Chromosomal Integration of HHV-6 in a Preterm Neonate: A Rare Case of Hyperleukocytosis and Clinical Implications
Abstract
:1. Introduction
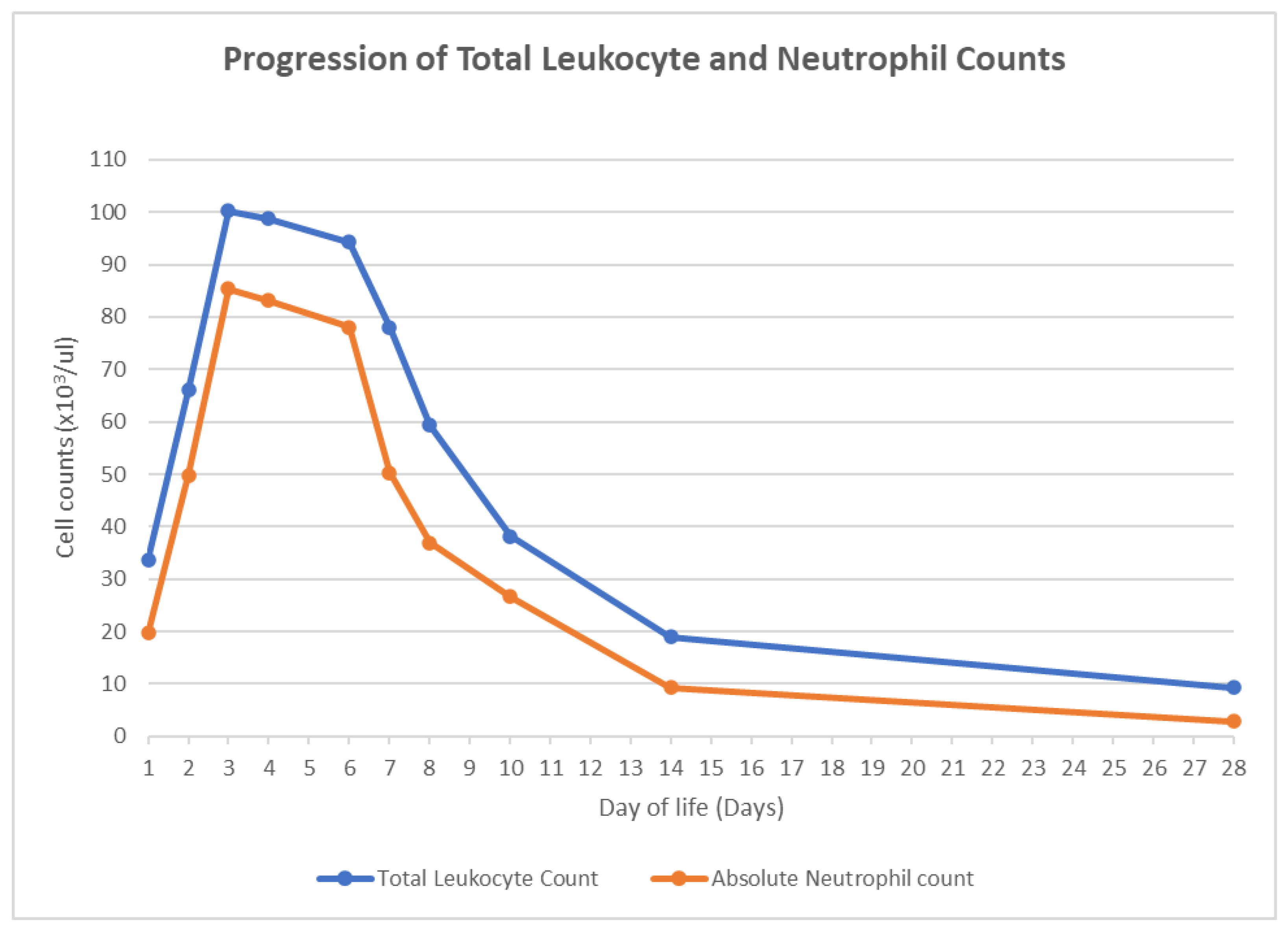
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortega, L.; Ramzanali, S.; Mora, P.; Kaylani, S.-Z.; Adesanya, O. Extreme Hyperleukocytosis in an Extremely Preterm Infant. J. Rare Dis. Orphan Drugs 2022, 3, 6–10. [Google Scholar] [CrossRef]
- Jansen, E.; Emmen, J.; Mohns, T.; Donker, A. Extreme hyperleucocytosis of the premature. BMJ Case Rep. 2013, 2013, bcr2012008385. [Google Scholar] [CrossRef] [PubMed]
- Parvez, Y.; Mathew, A.G. Hyperleukocytosis in newborn: A diagnosis of concern. Indian J. Hematol. Blood Transfus. 2014, 30 (Suppl. S1), 131–132. [Google Scholar] [CrossRef] [PubMed]
- Sakka, V.; Tsiodras, S.; Giamarellos-Bourboulis, E.J.; Giamarellou, H. An update on the etiology and diagnostic evaluation of a leukemoid reaction. Eur. J. Intern. Med. 2006, 17, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Ablashi, D.; Agut, H.; Alvarez-Lafuente, R.; Clark, D.A.; Dewhurst, S.; DiLuca, D.; Flamand, L.; Frenkel, N.; Gallo, R.; Gompels, U.A.; et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch. Virol. 2014, 159, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Agut, H.; Bonnafous, P.; Gautheret-Dejean, A. Update on infections with human herpesviruses 6A, 6B, and 7. Med. Mal. Infect. 2017, 47, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.C.; Micali, G.A.; Schwartz, R.A. Roseola infantum and its causal human herpesviruses. Int. J. Dermatol. 2014, 53, 397–403. [Google Scholar] [CrossRef]
- Zerr, D.M. Human Herpesvirus 6B in the Transplant Recipient: When to Worry, When to Act. J. Pediatric Infect. Dis. Soc. 2018, 7 (Suppl. S2), S75–S78. [Google Scholar] [CrossRef]
- Pantry, S.N.; Medveczky, P.G. Latency, Integration, and Reactivation of Human Herpesvirus-6. Viruses 2017, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Aimola, G.; Beythien, G.; Aswad, A.; Kaufer, B.B. Current understanding of human herpesvirus 6 (HHV-6) chromosomal integration. Antiviral Res. 2020, 176, 104720. [Google Scholar] [CrossRef] [PubMed]
- Kaufer, B.B.; Flamand, L. Chromosomally integrated HHV-6: Impact on virus, cell and organismal biology. Curr. Opin. Virol. 2014, 9, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A. Clinical and laboratory features of human herpesvirus 6 chromosomal integration. Clin. Microbiol. Infect. 2016, 22, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Caserta, M.T.; Schnabel, K.; Shelley, L.M.; Marino, A.S.; Carnahan, J.A.; Yoo, C.; Lofthus, G.K.; McDermott, M.P. Chromosomal integration of human herpesvirus 6 is the major mode of congenital human herpesvirus 6 infection. Pediatrics 2008, 122, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Taya, K.; Sashihara, J.; Kurahashi, H.; Amo, K.; Miyagawa, H.; Kondo, K.; Okada, S.; Yamanishi, K. Human herpesvirus 6 (HHV-6) is transmitted from parent to child in an integrated form characterization of cases with chromosomally integrated HHV-6 DNA. J. Med. Virol. 2004, 73, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kosugi, S.; Koide, R.; Kawamura, Y.; Ito, J.; Miura, H.; Matoba, N.; Matsuzaki, M.; Fujita, M.; Kamada, A.J.; et al. Endogenization and excision of human herpesvirus 6 in human genomes. PLoS Genet. 2020, 16, e1008915. [Google Scholar] [CrossRef] [PubMed]
- Flamand, L.; Komaroff, A.L.; Arbuckle, J.H.; Medveczky, P.G.; Ablashi, D.V. Review, part 1: Human herpesvirus-6-basic biology, diagnostic testing, and antiviral efficacy. J. Med. Virol. 2010, 82, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
| Laboratory Test | Day of Life (DOL) Collected | Laboratory Value |
|---|---|---|
| CSF HHV-6 PCR | DOL 4 | 1600 copies/mL |
| Plasma/Serum HHV-6 PCR | DOL 7 | 470,000 copies/mL |
| Whole-Blood HHV-6 PCR | DOL 7 | 5,500,000 copies/mL |
| Whole-Blood HHV-6 DNA Copies to Nucleated Cell Count Ratio | DOL 7 | 0.96 |
| Serum HHV-6 IgG | DOL 7 | Positive |
| Serum HHV-6 IgM | DOL 7 | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasundaram, P.; Sakr, M. Chromosomal Integration of HHV-6 in a Preterm Neonate: A Rare Case of Hyperleukocytosis and Clinical Implications. Pediatr. Rep. 2024, 16, 432-437. https://doi.org/10.3390/pediatric16020037
Balasundaram P, Sakr M. Chromosomal Integration of HHV-6 in a Preterm Neonate: A Rare Case of Hyperleukocytosis and Clinical Implications. Pediatric Reports. 2024; 16(2):432-437. https://doi.org/10.3390/pediatric16020037
Chicago/Turabian StyleBalasundaram, Palanikumar, and Mohamed Sakr. 2024. "Chromosomal Integration of HHV-6 in a Preterm Neonate: A Rare Case of Hyperleukocytosis and Clinical Implications" Pediatric Reports 16, no. 2: 432-437. https://doi.org/10.3390/pediatric16020037
APA StyleBalasundaram, P., & Sakr, M. (2024). Chromosomal Integration of HHV-6 in a Preterm Neonate: A Rare Case of Hyperleukocytosis and Clinical Implications. Pediatric Reports, 16(2), 432-437. https://doi.org/10.3390/pediatric16020037







