | |
| Nightingale (Luscinia megarhynchos) | 2 (3.5%) | 3 (3%) | |
| Song Thrush (Turdus philomelos) | 1 (1.8%) | 2 (2%) | |
| Redstart (Saxicola rubetra) | 1 (1.8%) | 1 (1%) | |
| Tyrrhenian Spotted Flycatcher (Muscicapa Striata Tyrrhenica) | 1 (1.8%) | 1 (1%) | |
| Magpie (Pica pica) | 1 (1.8%) | 1 (1%) | |
| Southern Grey Shrike (Lanius senator) | 1 (1.8%) | 1 (1%) | |
| Total Spring | 56 | 99 | |
| TOTAL | 461 | 961 |
| Season | Tick Species | Number of Ticks Collected (%) | Larva (n°/%) | Nimph | Adult |
|---|---|---|---|---|---|
| Fall | Hyalomma marginatum | 5 (0.5%) | 2 (40%) | 2 (40%) | 1 (20%) |
| Ixodes inopinatus | 3 (0.2%) | 3 (100%) | 0 | 0 | |
| Ixodes ventalloi | 5 (0.5%) | 4 (80%) | 1 (20%) | 0 | |
| Ixodes frontalis | 117 (13.6%) | 83 (71%) | 25 (21%) | 9 (8%) | |
| Ixodes ricinus | 60 (77.2%) | 461 (53.4%) | 179 (20.8%) | 26 (3%) | |
| Ixodes spp. | 66 (7.7%) | 39 (59%) | 24 (36%) | 3 (5%) | |
| TOTAL | 862 (90%) | 592 (69%) | 231 (27%) | 39 (4%) | |
| Spring | Amblyomma marmoreum | 1 (1%) | 0 | 0 | 1 (100%) |
| Hyalomma marginatum | 11 (11.1%) | 3 (27%) | 8 (73%) | ||
| Hyalomma rufipes | 17 (17.2%) | 2 (12%) | 1 (6%) | 14 (82%) | |
| Rhipicephalus bursa | 1 (1%) | 0 | 0 | 1 (100%) | |
| Ixodes frontalis | 4 (4%) | 2 (50%) | 1 (25%) | 1 (25%) | |
| Ixodes inopinatus | 1 (1%) | 1 (100%) | 0 | 0 | |
| Ixodes ventalloi | 1 (1%) | 0 | 1 (100%) | 0 | |
| Hyalomma spp. | 63 (63.7%) | 25 (40%) | 7 (11%) | 31 (49%) | |
| TOTAL | 99 (10%) | 30 (30%) | 14 (14%) | 55 (55%) | |
| TOTAL | 961 | 622 (65%) | 245 (26%) | 94 (9%) |
| Season | Bird Species (n.) | Tick Species | Tick Life Stage | Sequencing Result |
|---|---|---|---|---|
| Fall | Redbreast (10) | I. ricinus (9) | Larva (9) | B. valaisiana (1) B. garinii (7) B. afzelii (1) |
| I. frontalis (1) | Larva (1) | B. garinii (1) | ||
| Song Thrush (13) | I. ricinus (11) | Larva (8) | B. garinii (7) B. valaisiana (1) | |
| Nymph (3) | B. garinii (3) | |||
| I. frontalis (2) | Larva (2) | B. turdi (1) B. garinii (1) | ||
| Blackbird (33) | I. ricinus (27) | Larva (17) | B. valaisiana (4) B. garinii (13) | |
| Nymph (10) | B. garinii (9) B. valaisiana (1) | |||
| I. frontalis (5) | Adult (1) | B. turdi (1) | ||
| Larva (4) | B. garinii (4) | |||
| I. inopinatus (1) | Larva (1) | B. garinii (1) |
| Sequence Type | Host | Collection Host | Gen Bank Accession Numbers | Blast Analysis |
|---|---|---|---|---|
| ST1 | I. ricinus 105 | European Robin | PV037008 | B. valaisiana |
| I. ricinus 240 | Blackbird | PV037009 | ||
| I. ricinus 349A | Blackbird | PV037010 | ||
| I. ricinus 349B | Blackbird | PV037011 | ||
| I. ricinus 350D | Song trush | PV037012 | ||
| ST2 | I. ricinus 141 | European Robin | PV036970 | B. garinii |
| I. ricinus 308B | Common Blackbird | PV036971 | ||
| I. frontalis 185b | Common Blackbird | PV036973 | ||
| I. frontalis 185d | Common Blackbird | PV036974 | ||
| I. ricinus 186 | Common Blackbird | PV036977 | ||
| I. ricinus 199a | Common Blackbird | PV036978 | ||
| I. frontalis 272a | European Robin | PV036984 | ||
| I. ricinus 351b | Common Blackbird | PV036991 | ||
| I. ricinus 314b | Common Blackbird | PV036994 | ||
| I. ricinus 315A | Common Blackbird | PV036995 | ||
| I. ricinus 317B | Common Blackbird | PV036997 | ||
| I. ricinus 317E | Common Blackbird | PV036998 | ||
| I. ricinus 28B | Common Blackbird | PV037002 | ||
| I. ricinus 41 | Song Thrush | PV037003 | ||
| I. ricinus 42 | Song Thrush | PV037004 | ||
| I. ricinus 70 | Song Thrush | PV037005 | ||
| ST3 | I. ricinus 233A | European Robin | PV036972 | B. garinii |
| I. ricinus 185H | Common Blackbird | PV036976 | ||
| ST4 | I. frontalis 185F | Common Blackbird | PV036975 | B. garinii |
| ST5 | I. ricinus 200A | Common Blackbird | PV036979 | B. garinii |
| I. ricinus 210B | European Robin | PV036983 | ||
| ST6 | I. ricinus 201 | Common Blackbird | PV036980 | B. garinii |
| ST7 | I. ricinus 206A | Song Thrush | PV036981 | B. garinii |
| ST8 | I. frontalis 206B | Song Thrush | PV037013 | B. turdi |
| I. frontalis 10A | Common Blackbird | PV037014 | ||
| ST9 | I. ricinus 263C | European Robin | PV068046 | B. afzelii |
| ST10 | I. ricinus 342A | European Robin | PV036987 | B. garinii |
| I. frontalis 345C | Common Blackbird | PV036988 | ||
| I. frontalis 345D | Common Blackbird | PV036989 | ||
| I. ricinus 347B | European Robin | PV036990 | ||
| I. ricinus 351C | Song Thrush | PV036992 | ||
| I. ricinus 351D | Song Thrush | PV036993 | ||
| I. ricinus 317A | Common Blackbird | PV036996 | ||
| I. ricinus 317F | Common Blackbird | PV036999 | ||
| I. ricinus 317H | Common Blackbird | PV037000 | ||
| I. ricinus 317Q | Common Blackbird | PV037001 | ||
| I. ricinus 43A | Common Blackbird | PV037006 | ||
| I. ricinus 43B | Common Blackbird | PV037007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chisu, V.; Giua, L.; Bianco, P.; Foxi, C.; Chessa, G.; Masala, G.; Piredda, I. Tick Dispersal and Borrelia Species in Ticks from Migratory Birds: Insights from the Asinara National Park, Sardinia, Italy. Microbiol. Res. 2025, 16, 88. https://doi.org/10.3390/microbiolres16050088
Chisu V, Giua L, Bianco P, Foxi C, Chessa G, Masala G, Piredda I. Tick Dispersal and Borrelia Species in Ticks from Migratory Birds: Insights from the Asinara National Park, Sardinia, Italy. Microbiology Research. 2025; 16(5):88. https://doi.org/10.3390/microbiolres16050088
Chicago/Turabian StyleChisu, Valentina, Laura Giua, Piera Bianco, Cipriano Foxi, Giovanna Chessa, Giovanna Masala, and Ivana Piredda. 2025. "Tick Dispersal and Borrelia Species in Ticks from Migratory Birds: Insights from the Asinara National Park, Sardinia, Italy" Microbiology Research 16, no. 5: 88. https://doi.org/10.3390/microbiolres16050088
APA StyleChisu, V., Giua, L., Bianco, P., Foxi, C., Chessa, G., Masala, G., & Piredda, I. (2025). Tick Dispersal and Borrelia Species in Ticks from Migratory Birds: Insights from the Asinara National Park, Sardinia, Italy. Microbiology Research, 16(5), 88. https://doi.org/10.3390/microbiolres16050088






