Simple Summary
Lactobacillus acidophilus, a Gram-positive probiotic belonging to the Lactobacillus, has garnered significant attention for its applications in dairy products, dietary supplements, and animal feed additives. This bacterium is commonly found in the natural environment and the digestive systems of various animals. It has the ability to regulate pH levels, enhance growth performance and feed efficiency, and bolster the immune response in animals. This paper provides a comprehensive analysis of recent advancements in the use of L. acidophilus in aquaculture, specifically highlighting its role in promoting growth, improving immune function and overall health in aquatic organisms, as well as enhancing water quality. Furthermore, the paper offers valuable recommendations for future research directions aimed at advancing the development of L. acidophilus products and supporting the sustainable growth of the aquaculture industry.
Abstract
Microbial feed additives can effectively promote the healthy development of aquaculture, and Lactobacillus acidophilus can be utilized to mitigate disease risks and enhance productivity while minimizing antibiotic use. This article summarizes research on the application of L. acidophilus in aquaculture, focusing on growth and nutrient utilization, intestinal structure and microbial communities, disease prevention and control in aquatic organisms, and the regulation of water quality. This review holds significant implications for the development of compound feed additives and environmental regulators involving L. acidophilus, as well as for future aquatic food safety.
1. Introduction
Lactobacillus acidophilus is a Gram-positive bacterium that belongs to the Lactobacillus genus [1,2]. This genus also includes other species, such as Lactobacillus plantarum and Lactobacillus bulgaricus [3,4]. These bacteria are capable of producing lactic acid, acetic acid, and certain antibiotics that help combat harmful bacteria [5,6,7,8,9,10]. As a probiotic, L. acidophilus demonstrates strong intestinal colonization abilities and enhances both growth performance and immune function in animals [11,12]. Furthermore, it has been utilized as an adjunct treatment for allergic diseases, digestive disorders, and viral infections in animals [1,13,14]. In recent years, L. acidophilus has been the subject of extensive research and application across various domains, including dairy products [15], dietary supplements [16], animal feed additives [1], and clinical medicine [13]. The widespread adoption of L. acidophilus in aquaculture has demonstrated its beneficial effects on aquatic animals [17,18,19]. This review highlights the various advantages of L. acidophilus in enhancing fish growth and immunity, as well as improving water quality in aquaculture. The aim of this study is to summarize the role of L. acidophilus in the aquaculture process to promote the sustainable development of the aquaculture industry.
2. The Relationship Between L. acidophilus and the Growth of Aquatic Animals
Probiotics can enhance the activities of digestive and brush border enzymes, thereby improving the digestive and absorptive capacity of fish [20]. Adding an appropriate amount of L. acidophilus to the feed can effectively promote the growth of aquatic organisms. The inclusion of 1 × 107 CFU/g of L. acidophilus in the diet of juvenile Pangasianodon hypophthalmus resulted in a specific growth rate of 1.35%, compared to 0.92% in the control group [21]. Similarly, the inclusion of 3.01 × 107 CFU/g of L. acidophilus in the diet of juvenile Clarias gariepinus significantly increased their specific growth rate to 4.17%, whereas the control group exhibited a rate of 3.86% [8]. Weng [22] supplemented the basic diet of Penaeus vannamei with 1 × 106 CFU/g of L. acidophilus, resulting in a 6.25% increase in the specific growth rate compared to the control group. Liu et al. [23] and Li et al. [24] supplemented the basic diet of Litopenaeus vannamei with varying proportions of L. acidophilus and observed a higher specific growth rate in the experimental groups compared to the control group. Moreover, the combination of L. acidophilus and other probiotics can also improve the growth performance of aquatic animals. The addition of a bacterial compound consisting of L. acidophilus and Bacillus subtilis to the feed resulted in a 5.15% increase in the weight gain rate of juvenile red crucian carp [25], and the specific growth rate of P. vannamei exhibited a 4.79% increase compared to the control group [22].
Lactobacillus acidophilus may promote the growth of aquatic animals by enhancing their digestive capabilities and nutrient absorption [26]. The inclusion of L. acidophilus in the diet has been shown to increase the activities of protease and amylase in the liver and intestines of Epinephelus coioides [27], and it significantly enhances the activity of intestinal amylase in Scophthalmus maximus [28]. Furthermore, L. acidophilus has been demonstrated to significantly increase the activities of intestinal amylase and protease in P. hypophthalmus, surpassing those of the control group by more than 1-fold and 0.9-fold, respectively [21]. Additionally, L. acidophilus can decrease the pH levels in the gut and stomach, promoting the digestion and absorption of nutrients, which in turn increases the weight gain rate and specific growth rate of Oreochromis niloticus [17,18]. The inclusion of 1 × 107 CFU/g of L. acidophilus in the diet of juvenile P. hypophthalmus resulted in an apparent protein digestibility of 85.27%, compared to 79.89% in the control group [21]. The addition of 3.01 × 107 CFU/g of L. acidophilus in the diet of juvenile C. gariepinus significantly increased their protein efficiency to 2.57%, while the control group exhibited a protein efficiency of 2.23% [8]. Incorporating 1 × 106 CFU/g of L. acidophilus into the basic feed of P. vannamei led to a 6.98% reduction in the feed conversion ratio compared to the control group [22]. Furthermore, the addition of L. acidophilus at a concentration of 1 × 1010 CFU/kg to the basal diet resulted in an apparent digestibility of 80.46% for L. vannamei, whereas the control group showed values of only 74.14% [26]. Additionally, L. acidophilus positively impacts the meat quality of fish; long-term feeding of diets supplemented with L. acidophilus can enhance the fat and protein content in the muscles of Silurus asotus [29].
Nevertheless, L. acidophilus does not universally promote the growth of aquatic animals, and its improper use may not effectively enhance their growth performance. Wu et al. [30] found that excessive addition of L. acidophilus can lead to a decline in the growth performance of S. maximus. Furthermore, as the feeding duration increases, the growth-promoting effect of L. acidophilus on S. maximus may gradually diminish. The effectiveness of L. acidophilus in promoting growth among aquatic animals is influenced by several factors, including dosage, feeding duration, as well as the species and size of the animals. Therefore, it is essential to conduct research on the optimal feeding dosage and timing of L. acidophilus for different cultured varieties to meet their growth requirements at various developmental stages. Additional information on growth and nutrient utilization is presented in Table 1.

Table 1.
Beneficial effects of L. acidophilus on the growth and nutrient utilization of aquatic organisms.
3. The Relationship Between L. acidophilus and the Intestinal Structure and Microbiota Composition of Aquatic Animals
Probiotic-friendly bacteria, such as Lactobacillus, present in symbiotic colonization, facilitate ultrastructural improvements by enhancing the regulation of epithelial cell turnover [38]. Probiotics can influence intestinal villi height and crypt depth, as well as regulate the expression of tight junction proteins, thereby altering the structure of the animal’s intestines [39]. Research has demonstrated that probiotics promote intestinal development and induce significant histological changes in the intestine [40]. L. acidophilus has the capacity to enhance the development of intestinal villi and increase the intestinal absorption area in fish [41]. Akter et al. [21] introduced various concentrations of L. acidophilus into the feed of P. hypophthalmus over a 12-week period and found that the addition of 1 × 105 CFU/g or 1 × 107 CFU/g of L. acidophilus significantly increased the length of intestinal villi, while the addition of 1 × 109 CFU/g significantly increased the width of the villi. Adeshina et al. [41] supplemented the feed of juvenile Cyprinus carpio with 1 × 106 CFU/g of L. acidophilus and observed that the fish in the L. acidophilus group exhibited significantly higher mean values of 0.78 cm for length, 0.25 cm for width, and 0.20 cm2 for the absorption area of intestinal villi, compared to the control group, which had mean values of 0.45 cm, 0.18 cm, and 0.08 cm2, respectively. Furthermore, the combined use of L. acidophilus and B. subtilis also increased the thickness of the posterior intestinal muscle layer, as well as the height and width of villi in hybrid grouper [42].
Probiotics can inhibit the adhesion and colonization of pathogens by competing for limited nutrients and adhesion sites, as well as by upregulating the expression of mucins that protect the gastrointestinal tract [43]. Specifically, L. acidophilus A4 enhances the expression of the MUC2 mucin by increasing MUC2 mRNA levels in the intestine, thereby forming a protective barrier against bacterial attachment and penetration. This mechanism effectively inhibits the adhesion of Escherichia coli O157:H7 to intestinal epithelial cells [44].
Lactobacillus acidophilus is commonly found in the intestinal tract of fish, where it plays a vital role in both intestinal development and the promotion of beneficial bacterial growth while inhibiting the proliferation of harmful bacteria. This regulation of the fish’s intestinal microbial community is crucial for maintaining intestinal health [26]. A diet enriched with L. acidophilus for Apostichopus japonicus can enhance the structure of their intestinal microflora, increase the abundance of potentially beneficial bacteria such as Lactobacillus and Clostridium, suppress the prevalence of opportunistic pathogens like Pseudomonas and Vibrio, and improve the digestive capacity and feed utilization efficiency of A. japonicus [35]. Feeding juvenile O. niloticus with plant protein feed fermented by L. acidophilus results in an increased abundance of Lactobacillus and a decreased abundance of pathogenic Vibrio in the intestine compared to unfermented feed [45]. Li et al. [46] found that the addition of soybean meal fermented by L. acidophilus improved the structure of the intestinal flora and mitigated the negative impact of soybean meal on this structure. Additionally, supplementing the diet of Cyrinus carpio with B. subtilis and L. acidophilus significantly enhances the intestinal flora structure, stimulates the proliferation of beneficial bacteria within the intestine, and reduces the abundance of Actinomycetes bacteria [47]. Therefore, L. acidophilus has the capacity to influence the structure of intestinal flora and digestive function in aquatic animals. It can inhibit the growth of pathogenic bacteria and enhance digestive function, whether applied as a direct feed additive or used as a feed starter. More information on changes in intestinal tissues and microbial communities is presented in Table 2.

Table 2.
Beneficial effects of L. acidophilus on the intestinal structure and gut microbial community of aquatic organisms.
4. The Relationship Between L. acidophilus and the Immune System of Aquatic Animals
Probiotics can exert immune functions within the animal body, acting not only as non-specific immune regulatory factors that activate the host’s immune cells but also exhibiting specific immune functions by stimulating B cells to produce antibodies [51]. Fish mucus contains lectins, lysozymes, complement proteins, antimicrobial peptides, and immunoglobulin M, all of which play a crucial role in preventing pathogen invasion [52]. Microecological preparations modulate intestinal mucosal immune function by activating pattern recognition receptors (PRRs) on intestinal epithelial and mucosal immune cells. This activation induces the secretion of various cytokines, such as interferon (IFN), colony-stimulating factor (CSF), interleukin (IL), and tumor necrosis factor (TNF). These cytokines promote the proliferation and differentiation of neutrophils and intestinal T cells, influence the accumulation of B cells producing specific antibodies, and increase IgA levels in gut-associated lymphoid tissue, thereby eliciting protective immune responses [53,54]. The inclusion of L. acidophilus in the diet enhances antibacterial activity and increases mucus protein levels in the skin mucus of fish, thereby improving the immune response following probiotic administration [37]. The intestinal tract plays a significant immune role in fish and serves as a vital site for probiotic activity [55]. L. acidophilus can compete with pathogenic bacteria for adhesion sites, produce antibacterial substances, and improve the mechanical and immune barriers of the intestinal mucosa, thereby enhancing host immunity [1]. Hoseinifar et al. [37] reported that the addition of L. acidophilus to the diet had beneficial effects on mucosal immune parameters, stress resistance, and growth metrics in Xiphophorus helleri. Furthermore, incorporating probiotics into the feed can induce changes in the expression levels of immune-related genes in the host’s intestinal tract, assisting the host in resisting pathogenic invasion through immune enhancement [56]. L. acidophilus has the ability to reduce levels of intestinal inflammatory factors, thereby regulating intestinal inflammation [57], and it can exhibit antiviral effects by altering the gene expression profile in induced dendritic cells [58]. Hosseini et al. [52] observed a significant upregulation of mucoprotein tumor necrosis factor 1α (TNF-1α) and 2α (TNF-2α) gene expression in the head kidney of Carassius auratus gibelio following dietary supplementation with 6 × 108 CFU/g of L. acidophilus. Adeshina et al. [41] also found that L. acidophilus could regulate the expression of tumor necrosis factor α (TNF-α), transforming growth factor β (TGF-β), and interleukin 8 (IL-8) in C. carpio, thereby enhancing the host’s immune response. Additionally, the inclusion of L. acidophilus in the diet can elevate the activities of lysozyme, superoxide dismutase, and catalase in C. carpio [41], as well as the activities of acid phosphatase, alkaline phosphatase, and superoxide dismutase in the GIFT strain of O. niloticus, thereby improving the host’s ability to resist pathogens [59]. More Information on immune function is presented in Table 3.

Table 3.
Beneficial effects of L. acidophilus on the immune function of aquatic organisms.
5. The Prevention and Control Effect of L. acidophilus on Bacterial Diseases in Aquatic Animals
Bacterial diseases in aquaculture significantly impact both economic and social development in many countries [62]. Probiotics play a crucial role in preventing and controlling these diseases by enhancing host immunity, competing with pathogenic microorganisms for nutrients and living space, and secreting active substances with antimicrobial properties [40]. Probiotics can competitively exclude pathogenic microorganisms by occupying binding sites in aquatic animals, thereby reducing the population and density of pathogens [63]. Upon colonizing, growing, and reproducing in the host’s intestinal tract, probiotics secrete various extracellular antimicrobial substances, such as superoxide dismutase, lysozyme, organic acids, and hydrogen peroxide, which exhibit strong inhibitory effects against pathogen proliferation. Furthermore, probiotics often outcompete harmful pathogens for resources, placing the pathogens at a competitive disadvantage that ultimately leads to their elimination [64]. Iron is an essential element for critical biochemical reactions, including ATP synthesis, DNA precursor reduction, and heme formation. Under iron-limited conditions, such as those found in the intestinal environment, probiotics prefer Bacillus to Lactobacillus Fe3+. These siderophores competitively bind Fe3+ from host iron-binding proteins (e.g., transferrin and lactoferrin), forming soluble Fe3+-siderophore complexes that are transported into bacterial cells where Fe3+ is reduced by ferric reductases to meet bacterial iron demands [65]. Certain probiotic siderophores (e.g., those produced by Bacillus DET9) contain 2,3-dihydroxybenzoic acid, which exhibits high iron affinity, preferentially chelating environmental iron and consequently limiting pathogen growth due to iron deprivation [66,67]. Lactobacillus acidophilus produces metabolites including lactic acid, digestive enzymes, vitamins, hydrogen peroxide, and bacteriocins. Through mechanisms such as pH modulation, antimicrobial production, and niche competition, it inhibits the growth of harmful microorganisms, thereby reducing disease incidence [8,40,68]. L. acidophilus has been shown to secrete bacteriostatic substances with potent activity, effectively inhibiting the growth of pathogens such as Aeromonas hydrophila, Pseudomonas aeruginosa, and various pathogenic species of Vibrio [48,69,70].
Research on A. hydrophila as a pathogen is extensive. L. acidophilus E downregulates the mRNA expression of inflammatory cytokines IL-6 and IL-8 in an E. coli O157-infected human colon adenocarcinoma cell line (Caco-2 cells ATCC-HTB-37) through the TLR4/MyD88/NF-κB pathway. Additionally, it reduces the apparent permeability coefficient and monolayer permeability of Caco-2 cells, thereby mitigating E. coli-induced inflammatory responses [71]. Villamil et al. [72] discovered that L. acidophilus promotes the expression of immune-related genes, including IL-1β and transferrin-related genes in the spleen and kidney, thereby enhancing the survival rate of O. niloticus infected with A. hydrophila. Khalil et al. [73] also observed that O. niloticus exhibited improved health, immune status, and increased resistance against A. hydrophila when fed L. acidophilus. Similarly, Akter et al. [19] found that juvenile when P. hypophthalmus was fed L. acidophilus, it showed a survival rate of 93.33% after being injected with 1 × 106 CFU/mL of A. hydrophila, compared to only 80.00% in the control group. According to Adeshina [74], long-term feeding of L. acidophilus significantly reduced the mortality rate of Cyprinos carpio from 76.67% to 16.67% after injection with 1 × 107 CFU/mL of A. hydrophila, and reduced the mortality rate of C. carpio from 63.33% to 13.33% after injection with 1 × 107 CFU/mL of P. aeruginosa. Vibriosis is a highly prevalent bacterial disease that affects a variety of aquatic organisms [62]. Fermenting soybean meal with L. acidophilus has been found to be effective in reducing the concentration of Vibrio bacteria in fish intestines and promoting the control of intestinal pathogens [75]. The inclusion of L. acidophilus in the diet can decrease mortality caused by pathogenic Vibrio in aquatic animals [27]. For L. vannamei, the addition of L. acidophilus to the diet enhanced the activities of serum polyphenol oxidase, alkaline phosphatase, and acid phosphatase, while reducing mortality after Vibrio harveyi infection [24]. The addition of L. acidophilus in the feed significantly reduces morbidity and mortality in Macrobrachium rosenbergii after injection with V. harveyi, Vibrio vulnificus, and Vibrio anguillarum [76]. Moreover, supplementing the diet with L. acidophilus can improve the physiological and biochemical blood parameters of fish infected with pathogens such as Enterococcus faecalis, Staphylococcus xylosus, and Streptococcus agalactiae, while enhancing the fish’s immune response [18,77]. Faramarzi et al. [78] found that the mortality rate of O. mykiss injected with 1 × 107 CFU/mL of P. aeruginosa decreased by 26.7% to 33.4% when fed L. acidophilus compared to the control group.
Furthermore, the combination of L. acidophilus with other probiotics has demonstrated inhibitory effects on various pathogenic bacteria. The inclusion of a bacterial combination of L. acidophilus and B. subtilis in the feed has been shown to enhance the resistance of C. carpio to A. hydrophila [47], as well as the resistance of O. niloticus to Streptococcus [69]. Additionally, L. acidophilus La-14 and Lactobacillus rhamnosus can effectively mitigate the inflammation caused by E. coli in zebrafish [79]. These findings underscore the effectiveness of L. acidophilus in improving the overall health status of aquatic animals, enhancing their disease resistance, and increasing their survival rates. Consequently, L. acidophilus holds significant value in the field of aquaculture and has important applications. More bacteriostatic information is presented in Table 4.

Table 4.
Bacteriostatic effects of L. acidophilus in aquatic organisms.
6. The Regulatory Effect of L. acidophilus on Water Environment
There is a diverse array of microorganisms in aquatic environments that play a crucial role in decomposing residual feed, feces, and other organic matter, thereby contributing to water purification [83,84]. Additionally, these microorganisms can accelerate the breakdown of substances such as ammonia nitrogen, nitrate, and hydrogen sulfide, which leads to improved water quality and the maintenance of a healthy aquatic ecosystem [85]. The abundance and ratio of pathogenic bacteria to probiotics among these microorganisms can serve as biomarkers for assessing the health of aquatic ecosystems, as probiotics have the ability to inhibit pathogenic bacteria [86].
Currently, commonly used probiotics for improving water quality in aquaculture include lactic acid bacteria, photosynthetic bacteria, and Bacillus strains [87]. However, there is relatively little research on the role of lactic acid bacteria in regulating water quality. L. acidophilus can regulate pH levels through the production of acidic substances via its metabolic processes [88]. Additionally, L. acidophilus inhibits the growth and reproduction of harmful microorganisms by producing antibacterial substances, competing for nutrients, and occupying ecological niches, thereby reducing the incidence of diseases [89]. Furthermore, L. acidophilus has the ability to remove heavy metals such as arsenic, cadmium, and lead from water [90]. Even at low arsenic concentrations, L. acidophilus can reduce the arsenic level in water by 60% within three hours [91]. L. acidophilus also possesses the capability to remove, absorb, and decompose biodegradable organic matter in wastewater, resulting in a reduction of total suspended solids by 91.0% and total dissolved solids by 74.2% in industrial wastewater [92]. In aquaculture, the use of biological flocs containing L. acidophilus can effectively decrease nitrogen and phosphorus levels in aquaculture water, thereby improving its environmental quality [93]. While adding L. acidophilus to the diet of C. gariepinus has a positive effect on water quality in aquaculture, the impact is not significant [6]. However, further research is needed to explore the regulatory effects and mechanisms of L. acidophilus on aquaculture water quality.
7. Conclusions and Perspectives
Lactobacillus acidophilus plays a crucial role in enhancing the composition of intestinal microbiota in aquatic animals, thereby improving their digestive capabilities, immune function, and water quality (Figure 1). However, the effectiveness of L. acidophilus can be influenced by various factors, including the species of aquatic animals, application methods, and dosage. Further research is needed to investigate the form and quantity of additives used to assess the impact of L. acidophilus on the growth and health of different aquatic organisms. When incorporated into compound feed, it is essential to explore the timing of the addition to ensure maximum activity. If added during feeding, the optimal dosage must be determined to ensure the safety of aquatic animals. Additionally, measures should be taken to maintain the activity of the microbial community when L. acidophilus is directly introduced into the water environment. Effective application methods and optimal dosages can significantly promote the growth of aquatic animals and serve as a foundation for exploring the mechanisms by which L. acidophilus affects aquatic organisms.
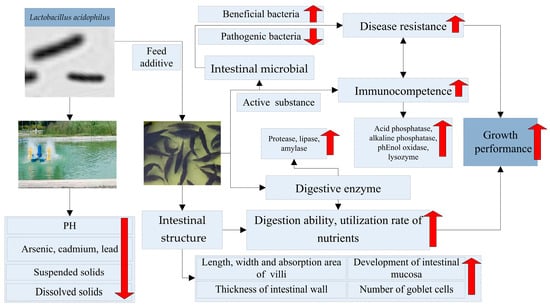
Figure 1.
The functions of Lactobacillus acidophilus in aquaculture.
Author Contributions
Conceptualization, Y.D. and J.Z.; methodology, L.Z., Q.L. and Z.H.; software, Z.H.; validation, Z.H., Z.Z. and L.Z.; formal analysis, Y.D., H.L. and L.Z.; investigation, Z.Z., H.Z., C.M. and Y.F.; resources, Y.D., H.Z., Q.L. and J.Z.; data curation, Y.D., Z.Z., Q.L. and L.Z.; writing—original draft preparation, L.Z., Q.L. and Y.D.; writing—review and editing, Y.D., Q.L. and J.Z.; visualization, Y.D.; supervision, J.Z.; project administration, Z.H. and J.Z.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Modern Agricultural Technology System—Specialty Freshwater Fish Industry Technology System (CARS-46); Sichuan Freshwater Fish Innovation Team of the National Modern Agricultural Industrial Technology System (SCCXTD-2025-15).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to acknowledge the support provided by the National Modern Agricultural Technology System—Specialty Freshwater Fish Industry Technology System (CARS-46); Sichuan Freshwater Fish Innovation Team of the National Modern Agricultural Industrial Technology System (SCCXTD-2025-15).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, C.; Li, J.; Wang, B.; Xing, X. Application research progress of Lactobacillus acidophilus. J. Tradit. Chin. Vet. Med. 2023, 42, 41–44. [Google Scholar]
- Bull, M.; Plummer, S.; Marchesi, J.; Mahenthiralingam, E. The life history of Lactobacillus acidophilus as a probiotic: A tale of revisionary taxonomy, misidentification and commercial success. FEMS Microbiol. Lett. 2013, 349, 77–87. [Google Scholar] [CrossRef]
- Alishahi, M.; Dezfuly, Z.T.; Mesbah, M.; Mohammadian, T. Effects of two probiotics, Lactobacillus plantarum and Lactobacillus bulgaricus on growth performance and intestinal lactic acid bacteria of Cyprinus carpio. Iran. J. Vet. Med. 2018, 12, 207–217. [Google Scholar]
- Foysal, M.J.; Fotedar, R.; Siddik, M.A.; Tay, A. Lactobacillus acidophilus and L. plantarum improve health status, modulate gut microbiota and innate immune response of marron (Cherax cainii). Sci. Rep. 2020, 10, 5916. [Google Scholar] [CrossRef]
- Jafarei, P.; Ebrahimi, M.T. Lactobacillus acidophilus cell structure and application. Afr. J. Microbiol. Res. 2011, 5, 4033–4042. [Google Scholar] [CrossRef]
- Wang, L.; Pan, D.; Yang, Y.; Wu, Z.; Zeng, X. Isolation and functional analysis of Lactobacillus acidophilus from fish intestine. J. Chin. Inst. Food Sci. Technol. 2014, 14, 20–25. [Google Scholar]
- Al-Dohail, M.A. Effects of Probiotic, Lactobacillus acidophilus on Pathogenic Bacteria, Growth, Hematological Parameters, and Histopathology of African Catfish (Clarias gariepinus). Ph.D. Thesis, Universiti Sains Malaysia, Pulau Pinang, Malaysia, 2010. [Google Scholar]
- Al-Dohail, M.A.; Hashim, R.; Aliyu-Paiko, M. Effects of the probiotic, Lactobacillus acidophilus, on the growth performance, haematology parameters and immunoglobulin concentration in African Catfish (Clarias gariepinus, Burchell 1822) fingerling. Aquac. Res. 2009, 40, 1642–1652. [Google Scholar] [CrossRef]
- Xing, Z.; Fu, X.; Huang, H.; Xu, Y.; Wei, L.; Shan, C.; Du, Y. Recent advances in Lactobacillus plantarum fermentation in modifying fruit-based products: Flavor property, bioactivity, and practical production applications. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70160. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Li, Y.; Hao, J.; Lu, H.; He, Y.; Wei, L.; Ai, L.; Wang, S. Co-culture of Lactobacillus bulgaricus with Streptococcus thermophilus and Bifidobacterium impact the metabolism and flavor of fermented milk. Food Sci. Nutr. 2025, 13, e70182. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, K.; Zhang, A.; Chang, W.; Zheng, A.; Chen, Z.; Cai, H.; Liu, G. Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O157. Poult. Sci. 2021, 100, 101323. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, W.; Ci, W.; Zheng, Y.; Han, X.; Huang, J.; Zhu, J. Effects of dietary supplementation with Lactobacillus acidophilus and Bacillus subtilis on mucosal immunity and intestinal barrier are associated with its modulation of gut metabolites and microbiota in late-phase laying hens. Probiotics Antimicrob. Proteins 2023, 15, 912–924. [Google Scholar] [CrossRef]
- María Remes-Troche, J.; Coss-Adame, E.; Ángel Valdovinos-Díaz, M.; Gómez-Escudero, O.; Eugenia Icaza-Chávez, M.; Antonio Chávez-Barrera, J.; Zárate-Mondragón, F.; Antonio Velarde-Ruíz Velasco, J.; Rafael Aceves-Tavares, G.; Antonio Lira-Pedrín, M. Lactobacillus acidophilus LB: A useful pharmabiotic for the treatment of digestive disorders. Ther. Adv. Gastroenterol. 2020, 13, 1756284820971201. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, L.; Guo, R.; Cui, S.; Lu, W.; Yang, L.; Lu, J.; Sun, X.; Zhao, L.; He, F. Anti-allergic effects of Lactobacillus acidophilus La28 and L.plantoplantum LP45 in mice. Acta Microbiol. Sin. 2022, 62, 797–805. [Google Scholar]
- Kim, H.S.; Gilliland, S.E. Lactobacillus acidophilus as a dietary adjunct for milk to aid lactose digestion in humans. J. Dairy Sci. 1983, 66, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.; Maqsood, S.; Masud, T.; Ahmad, A.; Sohail, A.; Momin, A. Lactobacillus acidophilus: Characterization of the species and application in food production. Crit. Rev. Food Sci. Nutr. 2014, 54, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.S.; El-Sayed, A.; Mohammady, E.Y.; Zaki, M.A.; Elkhyat, M.M.; Jarmołowicz, S.; El-Haroun, E.R. Eubiotic effect of a dietary potassium diformate (KDF) and probiotic (Lactobacillus acidophilus) on growth, hemato-biochemical indices, antioxidant status and intestinal functional topography of cultured Nile tilapia Oreochromis niloticus fed diet free fishmeal. Aquaculture 2021, 533, 736147. [Google Scholar] [CrossRef]
- Mousa, M.; El-Keredy, A.M.; Rashed, M.A. Using Lactobacillus acidophilus in fish feed to improve disease resistance and immune status of cultured Nile tilapia. Alex. J. Vet. Sci. 2021, 70, 15–28. [Google Scholar]
- Akter, N.; Hashim, R.; Sutriana, A.; Mohd Nor, S.A.; Janaranjani, M. Lactobacillus acidophilus supplementation improves the innate immune response and disease resistance of striped catfish (Pangasianodon hypophthalmus Sauvage, 1878) juveniles against Aeromonas hydrophila. Trends Sci. 2023, 20, 4932. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, W.; Wu, P.; Liu, Y.; Ma, Y.; Ren, H.; Jin, X.; Jiang, J.; Zhang, R.; Li, H.; et al. Probiotic efficacy of Cetobacterium somerae (CGMCC No. 28843): Promoting intestinal digestion, absorption, and structural integrity in juvenile grass carp (Ctenopharyngodon idella). J. Anim. Sci. Biotechnol. 2025, 16, 103. [Google Scholar] [CrossRef]
- Akter, M.N.; Hashim, R.; Sutriana, A.; Siti Azizah, M.N.; Asaduzzaman, M. Effect of Lactobacillus acidophilus supplementation on growth performances, digestive enzyme activities and gut histomorphology of striped catfish (Pangasianodon hypophthalmus Sauvage, 1878) juveniles. Aquac. Res. 2019, 50, 786–797. [Google Scholar] [CrossRef]
- Weng, Z. Effect of probiotics on growth performance of Penaeus vannamei. Henan Aquat. Prod. 2022, 21–22+30. [Google Scholar]
- Liu, D.; Dong, X.; Tan, B.; Yang, Q.; Chi, S.; Liu, H.; Zhang, S. Effects of Lactobacillus acidophilus on growth performance, immunity and disease resistance in Pacific white leg shrimp Litopenaeus vannamei. Fish. Sci. 2016, 35, 37–42. [Google Scholar]
- Li, J.; Yang, Q.; Tan, B.; Dong, X.; Chi, S.; Liu, H.; Zhang, S.; Zhang, H. Effects of Lactobacillus acidophilus supplementation on the growth, enzyme activities, and mRNA expression of disease-resistance related enzymes of juvenile Litopenaeus vannamei. J. Fish. Sci. China 2018, 25, 1022–1031. [Google Scholar] [CrossRef]
- Ma, X.; Wu, X.; Hu, L. Effects of two species of dietary viable bacteria on microbiota in water and intestinal of juvenile red crucian carp Carassius auratus. Fish. Sci. 2018, 37, 316–323. [Google Scholar]
- Li, J.; Wang, M.; Tian, X.; Liu, L.; Li, H.; Li, L.; Dong, S.; Xue, L. Effects of different Lactic acid bacteria added to feed on the growth performance, disease resistance and intestinal microflora of Litopenaeus vannamei. Period. Ocean Univ. China 2021, 51, 44–54. [Google Scholar]
- Li, J.; Yang, Q.; Tan, B.; Dong, X.; Chi, S.; Liu, H.; Zhang, S.; Zhang, H. Effects of Lactobacillus acidophilus (gim:1.730) on growth, digestive capacity, disease resistance of juvenile, epinephelus coioides. Acta Hydrobiol. Sin. 2019, 43, 992–1000. [Google Scholar]
- Gao, F.; Guo, W.; Pan, L.; Hu, F.; Jian, Y.; Zhang, S.; Wang, X. Effects of dietary probiotics on growth and digestive enzyme activities of juvenile Scophthal musmaximus. Mar. Sci. 2011, 35, 10–16. [Google Scholar]
- Xu, Q. Preparation and Application of Lactobacillus acidophilus Microecological Preparations. Master’s Thesis, Xihua University, Chengdu, China, 2015. [Google Scholar]
- Wu, Z.; Wang, Y.; Hu, F.; Guan, J.; Nie, A.; Sun, F. Study on the effect of Lactobacillus acidophilus on promoting growth of turbot Scophthalmus maximus (Linnaeus). Fish. Mod. 2013, 40, 28–31+50. [Google Scholar]
- Khalafalla, M.M.; Zayed, N.F.A.; Amer, A.A.; Soliman, A.A.; Zaineldin, A.I.; Gewaily, M.S.; Hassan, A.M.; Van Doan, H.; Tapingkae, W.; Dawood, M.A.O. Dietary Lactobacillus acidophilus ATCC 4356 relieves the impacts of aflatoxin B(1) toxicity on the growth performance, hepatorenal functions, and antioxidative capacity of thinlip grey mullet (Liza ramada) (Risso 1826). Probiotics Antimicrob Proteins 2022, 14, 189–203. [Google Scholar] [CrossRef]
- Abidin, Z.; Huang, H.T.; Hu, Y.F.; Chang, J.J.; Huang, C.Y.; Wu, Y.S.; Nan, F.H. Effect of dietary supplementation with Moringa oleifera leaf extract and Lactobacillus acidophilus on growth performance, intestinal microbiota, immune response, and disease resistance in whiteleg shrimp (Penaeus vannamei). Fish Shellfish Immunol. 2022, 127, 876–890. [Google Scholar] [CrossRef]
- Nikiforov-Nikishin, A.; Nikiforov-Nikishin, D.; Kochetkov, N.; Smorodinskaya, S.; Klimov, V. The influence of probiotics of different microbiological composition on histology of the gastrointestinal tract of juvenile Oncorhynchus mykiss. Microsc. Res. Tech. 2022, 85, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, T.; Nasirpour, M.; Tabandeh, M.R.; Heidary, A.A.; Ghanei-Motlagh, R.; Hosseini, S.S. Administrations of autochthonous probiotics altered juvenile rainbow trout Oncorhynchus mykiss health status, growth performance and resistance to Lactococcus garvieae, an experimental infection. Fish Shellfish Immunol. 2019, 86, 269–279. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Guan, X.; Dong, Y.; Zhao, Z.; Jiang, J.; Li, S.; Jiang, B.; Wang, B.; Zhang, G. Effects of dietary Lactobacillus acidophilus and tussah immunoreactive substances supplementation on physiological and immune characteristics of sea cucumber (Apostichopus japonicus). Aquaculture 2021, 542, 736897. [Google Scholar] [CrossRef]
- Munir, M.B.; Hashim, R.; Abdul Manaf, M.S.; Nor, S.A. Dietary prebiotics and probiotics influence the growth performance, feed utilisation, and body indices of snakehead (Channa striata) fingerlings. Trop. Life Sci. Res. 2016, 27, 111–125. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Roosta, Z.; Hajimoradloo, A.; Vakili, F. The effects of Lactobacillus acidophilus as feed supplement on skin mucosal immune parameters, intestinal microbiota, stress resistance and growth performance of black swordtail (Xiphophorus helleri). Fish Shellfish Immunol. 2015, 42, 533–538. [Google Scholar] [CrossRef]
- Yitbarek, A.; Rodriguez-Lecompte, J.C.; Echeverry, H.M.; Munyaka, P.; Barjesteh, N.; Sharif, S.; Camelo-Jaimes, G. Performance, histomorphology, and toll-like receptor, chemokine, and cytokine profile locally and systemically in broiler chickens fed diets supplemented with yeast-derived macromolecules. Poult. Sci. 2013, 92, 2299–2310. [Google Scholar] [CrossRef]
- Hernández-Coronado, A.C.; Cervantes, M.; González, F.; Valle, A.; Arce, N.; Vásquez, N.; Bernal, H.; Morales, A. Effect of probiotic supplementation on productive performance and epithelial intestinal integrity of broiler chickens exposed to heat stress. Trop. Anim. Health Prod. 2025, 57, 235. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.X.H.; Tan, L.T.H.; Law, J.W.F.; Khaw, K.Y.; Zengin, G.; Chan, K.G.; Letchumanan, V.; Lee, L.H.; Goh, B.H. Probiotics: Comprehensive exploration of the growth promotion mechanisms in shrimps. Prog. Microbes Mol. Biol. 2023, 6, a0000324. [Google Scholar] [CrossRef]
- Adeshina, I.; Abubakar, M.I.O.; Ajala, B.E. Dietary supplementation with Lactobacillus acidophilus enhanced the growth, gut morphometry, antioxidant capacity, and the immune response in juveniles of the common carp, Cyprinus carpio. Fish Physiol. Biochem. 2020, 46, 1375–1385. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Y.; Huang, J.; Ou, G.; Wen, Z.; Li, Y.; Ma, Q.; Chen, G. Effects of compound probiotics on growth performance, antioxidant capacity and intestinal health of hybrid grouper (Epinephelus fuscogutatus ♀ × Epinephelus polyphekadion ♂). J. Guangdong Ocean Univ. 2023, 43, 81–91. [Google Scholar]
- Cai, Y.; Wu, G.; Yang, H.; Fang, Y.; Chen, Z.; Zhan, X. Mechanisms of action and current applications of probiotics in animal health management. Chin. J. Anim. Sci. Vet. Med. 2025, 52, 2101–2114. [Google Scholar]
- Kim, Y.; Kim, S.H.; Whang, K.Y.; Kim, Y.J.; Oh, S. Inhibition of Escherichia coli O157:H7 attachment by interactions between lactic acid bacteria and intestinal epithelial cells. J. Microbiol. Biotechnol. 2008, 18, 1278–1285. [Google Scholar]
- Neves, N.O.D.S.; De Dea Lindner, J.; Stockhausen, L.; Delziovo, F.R.; Bender, M.; Serzedello, L.; Cipriani, L.A.; Ha, N.; Skoronski, E.; Gisbert, E. Fermentation of Plant-Based Feeds with Lactobacillus acidophilus Improves the Survival and Intestinal Health of Juvenile Nile Tilapia (Oreochromis niloticus) Reared in a Biofloc System. Animals 2024, 14, 332. [Google Scholar] [CrossRef]
- Li, C.; Tian, Y.; Wang, L.; Zhang, B.; Ma, Q. Effects of replacing fishmeal by raw or Lactobacillus acidophilus-fermented soybean meal on growth, intestinal digestive and immune-related enzyme activities, morphology, and microbiota in turbot (Scophthalmus maximus L.). Aquac. Nutr. 2022, 2022, e2643235. [Google Scholar] [CrossRef]
- Bi, Y. Preliminary Study on Intestinal Microflora Response of Yellow River Carp Based on Probiotics Regulation. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2016. [Google Scholar]
- Liu, G.; Hu, X.; Su, H.; Xu, W.; Xu, Y.; Wen, G.; Cao, Y. Antagonism of Lactobacillus acidophilus against three Vibrio species and its influence on gut microbiota of Litopenaeus vannamei. South China Fish. Sci. 2024, 20, 83–91. [Google Scholar]
- Ehsannia, S.; Ahari, H.; Kakoolaki, S.; Anvar, S.A.; Yousefi, S. Effects of probiotics on Zebrafish model infected with Aeromonas hydrophila: Spatial distribution, antimicrobial, and histopathological investigation. BMC Microbiol. 2022, 22, 167. [Google Scholar] [CrossRef]
- Wang, T.; Dai, M.Z.; Liu, F.S.; Cao, B.B.; Guo, J.; Shen, J.Q.; Li, C.Q. Probiotics modulate intestinal motility and inflammation in Zebrafish models. Zebrafish 2020, 17, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z. Application of probiotics in livestock husbandry. China Swine Ind. 2023, 18, 44–47. [Google Scholar]
- Hosseini, M.; Miandare, H.; Hoseinifar, S.; Yarahmadi, P. Dietary Lactobacillus acidophilus modulated skin mucus protein profile, immune and appetite genes expression in gold fish (Carassius auratus gibelio). Fish Shellfish Immunol. 2016, 59, 149–154. [Google Scholar] [CrossRef]
- Kayama, H.; Takeda, K. Manipulation of epithelial integrity and mucosal immunity by host and microbiota-derived metabolites. Eur. J. Immunol. 2020, 50, 921–931. [Google Scholar] [CrossRef]
- Wang, W.S.; Wu, H.; Chen, Z.X.; Liu, J.Y. Mechanisms of action of microecological preparations and their effects on immune function in livestock and poultry. Feed Res. 2024, 47, 143–147. [Google Scholar]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef]
- Xu, Q.; Li, R.; Wang, G. Research progress of the effect of probiotics on mucosal immunity of fish. Feed Ind. 2022, 43, 5–8. [Google Scholar]
- Ye, L.L. Study on the Mechanism of Regulating Wnt/β-Catenin Signaling Pathway by Lactobacillus acidophilus to Maintain Intestinal Mucosal Barrier. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2018. [Google Scholar]
- Weiss, G.; Rasmussen, S.; Zeuthen, L.H.; Nielsen, B.N.; Jarmer, H.; Jespersen, L.; Frøkiær, H. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology 2010, 131, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, J.; Kuang, Z.; Wang, G.; Wu, C.; Fu, J.; Mo, W.; Huang, Y. Effect of dietary Lactobacillus on growth performance, non-specific immune enzymes activities and intestinal microflora of Oreochromis niloticu. Guangdong Agric. Sci. 2013, 40, 123–126. [Google Scholar]
- Abu-Braka, A.Z.; Zaki, M.S.; Abbas, H.H.; Ismail, N.; Khalil, R.; Tanekhy, M.; Saad, T. Filed studies on some probiotics to minimize hazard effects of prevailing heavy metals contamination for improving immunity and growth performance of Oreochromis niloticus. Electron Physician 2017, 9, 4138–4144. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ren, P.; He, S.; Xu, L.; Yang, Y.; Gu, Z.; Zhou, Z. Comparison of adhesive gut bacteria composition, immunity, and disease resistance in juvenile hybrid tilapia fed two different Lactobacillus strains. Fish Shellfish Immunol. 2013, 35, 54–62. [Google Scholar] [CrossRef]
- Ina-Salwany, M.; Al-saari, N.; Mohamad, A.; Mursidi, F.A.; Mohd-Aris, A.; Amal, M.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in fish: A review on disease development and prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef]
- Vandaan, H.; Hoseinifar, S.H.; Ringø, E.; Angeles Esteban, M.; Dadar, M.; Dawood, M.A.; Faggio, C. Host-associated probiotics: A key factor in sustainable aquaculture. Rev. Fish. Sci. Aquac. 2020, 28, 16–42. [Google Scholar] [CrossRef]
- Liu, W.Q.; Jin, Y.Q.; Dong, H.X.; Mai, Q.Y.; Sun, D.L.; Wu, Y.L.; Lai, M.J.; Zeng, W.W. Application of probiotics in disease prevention and control in aquaculture. Prog. Vet. Med. 2023, 44, 112–116. [Google Scholar]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Patel, A.K.; Ahire, J.J.; Pawar, S.P.; Chaudhari, B.L.; Shouche, Y.S.; Chincholkar, S.B. Evaluation of probiotic characteristics of siderophoregenic Bacillus spp. isolated from dairy waste. Appl. Biochem. Biotechnol. 2010, 160, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Kumar, R.S.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Probiotics and its functionally valuable products-A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 641–658. [Google Scholar] [CrossRef]
- Hong, M.; Liu, Y.Z.; Shi, B.J. Antibacterial activity and characteristics of a Lactobacillus acidophilus strain. Heilongjiang Anim. Sci. Vet. Med. 2017, 18, 122–124. [Google Scholar]
- Aly, S.M.; Ahmed, Y.A.G.; Ghareeb, A.A.A.; Mohamed, M.F. Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol. 2008, 25, 128–136. [Google Scholar] [CrossRef]
- Alinejad Moallem, S. Investigation of antagonistic effect of Lactobacillus acidophilus isolated from the gastrointestinal tract of oscar fish (Astronotus ocellatus) on Pseudomonas aeruginosa. Int. J. Mol. Clin. Microbiol. 2021, 11, 1464–1470. [Google Scholar]
- Wu, Z.K. Probiotic effects of Lactobacillus acidophilus in broilers and its mechanisms. Chin. Acad. Agric. Sci. 2021. [Google Scholar] [CrossRef]
- Villamil, L.; Reyes, C.; Martínez-Silva, M. In vivo and in vitro assessment of Lactobacillus acidophilus as probiotic for tilapia (Oreochromis niloticus, Perciformes: Cichlidae) culture improvement. Aquac. Res. 2014, 45, 1116–1125. [Google Scholar] [CrossRef]
- Khalil, R.; Talaat, T.; Tenikhy, M.; Sherif, A.; Amina, S. Effect of Lactobacillus acidophilus on the growth and immune response in cultured Oreochromis niloticus. Glob. J. Fish. Aquac. Res. 2014, 1, 57–69. [Google Scholar]
- Adeshina, I. The effect of Lactobacillus acidophilus as a dietary supplement on nonspecific immune response and disease resistance in juvenile common carp, Cyprinos carpio. Int. Food Res. J. 2018, 25, 2345–2351. [Google Scholar]
- Oliveira, N.S.d.; Ha, N.; Cunha, L.d.; Cipriani, L.A.; Neto, A.T.; Skoronski, E.; Gisbert, E.; Fabregat, T.E.H.P. Fermentation of soybean meal with Lactobacillus acidophilus allows greater inclusion of vegetable protein in the diet and can reduce vibrionacea in the intestine of the south American catfish (Rhamdia quelen). Animals 2022, 12, 690. [Google Scholar] [CrossRef]
- Khan, S.; Mahmud, S. The impact of probiotic bacterium Lactobacillus acidophilus in growth and survival of the juvenile fresh water river prawn (Macrobrachium rosenbergii) infected with pathogenic Vibrio spp. J. Microbiol. Biotechnol. Food Sci. 2015, 5, 225–229. [Google Scholar] [CrossRef]
- Al-Dohail, M.A.; Hashim, R.; Aliyu-Paiko, M. Evaluating the use of Lactobacillus acidophilus as a biocontrol agent against common pathogenic bacteria and the effects on the haematology parameters and histopathology in African catfish Clarias gariepinus juveniles. Aquac. Res. 2011, 42, 196–209. [Google Scholar] [CrossRef]
- Faramarzi, M.; Kiaalvandi, S.; Lashkarbolooki, M.; Iranshahi, F. The investigation of Lactobacillus acidophilus as probiotics on growth performance and disease resistance of rainbow trout (Oncorhychus mykiss). Am. Eurasian J. Sci. Res. 2011, 6, 32–38. [Google Scholar]
- Guo, Y.; Yu, Q.; Yan, J.; Gao, Y.; Li, X.; Mao, X. Anti-infection and anti-inflammatory effects of compound probiotics on zebrafish. Mod. Food 2023, 29, 176–180+193. [Google Scholar]
- Akter, N.; Hashim, R.; Pham, H.Q.; Choi, S.D.; Lee, D.W.; Shin, J.H.; Rajagopal, K. Lactobacillus acidophilus antimicrobial peptide is antagonistic to Aeromonas hydrophila. Front. Microbiol. 2020, 11, 570851. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.B.; Hashim, R.; Nor, S.A.M.; Marsh, T.L. Effect of dietary prebiotics and probiotics on snakehead (Channa striata) health: Haematology and disease resistance parameters against Aeromonas hydrophila. Fish Shellfish Immunol. 2018, 75, 99–108. [Google Scholar] [CrossRef]
- Patel, B.; Kumar, P.; Banerjee, R.; Basu, M.; Pal, A.; Samanta, M.; Das, S. Lactobacillus acidophilus attenuates Aeromonas hydrophila induced cytotoxicity in catla thymus macrophages by modulating oxidative stress and inflammation. Mol. Immunol. 2016, 75, 69–83. [Google Scholar] [CrossRef]
- Tian, Q.; Guo, Z.; Ji, L.; He, S.; Liu, P.; Zhu, X.; Li, X. Research progress of probiotics in aquaculture. Ind. Microorg. 2019, 49, 50–55. [Google Scholar]
- Huang, Y. Research progress of probiotics in fish-farming. Chin. Feed 2023, 5–8. [Google Scholar] [CrossRef]
- Su, Z.; Li, Y.; Pan, L.; Xue, F. An investigation on the immunoassays of an ammonia nitrogen-degrading bacterial strain in aquatic water. Aquaculture 2016, 450, 17–22. [Google Scholar] [CrossRef]
- Liu, P.; Luo, J.; Gao, Q. Research progress of environmental microorganisms in aquaculture. Curr. Biotechnol. 2022, 12, 690–695. [Google Scholar]
- Mi, S.; Xu, J.; Li, J.; Zhang, Z.; Xian, J.; Wang, D. Research progress on the application of common probiotics in fish and shrimp culture. Fish. Hebei 2021, 39–44. [Google Scholar]
- Zhang, W. Screening of Lactobacillus acidophilus of Animal Origin and Its Preparation Technology. Master’s Thesis, Nanchang University, Nanchang, China, 2021. [Google Scholar]
- Zhang, Y.; Zhang, L.; Li, Y. Study on the growth inhibition of some pathogens by the L. Acidophilus B. infantis. J. Northeast Agric. Univ. 2004, 35, 208–211. [Google Scholar]
- Afsharian, Z.; Salavatifar, M.; Khosravi_Daranic, K. Impact of simulated microgravity on bioremoval of heavy-metals by Lactobacillus acidophilus ATCC 4356 from water. Heliyon 2022, 8, e12307. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.L.; Sarma, P. Removal of Arsenic (III) from waste water using Lactobacillus acidophilus. Bioremediation J. 2010, 14, 92–97. [Google Scholar] [CrossRef]
- Vellingiri, K.; Ramachandran, T.; Thirugnanasambantham, A. Isolation and purification of Lactobacillus acidophilus and analyzing its influence on effluent treatment. Int. J. Eng. Technol. 2015, 5, 66–74. [Google Scholar]
- Li, H.; Zhang, Z.; Ge, H.; Pan, B.; Yang, Z. Application of biofloc technology in desalination culture of Litopenaeus vannamei in ponds. J. Fish. Res. 2022, 44, 562. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).