A Retrospective Observational Study of the Impact of HIV Status on the Outcome of Paediatric Intensive Care Unit Admissions at a Tertiary Hospital in South Africa (2015–2019)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
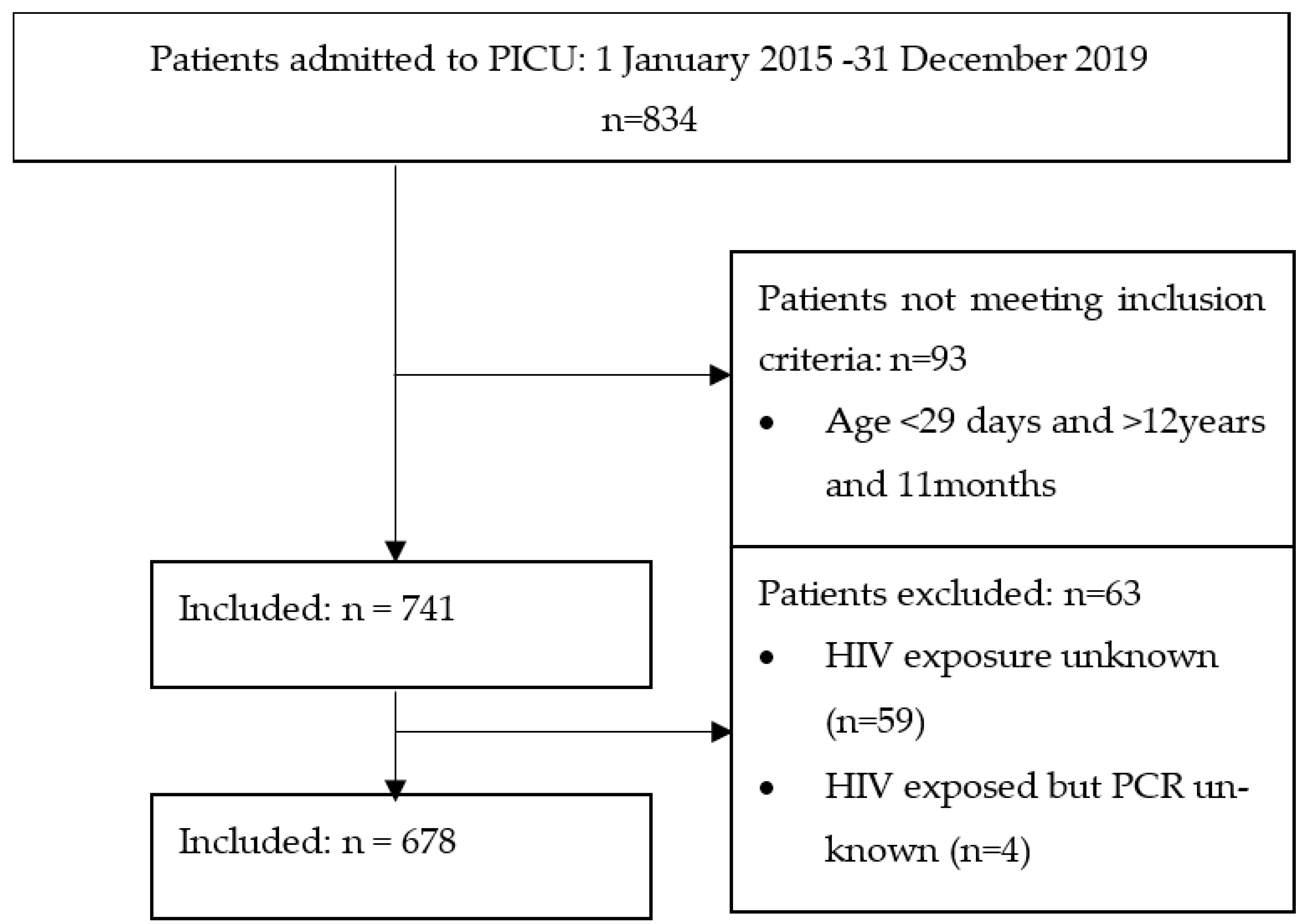
2.3. Participants
2.4. Study Procedure
- (1)
- HIV negative: The mother was negative, or the child had a negative HIV ELISA;
- (2)
- HEU: The mother was HIV positive, or the child less than 18 months had a positive ELISA AND the child had a negative HIV PCR;
- (3)
- HIV infected: A child less than 18 months with a positive HIV PCR or a child more than 18 months with a positive ELISA. HIV infection was confirmed with a second ELISA, PCR or HIV viral load.
HIV-Exposed Children Include Both HEU and HIV-Infected Children
- (1)
- Surgical: Any patient admitted with an underlying surgical condition;
- (2)
- Accidents (including trauma), drownings and poisonings (A, D + P);
- (3)
- Lower respiratory tract (LRT) conditions: An illness affecting the respiratory tract below the level of the larynx including pneumonia, bronchiolitis, asthma and pleural effusions;
- (4)
- Other medical conditions: Medical conditions other than lower respiratory tract conditions (e.g., gastrointestinal and cardiovascular conditions).
2.5. Statistical Methods
Ethical Consideration
3. Results
3.1. Descriptive Statistics
3.2. Comparison between HIV-Negative, HEU and HIV-Infected Patients
3.3. Comparison by HIV Status for Children Admitted with a Medical Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A, D and P | Accidents, drownings and poisonings |
| ART | Antiretroviral Therapy |
| CMJAH | Charlotte Maxeke Johannesburg Academic Hospital |
| CMV | Cytomegalovirus |
| CPAP | Continuous Positive Airway Pressure |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HEU | HIV Exposed Uninfected |
| HFOV | High-Frequency Oscillatory Ventilation |
| HIV | Human Immunodeficiency Virus |
| ICU | Intensive Care Unit |
| IPPV | Intermittent Positive Pressure Ventilation |
| IQR | Interquartile Range |
| LDH | Lactate Dehydrogenase |
| LMICs | Low- and Middle-Income Countries |
| LRT | Lower Respiratory Tract |
| PCR | Polymerase Chain Reaction |
| PICU | Paediatric Intensive Care Unit |
| PIM3 | Paediatric Index of Mortality 3 |
| PCP | Pneumocystis Pneumonia |
| PMTCT | Prevention of Mother-to-Child Transmission |
| REDCap | Research Electronic Data Capture |
| SPSS | Statistical Programme for the Social Sciences |
| WHO | World Health Organization |
References
- Bamford, L.; McKerrow, N.; Barron, P.; Aung, Y. Child mortality in South Africa: Fewer deaths, but better data are needed. S. Afr. Med. J. 2018, 108, 25–32. [Google Scholar] [CrossRef]
- UNAIDS. Country Factsheets South Africa. 2019. Available online: https://www.unaids.org/en/regionscountries/countries/southafrica (accessed on 20 September 2022).
- South African National Department of Health. Guideline for the Prevention of Mother to Child Transmission of Communicable Infections. 2019. Available online: http://www.nicd.ac.za/wp-content/uploads/2019/11/Guidelines-for-the-Prevention-of-Transmission-of-Communicable-Diseases-from-mother-to-child_28-October.pdf (accessed on 5 June 2021).
- Argent, A.C. Managing HIV in the PICU—The experience at the Red Cross War Memorial Children’s Hospital in Cape Town. Indian J. Pediatr. 2008, 75, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Jeena, P.M.; Adhikari, M. Provision of critical care services to HIV-infected children in an era of advanced intensive care and availability of combined antiretroviral therapy. Paediatr. Int. Child Health 2017, 37, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Vernon, D.D.; Holzman, B.H.; Lewis, P.; Scott, G.B.; Birriel, J.A.; Scott, M.B. Respiratory failure in children with acquired immunodeficiency syndrome and acquired immunodeficiency syndrome-related complex. Pediatrics 1988, 82, 223–228. [Google Scholar] [PubMed]
- Thirsk, E.R.; Kapongo, M.C.; Jeena, P.M.; Liebeschuetz, S.; York, D.F.; Vega, G.; van Vuuren, S.; Coovadia, H.M. HIV-exposed infants with acute respiratory failure secondary to acute lower respiratory infections managed with and without mechanical ventilation. S. Afr. Med. J. 2003, 93, 617–620. [Google Scholar] [PubMed]
- Jeena, P.M.; McNally, L.M.; Stobie, M.; Coovadia, H.M.; Adhikari, M.A.; Petros, A.J. Challenges in the provision of ICU services to HIV infected children in resource poor settings: A South African case study. J. Med. Ethics 2005, 31, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Pillay, V.; Davies, M.A.; King, S.; Eley, B. Short-term treatment outcomes of children starting antiretroviral therapy in the intensive care unit, general medical wards and outpatient HIV clinics at Red Cross War Memorial Children’s Hospital, Cape Town, South Africa: A retrospective cohort study. S. Afr. Med. J. 2015, 105, 220–227. [Google Scholar] [CrossRef]
- Hutton, H.K.; Zar, H.J.; Argent, A.C. Clinical Features and Outcome of Children with Severe Lower Respiratory Tract Infection Admitted to a Pediatric Intensive Care Unit in South Africa. J. Trop. Pediatr. 2019, 65, 46–54. [Google Scholar] [CrossRef]
- Mopeli, R.K.; White, D.A.; Ballot, D.E. An audit of primary medical conditions in children admitted to the paediatric intensive care unit of Charlotte Maxeke Johannesburg Academic Hospital. S. Afr. J. Child. Health 2016, 10, 221–226. [Google Scholar] [CrossRef]
- Argent, A.C.; Ahrens, J.; Morrow, B.M.; Reynolds, L.G.; Hatherill, M.; Salie, S.; Benatar, S.R. Pediatric intensive care in South Africa: An account of making optimum use of limited resources at the Red Cross War Memorial Children’s Hospital. Pediatr. Crit. Care Med. 2014, 15, 7–14. [Google Scholar] [CrossRef]
- Morrow, B.M.; Hsaio, N.Y.; Zampoli, M.; Whitelaw, A.; Zar, H.J. Pneumocystis pneumonia in South African children with and without human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Pediatr. Infect. Dis. J. 2010, 29, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Kitchin, O.P.; Masekela, R.; Becker, P.; Moodley, T.; Risenga, S.M.; Green, R.J. Outcome of human immunodeficiency virus-exposed and -infected children admitted to a pediatric intensive care unit for respiratory failure. Pediatr. Crit. Care Med. 2012, 13, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Zar, H.J.; Apolles, P.; Argent, A.; Klein, M.; Burgess, J.; Hanslo, D.; Bateman, E.D.; Hussey, G. The etiology and outcome of pneumonia in human immunodeficiency virus-infected children admitted to intensive care in a developing country. Pediatr. Crit. Care Med. 2001, 2, 108–112. [Google Scholar] [CrossRef] [PubMed]
- le Roux, D.M.; Myer, L.; Nicol, M.P.; Zar, H.J. Incidence and severity of childhood pneumonia in the first year of life in a South African birth cohort: The Drakenstein Child Health Study. Lancet Glob. Health 2015, 3, e95–e103. [Google Scholar] [CrossRef] [PubMed]
- Bhagwanjee, S.; Scribante, J. National audit of critical care resources in South Africa—Unit and bed distribution. S. Afr. Med. J. 2007, 97, 1311–1314. [Google Scholar]
- Ballot, D.; Ramdin, T.; White, D.; Dhai, A. Ethical dilemmas in paediatric intesive care in the South African public healthcare sector. S. Afr. J. Bioeth. Law. 2019, 12, 44–46. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inf. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- SA National Department of Health. National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults. Available online: http://www.kznhealth.gov.za/family/HIV-Guidelines-Jan2015.pdf (accessed on 10 June 2021).
- WHO. Weight-for-Age Charts. Available online: https://www.who.int/tools/child-growth-standards/standards/weight-for-age (accessed on 5 June 2021).
- Cooper, S.; Lyall, H.; Walters, S.; Tudor-Williams, G.; Habibi, P.; de Munter, C.; Britto, J.; Nadel, S. Children with human immunodeficiency virus admitted to a paediatric intensive care unit in the United Kingdom over a 10-year period. Intensive Care Med. 2004, 30, 113–118. [Google Scholar] [CrossRef]
- Rabie, H.; de Boer, A.; van den Bos, S.; Cotton, M.F.; Kling, S.; Goussard, P. Children with human immunodeficiency virus infection admitted to a paediatric intensive care unit in South Africa. J. Trop. Pediatr. 2007, 53, 270–273. [Google Scholar] [CrossRef][Green Version]
- Mussi-Pinhata, M.M.; Motta, F.; Freimanis-Hance, L.; de Souza, R.; Szyld, E.; Succi, R.C.; Christie, C.D.; Rolon, M.J.; Ceriotto, M.; Read, J.S. Lower respiratory tract infections among human immunodeficiency virus-exposed, uninfected infants. Int. J. Infect. Dis. 2010, 14 (Suppl. S3), e176–e182. [Google Scholar] [CrossRef]
- Ballot, D.E.; Ramdin, T.; White, D.A.; Lipman, J. A comparison between raw and predicted mortality in a paediatric intensive care unit in South Africa. BMC Res. Notes 2018, 11, 829. [Google Scholar] [CrossRef]
| HIV Negative | HIV Infected | p-Value | |
|---|---|---|---|
| Total Patients | 534 | 35 | |
| Admission age (months) Median (IQR) | 13 (51) | 2 (1) | p < 0.001 |
| Weight z-score Median (IQR) | −1.45 (3.20) | −2.42 (2.33) | p = 0.063 |
| Main Admission Diagnosis n (%): | p < 0.001 | ||
| Surgical | 257/534 (48.1) | 2/35 (5.7) | |
| A, P and D | 85/534 (15.9) | 0/35 (0) | |
| LRT | 100/534 (18.7) | 29/35 (82.9) | |
| Other medical | 92/534 (17.2) | 4/35 (11.4) | |
| Viral Infection n (%) | 25/517 (4.8) | 14/34 (41.2) | p < 0.001 |
| PCP/CMV n (%): | p < 0.001 | ||
| PCP only | 2/532 (0.4) | 13/35 (37.1) | |
| CMV only | 1/532 (0.2) | 2/35 (5.7) | |
| PCP + CMV | 0/532 (0) | 10/35 (28.6) | |
| TB n (%) | 8/531 (1.5) | 6/35 (17.1) | p < 0.001 |
| Ventilation mode n (%): | p < 0.001 | ||
| CPAP | 15/516 (2.9) | 0/33 (0) | |
| IPPV | 426/516 (82.6) | 23/33 (69.7) | |
| HFOV | 23/516 (4.5) | 10/33 (30.3) | |
| ICU duration (days) Median (IQR) | 3 (5) | 9 (10) | p < 0.001 |
| Ventilation length (days) Median (IQR) | 2 (5) | 7.5 (10) | p < 0.001 |
| Died in ICU n (%) | 120/529 (22.7) | 14/35 (40) | p = 0.02 |
| HIV Negative | HEU | p-Value | |
|---|---|---|---|
| Total Patients | 534 | 109 | |
| Admission age (months) Median (IQR) | 13 (51) | 3 (6) | p < 0.001 |
| Weight z-score Median (IQR) | −1.45 (3.20) | −2.71 (4.34) | p = 0.001 |
| Main Admission Diagnosis n (%): | p < 0.001 | ||
| Surgical | 257/534 (48.1) | 39/109 (35.8) | |
| A, P and D | 85/534 (15.9) | 2/109 (1.8) | |
| LRT | 100/534 (18.7) | 43/109 (39.4) | |
| Other medical | 92/534 (17.2) | 25/109 (22.9) | |
| Viral Infection n (%) | 25/517 (4.8) | 12/106 (11.3) | p < 0.001 |
| PCP/CMV n (%): | p < 0.001 | ||
| PCP only | 2/532 (0.4) | 5/109 (4.6) | |
| CMV only | 1/532 (0.2) | 2/109 (1.8) | |
| PCP + CMV | 0/532 (0) | 2/109 (1.8) | |
| TB n (%) | 8/531 (1.5) | 4/109 (3.7) | p = 0.129 |
| Ventilation mode n (%): | p = 0.163 | ||
| CPAP | 15/516 (2.9) | 4/105 (3.8) | |
| IPPV | 426/516 (82.6) | 83/105 (79) | |
| HFOV | 23/516 (4.5) | 10/105 (9.5) | |
| ICU duration (days) Median (IQR) | 3 (5) | 4 (8) | p = 0.163 |
| Ventilation length (days) Median (IQR) | 2 (5) | 3.5 (7) | p = 0.443 |
| Died in ICU n (%) | 120/529 (22.7) | 33/109 (30.3) | p = 0.091 |
| HEU | HIV Infected | p-Value | |
|---|---|---|---|
| Total Patients | 109 | 35 | |
| Admission age (months) Median (IQR) | 3 (6) | 2 (1) | p = 1.00 |
| Weight z-score Median (IQR) | −2.71 (4.34) | −2.42 (2.33) | p = 1.00 |
| Main Admission Diagnosis n (%): | p < 0.001 | ||
| Surgical | 39/109 (35.8) | 2/35 (5.7) | |
| A, P and D | 2/109 (1.8) | 0/35 (0) | |
| LRT | 43/109 (39.4) | 29/35 (82.9) | |
| Other medical | 25/109 (22.9) | 4/35 (11.4) | |
| Viral Infection n (%) | 12/106 (11.3) | 14/34 (41.2) | p < 0.001 |
| PCP/CMV n (%): | p < 0.001 | ||
| PCP only | 5/109 (4.6) | 13/35 (37.1) | |
| CMV only | 2/109 (1.8) | 2/35 (5.7) | |
| PCP + CMV | 2/109 (1.8) | 10/35 (28.6) | |
| TB n (%) | 4/109 (3.7) | 6/35 (17.1) | p = 0.006 |
| Ventilation mode n (%): | p = 0.009 | ||
| CPAP | 4/105 (3.8) | 0/33 (0) | |
| IPPV | 83/105 (79) | 23/33 (69.7) | |
| HFOV | 10/105 (9.5) | 10/33 (30.3) | |
| ICU duration (days) Median (IQR) | 4 (8) | 9 (10) | p = 0.009 |
| Ventilation length (days) Median (IQR) | 3.5 (7) | 7.5 (10) | p = 0.001 |
| Died in ICU n (%) | 33/109 (30.3) | 14/35 (40) | p = 0.286 |
| HIV Negative | HEU | HIV Infected | p-Value | |
|---|---|---|---|---|
| Weight z-score Median (IQR) | −2.11 (3.13) | −2.37 (4.48) | −2.42 (2.33) | 0.338 |
| Age (months) Median (IQR) | 5.5 (22) | 3 (6) | 2 (1) | 0.001 |
| Duration ventilation Median (IQR) | 4 (6) | 4 (7) | 8 (10) | 0.001 |
| Duration ICU Median (IQR) | 4 (7) | 5 (9) | 9 (10) | 0.001 |
| Died in ICU (%) | 62/190 (32.6) | 27/68 (39.7) | 14/33 (42.4) | 0.387 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitehead, K.; Ballot, D.E. A Retrospective Observational Study of the Impact of HIV Status on the Outcome of Paediatric Intensive Care Unit Admissions at a Tertiary Hospital in South Africa (2015–2019). Pediatr. Rep. 2023, 15, 679-690. https://doi.org/10.3390/pediatric15040061
Whitehead K, Ballot DE. A Retrospective Observational Study of the Impact of HIV Status on the Outcome of Paediatric Intensive Care Unit Admissions at a Tertiary Hospital in South Africa (2015–2019). Pediatric Reports. 2023; 15(4):679-690. https://doi.org/10.3390/pediatric15040061
Chicago/Turabian StyleWhitehead, Kim, and Daynia E. Ballot. 2023. "A Retrospective Observational Study of the Impact of HIV Status on the Outcome of Paediatric Intensive Care Unit Admissions at a Tertiary Hospital in South Africa (2015–2019)" Pediatric Reports 15, no. 4: 679-690. https://doi.org/10.3390/pediatric15040061
APA StyleWhitehead, K., & Ballot, D. E. (2023). A Retrospective Observational Study of the Impact of HIV Status on the Outcome of Paediatric Intensive Care Unit Admissions at a Tertiary Hospital in South Africa (2015–2019). Pediatric Reports, 15(4), 679-690. https://doi.org/10.3390/pediatric15040061





