Prenatal Tobacco Exposure and Behavioral Disorders in Children and Adolescents: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Studies
2.2. Data Analysis Plan
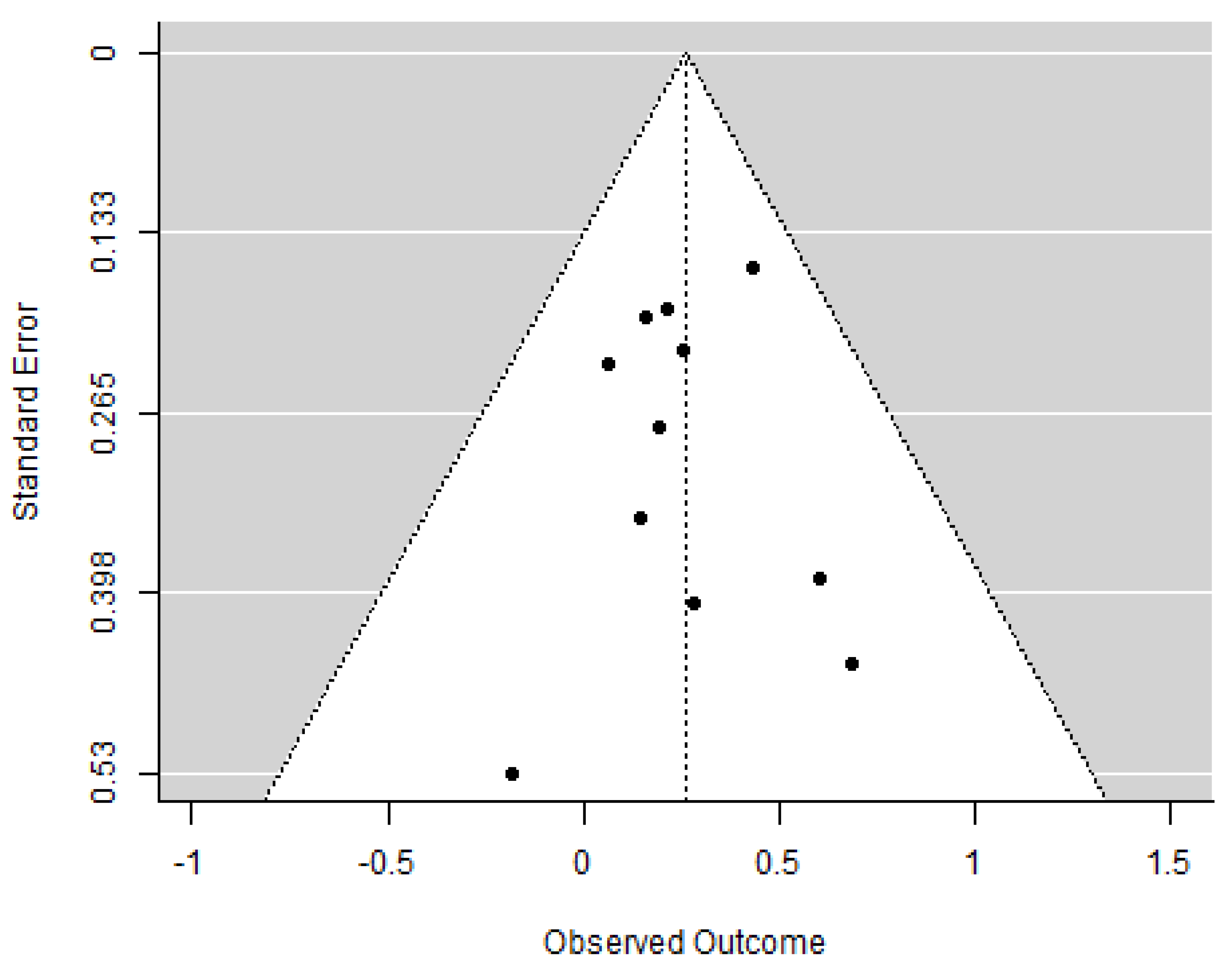
2.3. Publication Bias
2.4. Quality Assessment
3. Results
3.1. Search Results, Quality Assessment, and Coding Features
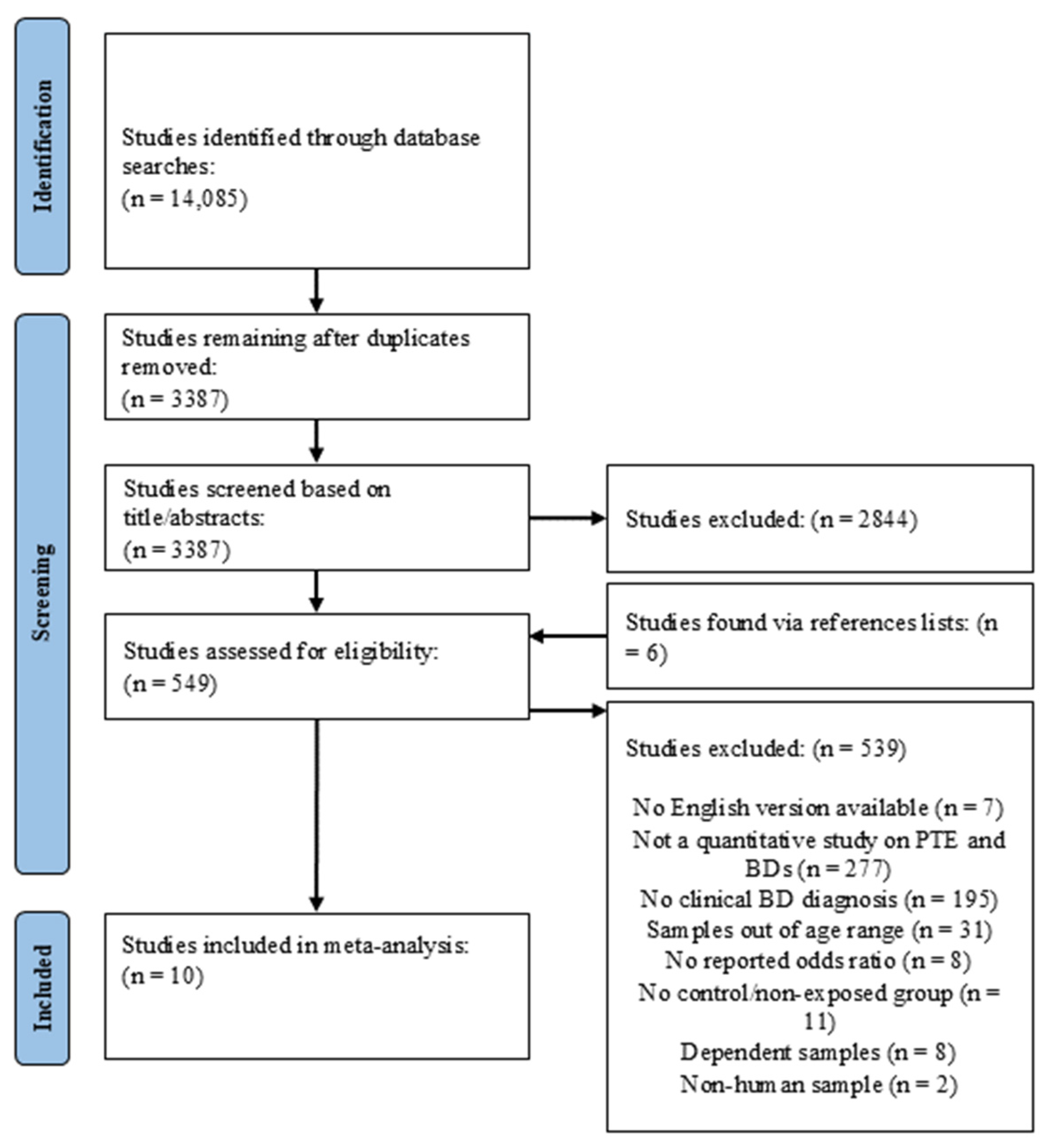
3.1.1. Search Outcomes
3.1.2. Study Inclusion Criteria
3.1.3. Studies Sharing Common Data
3.1.4. Risk of Bias Assessment
3.1.5. Coding Study Features
3.1.6. Prenatal Tobacco Exposure
3.1.7. Behavior Disorders
3.1.8. Effect Sizes
3.2. Effects of Prenatal Tobacco Exposure on BD Diagnoses
3.2.1. Effects of Prenatal Tobacco Exposure on ADHD Diagnosis
3.2.2. Effects of Prenatal Tobacco Exposure on ODD Diagnosis
3.2.3. Effects of Prenatal Tobacco Exposure on CD Diagnosis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Information for Health Care Providers and Public Health Professionals: Preventing Tobacco Use During Pregnancy; Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion: Atlanta, GA, USA, 2014. [Google Scholar]
- National Survey on Drug Use and Health. NSDUH Report: Substance Use During Pregnancy: 2002 and 2003 Update; Office of Applied Studies, Substance Abuse and Mental Health Services Administration (SAMHSA): Rockville, MD, USA, 2005. [Google Scholar]
- Centers for Disease Control and Prevention. QuickStats: Percentage of Births to Mothers Who Reported Smoking Cigarettes at Any Time During Pregnancy, by Urbanization Level of County of Residence—United States, 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 1652. [Google Scholar] [CrossRef]
- Banderali, G.; Martelli, A.; Landi, M.; Moretti, F.; Betti, F.; Radaelli, G.; Lassandro, C.; Verduci, E. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: A descriptive review. J. Transl. Med. 2015, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.D.; Kable, J.A.; Lynch, M.E. Examination of gender differences in effects of tobacco exposure. In Gender Differences in Prenatal Substance Exposure; Lewis, M., Kestler, L., Eds.; American Psychological Association: Washington, DC, USA, 2012; pp. 99–120. [Google Scholar]
- Cornelius, M.D.; Day, N.L. The effects of tobacco use during and after pregnancy on exposed children: Relevance of findings for alcohol research. Alcohol. Res. Health 2000, 24, 242–249. [Google Scholar] [PubMed]
- Cornelius, M.D.; Day, N.L. Developmental consequences of prenatal tobacco exposure. Curr. Opin. Neurol. 2009, 22, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, M.D.; Ryan, C.M.; Day, N.L.; Goldschmidt, L.; Willford, J.A. Prenatal tobacco effects on neuropsychological outcomes among preadolescents. J. Dev. Behav. Pediatr. 2001, 22, 217–225. [Google Scholar] [CrossRef]
- Day, N.L.; Richardson, G.A.; Goldschmidt, L.; Cornelius, M.D. Effects of prenatal tobacco exposure on preschoolers’ behavior. J. Dev. Behav. Pediatr. 2000, 21, 180–188. [Google Scholar] [PubMed]
- Wiebe, S.A.; Clark, C.A.C.; De Jong, D.M.; Chevalier, N.; Espy, K.A.; Wakschlag, L. Prenatal tobacco exposure and self-regulation in early childhood: Implications for developmental psychopathology. Dev. Psychopathol. 2015, 27, 397–409. [Google Scholar] [CrossRef]
- Eiden, R.D.; Perry, K.J.; Ivanova, M.Y.; Marcus, R.C. Prenatal Substance Exposure. Annu. Rev. Dev. Psychol. 2023, 5, 19–44. [Google Scholar] [CrossRef]
- Froggatt, S.; Covey, J.; Reissland, N. Infant neurobehavioural consequences of prenatal cigarette exposure: A systematic review and meta-analysis. Acta Paediatr. 2020, 109, 1112–1124. [Google Scholar] [CrossRef]
- Clark, C.A.; Massey, S.H.; Wiebe, S.A.; Espy, K.A.; Wakschlag, L.S. Does early maternal responsiveness buffer prenatal tobacco exposure effects on young children’s behavioral disinhibition? Dev. Psychopathol. 2019, 31, 1285–1298. [Google Scholar] [CrossRef]
- Espy, K.A.; Fang, H.; Johnson, C.; Stopp, C.; Wiebe, S.A.; Respass, J. Prenatal tobacco exposure: Developmental outcomes in the neonatal period. Dev. Psychol. 2011, 47, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Huizink, A.C.; Mulder, E.J. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci. Biobehav. Rev. 2006, 30, 24–41. [Google Scholar] [CrossRef]
- Kotimaa, A.J.; Moilanen, I.; Taanila, A.; Ebeling, H.; Smalley, S.L.; Mcgough, J.J.; Hartikainen, A.-L.; Järvelin, M.-R. Maternal smoking and hyperactivity in 8-year-old children. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Kahn, R.S.; Froehlich, T.; Auinger, P.; Lanphear, B.P. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ. Health Perspect. 2006, 114, 1904–1909. [Google Scholar] [CrossRef]
- Han, J.-Y.; Kwon, H.-J.; Ha, M.; Paik, K.-C.; Lim, M.-H.; Lee, S.G.; Yoo, S.-J.; Kim, E.-J. The effects of prenatal exposure to alcohol and environmental tobacco smoke on risk for ADHD: A large population-based study. Psychiatry Res. 2015, 225, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Dolan, C.V.; Geels, L.; Vink, J.M.; van Beijsterveldt, C.E.M.; Neale, M.C.; Bartels, M.; Boomsma, D.I. Testing causal effects of maternal smoking during pregnancy on offspring’s externalizing and internalizing behavior. Behav. Genet. 2016, 46, 378–388. [Google Scholar] [CrossRef]
- Ellis, L.C.; Berg-Nielsen, T.S.; Lydersen, S.; Wichstrøm, L. Smoking during pregnancy and psychiatric disorders in preschoolers. Eur. Child Adolesc. Psychiatry 2012, 21, 635–644. [Google Scholar] [CrossRef]
- Gaysina, D.; Fergusson, D.M.; Leve, L.D.; Horwood, J.; Reiss, D.; Shaw, D.S.; Elam, K.K.; Natsuaki, M.N.; Neiderhiser, J.M.; Harold, G.T. Maternal smoking during pregnancy and offspring conduct problems: Evidence from 3 independent genetically sensitive research designs. JAMA Psychiatry 2013, 70, 956–963. [Google Scholar] [CrossRef]
- Wakschlag, L.S.; Lahey, B.B.; Loeber, R.; Green, S.M.; Gordon, R.A.; Leventhal, B.L. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch. Gen. Psychiatry 1997, 54, 670–676. [Google Scholar] [CrossRef]
- Berchiatti, M.; Ferrer, A.; Badenes-Ribera, L.; Longobardi, C. School adjustments in children with attention deficit hyperactivity disorder (ADHD): Peer relationships, the quality of the student-teacher relationship, and children’s academic and behavioral competencies. J. Appl. Sch. Psychol. 2022, 38, 241–261. [Google Scholar] [CrossRef]
- Cook, C.R.; Williams, K.R.; Guerra, N.G.; Kim, T.E.; Sadek, S. Predictors of bullying and victimization in childhood and adolescence: A meta-analytic investigation. Sch. Psychol. Q. 2010, 25, 65–83. [Google Scholar] [CrossRef]
- Erskine, H.E.; Norman, R.E.; Ferrari, A.J.; Chan, G.C.; Copeland, W.E.; Whiteford, H.A.; Scott, J.G. Long-term outcomes of attention-deficit/hyperactivity disorder and conduct disorder: A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Boylan, K.; Vaillancourt, T.; Boyle, M.; Szatmari, P. Comorbidity of internalizing disorders in children with oppositional defiant disorder. Eur. Child Adolesc. Psychiatry 2007, 16, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Su, T.-P.; Chen, Y.-S.; Hsu, J.-W.; Huang, K.-L.; Chang, W.-H.; Chen, T.-J.; Bai, Y.-M. Higher risk of developing mood disorders among adolescents with comorbidity of attention deficit hyperactivity disorder and disruptive behavior disorder: A nationwide prospective study. J. Psychiatr. Res. 2013, 47, 1019–1023. [Google Scholar] [CrossRef]
- Christenson, J.D.; Crane, D.R.; Malloy, J.; Parker, S. The cost of oppositional defiant disorder and disruptive behavior: A review of the literature. J. Child Fam. Stud. 2016, 25, 2649–2658. [Google Scholar] [CrossRef]
- Matza, L.S.; Paramore, C.; Prasad, M. A review of the economic burden of ADHD. Cost Eff. Resour. Alloc. 2005, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.H.; Yeh, M.; Slymen, D. Strain in caring for youths meeting diagnosis for disruptive behavior disorders. J. Emot. Behav. Disord. 2015, 23, 40–51. [Google Scholar] [CrossRef]
- Kashdan, T.B.; Jacob, R.G.; Pelham, W.E.; Lang, A.R.; Hoza, B.; Blumenthal, J.D.; Gnagy, E.M. Depression and anxiety in parents of children with adhd and varying levels of oppositional defiant behaviors: Modeling relationships with family functioning. J. Clin. Child Adolesc. Psychol. 2004, 33, 169–181. [Google Scholar] [CrossRef]
- Foster, E.M.; Jones, D.E.; The Conduct Problems Prevention Research Group. The high costs of aggression: Public expenditures resulting from conduct disorder. Am. J. Public Health 2005, 95, 1767–1772. [Google Scholar] [CrossRef]
- Guevara, J.P.; Mandell, D.S.; Rostain, A.L.; Zhao, H.; Hadley, T.R. National estimates of health services expenditures for children with behavioral disorders: An analysis of the medical expenditure panel survey. Pediatrics 2003, 112, e440–e446. [Google Scholar] [CrossRef]
- Schein, J.; Adler, L.A.; Childress, A.; Cloutier, M.; Gagnon-Sanschagrin, P.; Davidson, M.; Kinkead, F.; Guerin, A.; Lefebvre, P. Economic burden of attention-deficit/hyperactivity disorder among children and adolescents in the United States: A societal perspective. J. Med. Econ. 2022, 25, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Pelham, W.E.; Foster, E.M.; Robb, J.A. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. J. Pediatr. Psychol. 2007, 32, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Erskine, H.E.; Ferrari, A.J.; Polanczyk, G.V.; Moffitt, T.E.; Murray, C.J.L.; Vos, T.; Whiteford, H.A.; Scott, J.G. The global burden of conduct disorder and attention-deficit/hyperactivity disorder in 2010. J. Child Psychol. Psychiatry 2014, 55, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Melchior, M.; Hersi, R.; van der Waerden, J.; Larroque, B.; Saurel-Cubizolles, M.-J.; Chollet, A.; Galéra, C. Maternal tobacco smoking in pregnancy and children’s socio-emotional development at age 5: The EDEN mother-child birth cohort study. Eur. Psychiatry 2015, 30, 562–568. [Google Scholar] [CrossRef]
- Wang, Y.; Buckingham-Howes, S.; Nair, P.; Zhu, S.; Magder, L.S.; Black, M.M. Prenatal drug exposure, behavioral problems, and drug experimentation among African-American urban adolescents. J. Adolesc. Health 2014, 55, 423–431. [Google Scholar] [CrossRef]
- Arruda, M.A.; Querido, C.N.; Bigal, M.E.; Polanczyk, G.V. ADHD and mental health status in Brazilian school-age children. J. Atten. Disord. 2015, 19, 11–17. [Google Scholar] [CrossRef]
- Ball, S.W.; Gilman, S.E.; Mick, E.; Fitzmaurice, G.; Ganz, M.L.; Seidman, L.J.; Buka, S.L. Revisiting the association between maternal smoking during pregnancy and ADHD. J. Psychiatr. Res. 2010, 44, 1058–1062. [Google Scholar] [CrossRef]
- Hill, S.Y.; Lowers, L.; Locke-Wellman, J.; Shen, S.A. Maternal smoking and drinking during pregnancy and the risk for child and adolescent psychiatric disorders. J. Stud. Alcohol 2000, 61, 661–668. [Google Scholar] [CrossRef]
- Thapar, A.; Fowler, T.; Rice, F.; Scourfield, J.; van den Bree, M.; Thomas, H.; Harold, G.; Hay, D. Maternal Smoking During Pregnancy and Attention Deficit Hyperactivity Disorder Symptoms in Offspring. Am. J. Psychiatry 2003, 160, 1985–1989. [Google Scholar] [CrossRef]
- E Gilman, S.; Hornig, M. Invited Commentary: The Disillusionment of Developmental Origins of Health and Disease (DOHaD) Epidemiology. Am. J. Epidemiol. 2020, 189, 1–5. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Üstün, B.; Kennedy, C. What is “functional impairment”? Disentangling disability from clinical significance. World Psychiatry 2009, 8, 82–85. [Google Scholar] [CrossRef]
- Sciberras, E.; Mulraney, M.; Silva, D.; Coghill, D. Prenatal risk factors and the etiology of ADHD—Review of existing evidence. Curr. Psychiatry Rep. 2017, 19, 1. [Google Scholar] [CrossRef]
- Haan, E.; Westmoreland, K.E.; Schellhas, L.; Sallis, H.M.; Taylor, G.; Zuccolo, L.; Munafò, M.R. Prenatal smoking, alcohol and caffeine exposure and offspring externalizing disorders: A systematic review and meta-analysis. Addiction 2022, 117, 2602–2613. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, J.; Zhu, L.-H.; Hua, L.-L.; Ke, F.-F. Maternal Smoking During Pregnancy and ADHD: Results From a Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Atten. Disord. 2020, 24, 1637–1647. [Google Scholar] [CrossRef]
- Dong, T.; Hu, W.; Zhou, X.; Lin, H.; Lan, L.; Hang, B.; Lv, W.; Geng, Q.; Xia, Y. Prenatal exposure to maternal smoking during pregnancy and attention-deficit/hyperactivity disorder in offspring: A meta-analysis. Reprod. Toxicol. 2018, 76, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, Y.; Zhang, L.; Zheng, Z.; Zhu, T.; Qu, Y.; Mu, D. Maternal Smoking and Attention-Deficit/Hyperactivity Disorder in Offspring: A Meta-analysis. Pediatrics 2018, 141, e20172465. [Google Scholar] [CrossRef]
- Chang, B.-H.S.; Hoaglin, D.C. Meta-Analysis of Odds Ratios: Current Good Practices. Med. Care 2017, 55, 328–335. [Google Scholar] [CrossRef]
- Egger, H.L.; Angold, A. Common emotional and behavioral disorders in preschool children: Presentation, nosology, and epidemiology. J. Child Psychol. Psychiatry 2006, 47, 313–337. [Google Scholar] [CrossRef] [PubMed]
- Keenan, K.; Boeldt, D.; Chen, D.; Coyne, C.; Donald, R.; Duax, J.; Hart, K.; Perrott, J.; Strickland, J.; Danis, B.; et al. Predictive validity of DSM-IV oppositional defiant and conduct disorders in clinically referred preschoolers. J. Child Psychol. Psychiatry 2011, 52, 47–55. [Google Scholar] [CrossRef]
- Cooper, H. Research Synthesis and Meta-Analysis: A Step-by-Step Approach, 4th ed.; Sage Publications, Inc.: Thousand Oaks, CA, USA, 2010. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Cochran, W.G. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 21 May 2023).
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Rothstein, H.R.; Sutton, A.J.; Borenstein, M. Publication bias in meta-analysis. In Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments; Rothstein, H.R., Sutton, A.J., Borenstein, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Egger, M. Regression methods to detect publication and other bias in meta-analysis. In Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustment; Rothstein, H.R., Sutton, A.J., Borenstein, M., Eds.; Wiley: Hoboken, NJ, USA, 2005; pp. 99–110. [Google Scholar]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic Reviews of Effectiveness. In JBI Manual for Evidence Synthesis; Aromataris, E., Lockwood, C., Porritt, K., Pilla, B., Jordan, Z., Eds.; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 23 January 2024).
- Schlack, R.; Göbel, K.; Hölling, H.; Petermann, F.; Romanos, M. Prädiktoren der Stabilität des Elternberichts über die ADHS-Lebenszeitprävalenz und Inzidenz der elternberichteten ADHS-Diagnose im Entwicklungsverlauf über sechs Jahre–Ergebnisse aus der KiGGS-Studie. Z. Psychiatr. Psychol. Psychother. 2018, 66, 233–247. [Google Scholar] [CrossRef]
- Schmitz, J.C.; Cholemkery, H.; Medda, J.; Freitag, C.M. Prä-und perinatale Risikofaktoren bei Autismus-Spektrum-Störung und Aktivitäts-und Aufmerksamkeitsstörung. Z. Kinder. Jugendpsychiatr. Psychother. 2017, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brinksma, D.M.; Hoekstra, P.J.; Hoofdakker, B.v.D.; de Bildt, A.; Buitelaar, J.K.; Hartman, C.A.; Dietrich, A. Age-dependent role of pre- and perinatal factors in interaction with genes on ADHD symptoms across adolescence. J. Psychiatr. Res. 2017, 90, 110–117. [Google Scholar] [CrossRef]
- Slotkin, T.A. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol. Teratol. 2008, 30, 1–19. [Google Scholar] [CrossRef]
- Zhu, J.L.; Olsen, J.; Liew, Z.; Li, J.; Niclasen, J.; Obel, C. Parental smoking during pregnancy and ADHD in children: The Danish national birth cohort. Pediatrics 2014, 134, e382–e388. [Google Scholar] [CrossRef]
- Mick, E.; Biederman, J.; Faraone, S.V.; Sayer, J.; Kleinman, S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Owens, E.B.; Hinshaw, S.P. Perinatal problems and psychiatric comorbidity among children with ADHD. J. Clin. Child Adolesc. Psychol. 2013, 42, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Ford, T.; Rosenberg, R.; Kelly, S. The association of attention deficit hyperactivity disorder with socioeconomic disadvantage: Alternative explanations and evidence. J. Child Psychol. Psychiatry 2014, 55, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Wakschlag, L.S.; Pickett, K.E.; Kasza, K.E.; Loeber, R. Is prenatal smoking associated with a developmental pattern of conduct problems in young boys? J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 461–467. [Google Scholar] [CrossRef]
- Chen, K.; Budman, C.L.; Herrera, L.D.; Witkin, J.E.; Weiss, N.T.; Lowe, T.L.; Freimer, N.B.; Reus, V.I.; Mathews, C.A. Prevalence and clinical correlates of explosive outbursts in Tourette Syndrome. Psychiatry Res. 2013, 205, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Roigé-Castellví, J.; Morales-Hidalgo, P.; Voltas, N.; Hernández-Martínez, C.; van Ginkel, G.; Canals, J. Prenatal and perinatal factors associated with ADHD risk in schoolchildren: EPINED epidemiological study. Eur. Child Adolesc. Psychiatry 2020, 30, 347–358. [Google Scholar] [CrossRef]
- Tatarka, M.E. Neurobehavioral Characteristics of Infants Exposed to Cocaine; University of Washington: Washington, DC, USA, 1997. [Google Scholar]
- Joelsson, P.; Chudal, R.; Talati, A.; Suominen, A.; Brown, A.S.; Sourander, A. Prenatal smoking exposure and neuropsychiatric comorbidity of ADHD: A finnish nationwide population-based cohort study. BMC Psychiatry 2016, 16, 306. [Google Scholar] [CrossRef]
- Brander, G.; Rydell, M.; Kuja-Halkola, R.; de la Cruz, L.F.; Lichtenstein, P.; Serlachius, E.; Rück, C.; Almqvist, C.; D’Onofrio, B.M.; Larsson, H.; et al. Perinatal risk factors in Tourette’s and chronic tic disorders: A total population sibling comparison study. Mol. Psychiatry 2018, 23, 1189–1197. [Google Scholar] [CrossRef]
- Cochran, D.M.; Jensen, E.T.; Frazier, J.A.; Jalnapurkar, I.; Kim, S.; Roell, K.R.; Joseph, R.M.; Hooper, S.R.; Santos, H.P.; Kuban, K.C.K.; et al. Association of prenatal modifiable risk factors with attention-deficit hyperactivity disorder outcomes at age 10 and 15 in an extremely low gestational age cohort. Front. Hum. Neurosci. 2022, 16, 911098. [Google Scholar] [CrossRef]
- Desrosiers, C.; Boucher, O.; Forget-Dubois, N.; Dewailly, É.; Ayotte, P.; Jacobson, S.W.; Jacobson, J.L.; Muckle, G. Associations between prenatal cigarette smoke exposure and externalized behaviors at school age among Inuit children exposed to environmental contaminants. Neurotoxicol. Teratol. 2013, 39, 84–90. [Google Scholar] [CrossRef]
- Freitag, C.M.; Hänig, S.; Schneider, A.; Seitz, C.; Palmason, H.; Retz, W.; Meyer, J. Biological and psychosocial environmental risk factors influence symptom severity and psychiatric comorbidity in children with ADHD. J. Neural Transm. 2012, 119, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Sciberras, E.; Ukoumunne, O.C.; Efron, D. Predictors of parent-reported attention-deficit/hyperactivity disorder in children aged 6–7 years: A national longitudinal study. J. Abnorm. Child Psychol. 2011, 39, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Langley, K.; Heron, J.; Smith, G.D.; Thapar, A. Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: Testing for intrauterine effects. Am. J. Epidemiol. 2012, 176, 261–268. [Google Scholar] [CrossRef]
- Palmer, R.H.C.; Bidwell, L.C.; Heath, A.C.; Brick, L.A.; Madden, P.A.F.; Knopik, V.S. Effects of maternal smoking during pregnancy on offspring externalizing problems: Contextual effects in a sample of female twins. Behav. Genet. 2016, 46, 403–415. [Google Scholar] [CrossRef]
- Madley-Dowd, P.; Kalkbrenner, A.E.; Heuvelman, H.; Heron, J.; Zammit, S.; Rai, D.; Schendel, D. Maternal smoking during pregnancy and offspring intellectual disability: Sibling analysis in an intergenerational Danish cohort. Psychol. Med. 2022, 52, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Nigg, J.T.; Breslau, N. Prenatal smoking exposure, low birth weight, and disruptive behavior disorders. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 362–369. [Google Scholar] [CrossRef]
- Sagiv, S.K.; Epstein, J.N.; Bellinger, D.C.; Korrick, S.A. Pre- and postnatal risk factors for ADHD in a nonclinical pediatric population. J. Atten. Disord. 2013, 17, 47–57. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q.; Gu, X.; Hou, Y.; Wang, M.; Chen, X.; Wu, J. LPHN3 gene variations and susceptibility to ADHD in Chinese Han population: A two-stage case–control association study and gene–environment interactions. Eur. Child Adolesc. Psychiatry 2019, 28, 861–873. [Google Scholar] [CrossRef]
- Arnold, L.E.; Elliott, M.; Lindsay, R.L.; Molina, B.; Cornelius, M.D.; Vitiello, B.; Hechtman, L.; Elliott, G.R.; Newcorn, J.; Epstein, J.N.; et al. Gestational and postnatal tobacco smoke exposure as predictor of ADHD, comorbid ODD/CD, and treatment response in the MTA. Clin. Neurosci. Res. 2005, 5, 295–306. [Google Scholar] [CrossRef]
- Kim, S.; Arora, M.; Fernandez, C.; Landero, J.; Caruso, J.; Chen, A. Lead, mercury, and cadmium exposure and attention deficit hyperactivity disorder in children. Environ. Res. 2013, 126, 105–110. [Google Scholar] [CrossRef]
- Lipińska, E.; Słopień, A.; Pytlińska, N.; Słopień, R.; Wolańczyk, T.; Bryńska, A. The role of factors associated with the course of pregnancy and childbirth in attention deficit hyperactivity disorder (ADHD). Psychiatr. Polska 2021, 55, 659–673. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, D.E.; Mulkins, R.S.; Dean, R.S. Utilization of maternal perinatal risk indicators in the differential diagnosis of ADHD and UADD children. Int. J. Neurosci. 1995, 81, 35–46. [Google Scholar] [CrossRef]
- Nordström, T.; Hurtig, T.; Rodriguez, A.; Savolainen, J.; Rautio, A.; Moilanen, I.; Taanila, A.; Ebeling, H. Different Risk Factors Between Disruptive Behavior Disorders and ADHD in Northern Finland Birth Cohort 1986. J. Atten. Disord. 2017, 21, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Chen, X.-T.; Yang, B.; Ma, F.-L.; Wang, S.; Tang, M.-L.; Hao, M.-G.; Ruan, D.-Y. Case–control study of blood lead levels and attention deficit hyperactivity disorder in Chinese children. Environ. Health Perspect. 2008, 116, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed.; Text Revision; American Psychiatric Association: Washington, DC, USA, 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Costello, A.; Edelbrock, C.; Kalas, R.; Kessler, R.; Klaric, S. The Diagnostic Interview Schedule for Children, Parent Version (Revised); University of Massachusetts Medical Center: Worcester, MA, USA, 1982. [Google Scholar]
- Shaffer, D.; Fisher, P.; Piacentini, J.; Schwab-Stone, M.; Wicks, J. Diagnostic Interview Schedule for Children; Second Revision; New York State Psychiatric Institute: New York, NY, USA, 1989. [Google Scholar]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Birmaher, B.; Brent, D.A.; Ryan, N.D.; Rao, U. K-SADS-PL. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 1208. [Google Scholar] [CrossRef]
- Swanson, J.M.; Kraemer, H.C.; Hinshaw, S.P.; Arnold, L.E.; Conners, C.K.; Abikoff, H.B.; Clevenger, W.; Davies, M.; Elliott, G.R.; Greenhill, L.L.; et al. Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 168–179. [Google Scholar] [CrossRef]
- Wilson, D.B. Practical Meta-Analysis Effect Size Calculator [Online Calculator]. Available online: https://campbellcollaboration.org/research-resources/effect-size-calculator.html (accessed on 1 May 2023).
- Latimer, K.; Wilson, P.; Kemp, J.; Thompson, L.; Sim, F.; Gillberg, C.; Puckering, C.; Minnis, H. Disruptive behaviour disorders: A systematic review of environmental antenatal and early years risk factors. Child Care Health Dev. 2012, 38, 611–628. [Google Scholar] [CrossRef]
- Tien, J.; Lewis, G.D.; Liu, J. Prenatal risk factors for internalizing and externalizing problems in childhood. World J. Pediatr. 2020, 16, 341–355. [Google Scholar] [CrossRef]
- Tiesler, C.M.T.; Heinrich, J. Prenatal nicotine exposure and child behavioural problems. Eur. Child Adolesc. Psychiatry 2014, 23, 913–929. [Google Scholar] [CrossRef]
- Estabrook, R.; Massey, S.H.; Clark, C.A.C.; Burns, J.L.; Mustanski, B.S.; Cook, E.H.; O’Brien, T.C.; Makowski, B.; Espy, K.A.; Wakschlag, L.S. Separating family-level and direct exposure effects of smoking during pregnancy on offspring externalizing symptoms: Bridging the behavior genetic and behavior teratologic divide. Behav. Genet. 2016, 46, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Minei, L.J.; Johnson, E. Effect of nicotine upon uterine blood flow in the pregnant rhesus monkey. Am. J. Obstet. Gynecol. 1980, 136, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, T.A. Fetal nicotine or cocaine exposure: Which one is worse? J. Pharmacol. Exp. Ther. 1998, 285, 931–945. [Google Scholar] [PubMed]
- Mamiya, N.; Buchanan, R.; Wallace, T.; Skinner, R.D.; Garcia-Rill, E. Nicotine suppresses the P13 auditory evoked potential by acting on the pedunculopontine nucleus in the rat. Exp. Brain Res. 2005, 164, 109–119. [Google Scholar] [CrossRef][Green Version]
- Law, K.L.; Stroud, L.R.; LaGasse, L.L.; Niaura, R.; Liu, J.; Lester, B.M. Smoking during pregnancy and newborn neurobehavior. Pediatrics 2003, 111, 1318–1323. [Google Scholar] [CrossRef]
- Stroud, L.R.; McCallum, M.; Salisbury, A.L. Impact of maternal prenatal smoking on fetal to infant neurobehavioral development. Dev. Psychopathol. 2018, 30, 1087–1105. [Google Scholar] [CrossRef]
- Shields, A.; Cicchetti, D. Reactive aggression among maltreated children: The Contributions of attention and emotion dysregulation. J. Clin. Child Psychol. 1998, 27, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Kuja-Halkola, R.; D’onofrio, B.M.; Larsson, H.; Lichtenstein, P. maternal smoking during pregnancy and adverse outcomes in offspring: Genetic and environmental sources of covariance. Behav. Genet. 2014, 44, 456–467. [Google Scholar] [CrossRef]
- D’Onofrio, B.M.; van Hulle, C.A.; Waldman, I.D.; Rodgers, J.L.; Harden, K.P.; Rathouz, P.J.; Lahey, B.B. Smoking during pregnancy and offspring externalizing problems: An exploration of genetic and environmental confounds. Dev. Psychopathol. 2008, 20, 139–164. [Google Scholar] [CrossRef]
- Eiden, R.D.; Schuetze, P.; Coles, C.D. Maternal cocaine use and mother–infant interactions: Direct and moderated associations. Neurotoxicol. Teratol. 2011, 33, 120–128. [Google Scholar] [CrossRef]
- Scott, T.J.L.; Heil, S.H.; Higgins, S.T.; Badger, G.J.; Bernstein, I.M. Depressive symptoms predict smoking status among pregnant women. Addict. Behav. 2009, 34, 705–708. [Google Scholar] [CrossRef]
- Brook, J.S.; Brook, D.W.; Whiteman, M. The Influence of Maternal Smoking During Pregnancy on the Toddler’s Negativity. Arch. Pediatr. Adolesc. Med. 2000, 154, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Tandon, M.; Si, X.; Belden, A.; Spitznagel, E.; Wakschlag, L.S.; Luby, J. Parenting practices in pregnancy smokers compared to non smokers. J. Clin. Med. Res. 2013, 5, 84–91. [Google Scholar] [CrossRef]
- Fergusson, D.M.; Woodward, L.J.; Horwood, L.J. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch. Gen. Psychiatry 1998, 55, 721–727. [Google Scholar] [CrossRef]
- Schuetze, P.; Eiden, R.D.; Dombkowski, L. The association between cigarette smoking during pregnancy and maternal behavior during the neonatal period. Infancy 2006, 10, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Pickett, K.E.; Wakschlag, L.S.; Dai, L.; Leventhal, B.L. Fluctuations of maternal smoking during pregnancy. Obstet. Gynecol. 2003, 101, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Massey, S.H.; Clark, C.A.; Sun, M.Y.; Burns, J.L.; Mroczek, D.K.; Espy, K.A.; Wakschlag, L.S. Dimension- and context-specific expression of preschoolers’ disruptive behaviors associated with prenatal tobacco exposure. Neurotoxicol. Teratol. 2020, 81, 106915. [Google Scholar] [CrossRef]
- El Marroun, H.; Bolhuis, K.; A Franken, I.H.; Jaddoe, V.W.V.; Hillegers, M.H.; Lahey, B.B.; Tiemeier, H. Preconception and prenatal cannabis use and the risk of behavioural and emotional problems in the offspring; a multi-informant prospective longitudinal study. Leuk. Res. 2019, 48, 287–296. [Google Scholar] [CrossRef]
- Oga, E.A.; Mark, K.; Coleman-Cowger, V.H. Cigarette Smoking Status and Substance Use in Pregnancy. Matern. Child Health J. 2018, 22, 1477–1483. [Google Scholar] [CrossRef]

| Assessment Domain | Groups Similar and from Same Population | Tobacco Exposure Measured Similarly | Tobacco Exposure Measure Valid and Reliable | Confounding Factors Identified | Strategies to Deal with Confounding Factors | Groups Free of BD at the Time of Exposure | BD Measure Valid and Reliable | Follow Up Time Reported and Sufficient | Follow Up Complete and Reasons for Loss to Follow Up Described/Explored | Strategies to Address Incomplete Follow Up Utilized | Appropriate Statistical Analysis Used |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arnold et al. (2005) [89] | + | + | − | + | + | + | + | + | − | − | + |
| Arruda et al. (2015) [39] | Unclear | + | − | + | Unclear | + | + | + | Unclear | − | + |
| Huang et al. (2019) [88] | − | + | − | + | + | + | − | + | + | N/A | + |
| Kim et al. (2013) [90] | − | + | − | + | + | + | − | + | + | N/A | + |
| Lipinska et al. (2021) [91] | − | + | − | + | − | + | + | + | Unclear | − | + |
| McIntosh et al. (1995) [92] | Unclear | + | − | + | + | + | + | + | + | N/A | + |
| Nigg and Breslau (2007) [86] | Unclear | + | − | + | + | + | + | + | Unclear | + | + |
| Nordström et al. (2017) [93] | Unclear | + | − | + | + | + | + | + | − | − | + |
| Sagiv et al. (2013) [87] | − | + | − | + | + | + | − | + | − | − | + |
| Wang et al. (2008) [94] | − | + | − | + | + | + | + | + | + | N/A | + |
| Study | Site | Number Exposed/Total Participants | Age (Years) | Assessment of Prenatal Smoking | BD Diagnosis Outcome | Assessment of BD |
|---|---|---|---|---|---|---|
| Arnold et al. (2005) [89] | United States and Canada | 163/714 | 7–9.9 | Baseline assessment asked mothers about smoking in gestational period (yes/no) | ADHD; 215/468 with ADHD had comorbid ODD/CD | Diagnostic Interview Schedule for Children (DISC-IV) |
| Arruda et al. (2015) [39] | Brazil | 495/1830 | 5–13 | Parents completed a study questionnaire | ADHD | Parents and/or caregivers completed the MTA-SNAP-IV scale |
| Huang et al. (2019) [88] | China | Discovery Sample: 56/1058 Stage One Sample: 26/674 | 6–18 | Maternal questionnaire covering environmental risk factors including prenatal smoking (yes/no) | ADHD | Diagnosis of ADHD by psychiatrists using DSM-IV diagnostic criteria |
| Kim et al. (2013) [90] | United States | 39/129 | 5–12 | Parents completed a questionnaire about prenatal smoke exposure (yes/no) and other covariates | ADHD | A previous diagnosis by a physician based on DSM-IV diagnostic criteria |
| Lipinska et al. (2021) [91] | Poland | 57/282 | 7–17 | Mothers completed a pregnancy and perinatal history questionnaire, including prenatal smoking (yes/no) | ADHD | Diagnosis of ADHD using DSM-IV-TR or ICD-10 criteria, verified with a structured history questionnaire |
| McIntosh et al. (1995) [92] | United States | 41/265 | 6–13 | Mothers reported on smoking during pregnancy with the Maternal Perinatal Scale | ADHD | Diagnosis by physicians and psychologists of ADHD using DSM-III criteria, verified with school health and testing records |
| Nigg and Breslau (2007) [86] | United States | 247/600 with prenatal exposure vs. never exposed | 6–17 | Mothers reported on their daily smoking habits during pregnancy during an interview (yes/no) | ADHD age 6; ODD age 6; ADHD age 11; ODD age 11; ODD age 17; CD age 17 | Diagnostic Interview Schedule for Children (DISC; version 2.1) and Diagnostic Interview Schedule for Youth (DIS-Y) |
| Nordström et al. (2017) [93] | Finland | 97/316 with ADHD diagnosis data | 15–16 | Information was systematically collected at antenatal clinics and birth hospital via self-report questionnaires and the delivery records | ADHD and/or DBD | At 15–16, Schedule for Affective Disorders and Schizophrenia for School-age Children—present and lifetime version (K-SADS-PL) |
| Sagiv et al. (2013) [87] | United States | 166/560 with smoking data | 8 | Mothers reported on their prenatal smoking approximately two weeks after birth on a questionnaire | ADHD | Pediatric records were reviewed at the time of assessment for ADHD diagnoses |
| Wang et al. (2008) [94] | China | 15/1260 | 4–12 | Covariates and confounder assessed via clinical records or questionnaires completed by interviewing parents | ADHD | Schedule for Affective Disorders and Schizophrenia for School-age Children (K-SADS-E) modified to assess DSM-IV-R criteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godleski, S.; Shisler, S.; Colton, K.; Leising, M. Prenatal Tobacco Exposure and Behavioral Disorders in Children and Adolescents: Systematic Review and Meta-Analysis. Pediatr. Rep. 2024, 16, 736-752. https://doi.org/10.3390/pediatric16030062
Godleski S, Shisler S, Colton K, Leising M. Prenatal Tobacco Exposure and Behavioral Disorders in Children and Adolescents: Systematic Review and Meta-Analysis. Pediatric Reports. 2024; 16(3):736-752. https://doi.org/10.3390/pediatric16030062
Chicago/Turabian StyleGodleski, Stephanie, Shannon Shisler, Kassidy Colton, and Meghan Leising. 2024. "Prenatal Tobacco Exposure and Behavioral Disorders in Children and Adolescents: Systematic Review and Meta-Analysis" Pediatric Reports 16, no. 3: 736-752. https://doi.org/10.3390/pediatric16030062
APA StyleGodleski, S., Shisler, S., Colton, K., & Leising, M. (2024). Prenatal Tobacco Exposure and Behavioral Disorders in Children and Adolescents: Systematic Review and Meta-Analysis. Pediatric Reports, 16(3), 736-752. https://doi.org/10.3390/pediatric16030062






