Seasonal Variation of Leaf Ca, Fe, and Mn Concentration in Six Olive Varieties
Abstract
:1. Introduction
2. Materials and Methods
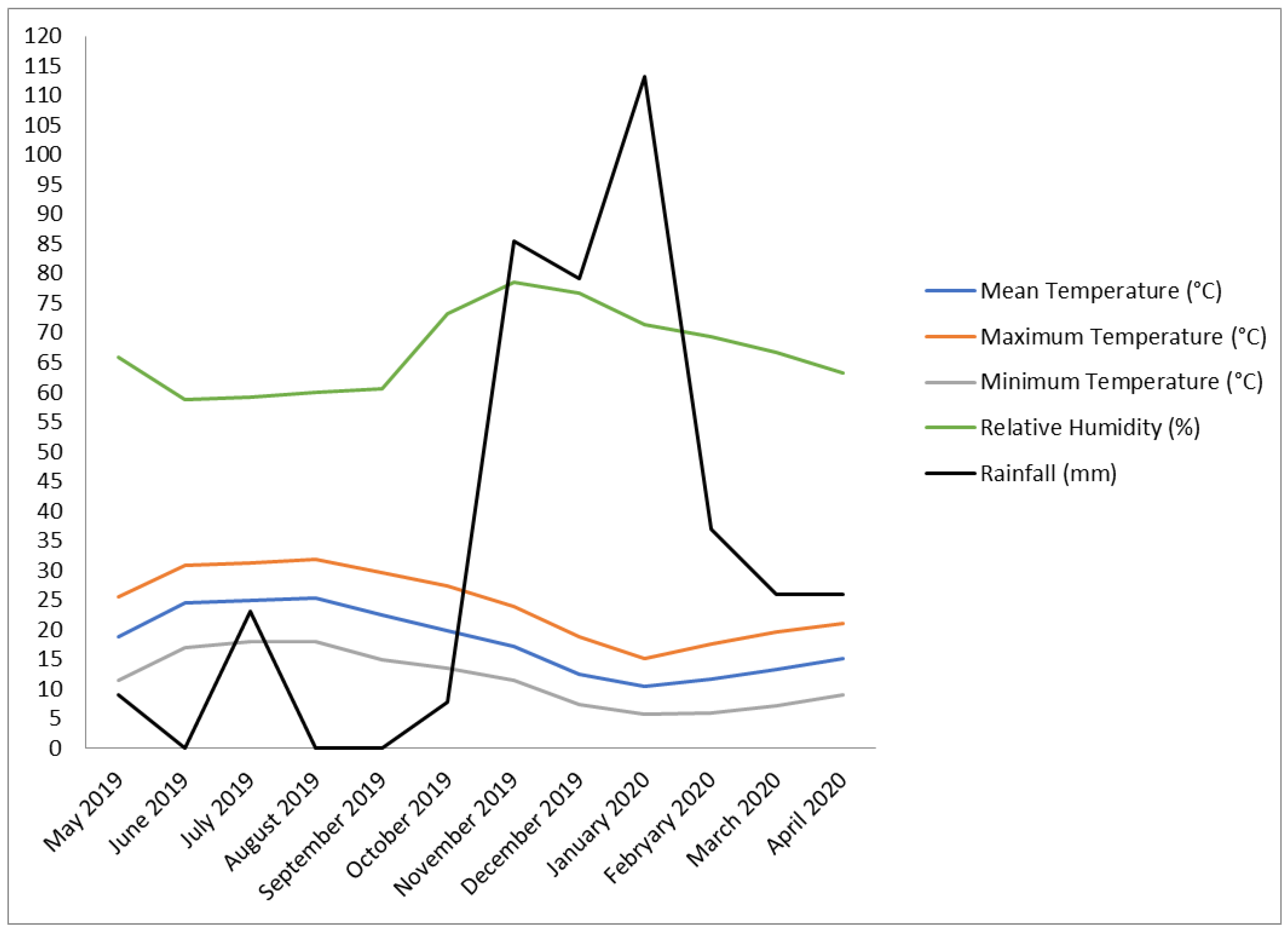
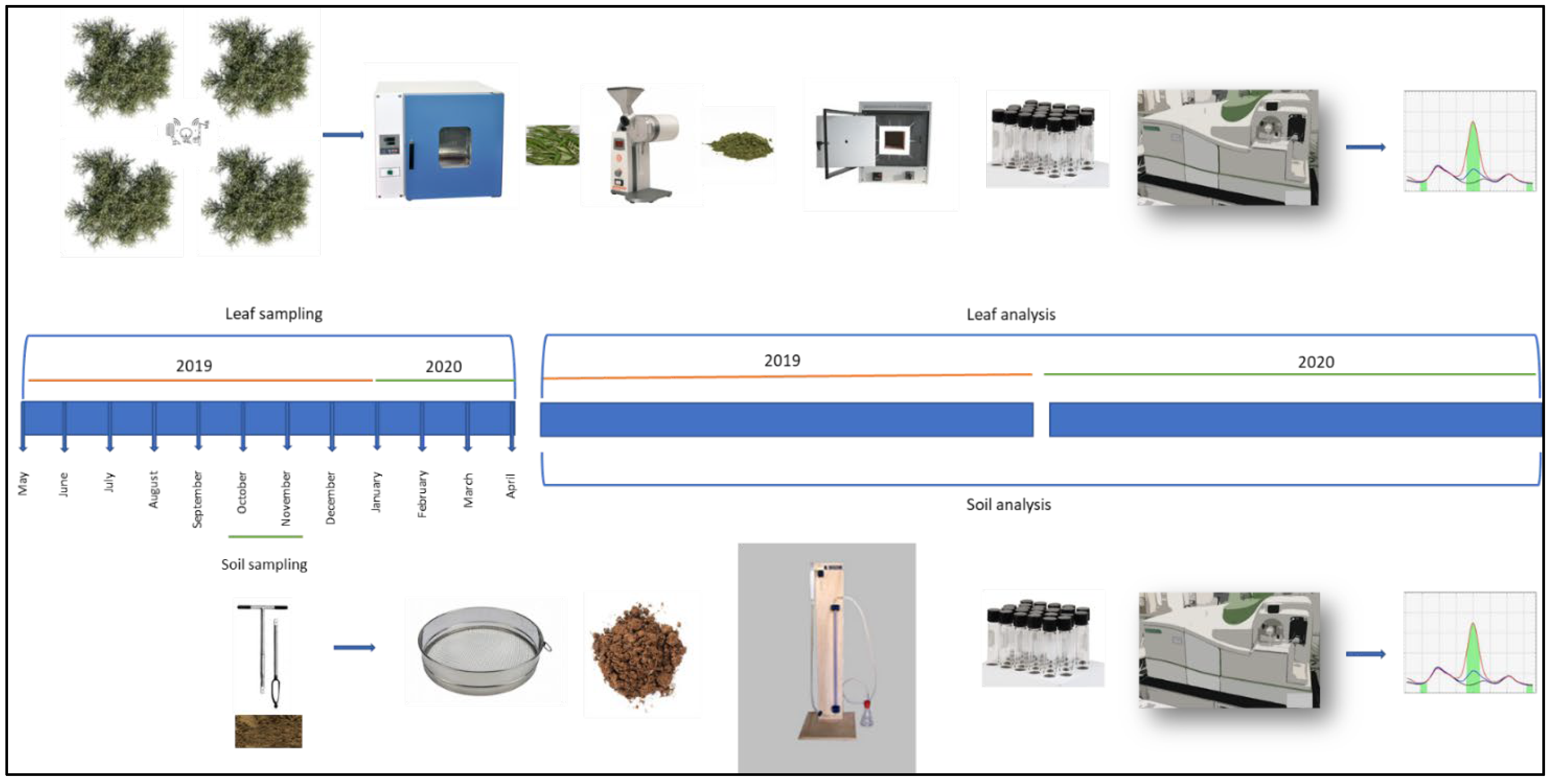
2.1. Experimental Site and Plant Material
2.2. Soil Physicochemical Properties
2.3. Leaf Mineral Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Genotypic and Seasonal Effect on Leaf Ca Concentration
3.2. Genotypic and Seasonal Effect on Leaf Fe Concentration
3.3. Genotypic and Seasonal Effect on Leaf Mn Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rallo, L.; Caruzo, T.; Diez, C.M.; Campisi, G. Olive growing in a time of change: From empiricism to genomics. In The Olive Tree Genome; Rugini, L.B., Muleo, R., Sebastiani, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 55–64. [Google Scholar]
- International Olive Council. World Olive Figures. Madrid, Spain. 2018. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/ (accessed on 18 March 2022).
- International Olive Council. World Olive Figures. Madrid, Spain. 2016. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/ (accessed on 18 March 2022).
- Zipori, I.; Erel, R.; Yermiyahu, U.; Ben-Gal, A.; Dag, A. Sustainable Management of Olive Orchard Nutrition: A Review. Agriculture 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Escobar, R.; Sánchez-Zamora, M.A.; García-Novelo, J.M.; Molina-Soria, C. Nutrient removal from olive trees by fruit yield and pruning. Hort. Sci. 2015, 50, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Escobar, R. Olive Nutritional Status and Tolerance to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1151. [Google Scholar] [CrossRef]
- Sanz-Cortés, F.; Martínez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llácer, G.; Meier, U. Phenological growth stages of olives trees (Olea europaea). Ann. App. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- Stateras, D.C.; Moustakas, N.K. Seasonal changes of macro- and micro-nutrients concentration in olive leaves. J. Plant Nutr. 2018, 41, 186–196. [Google Scholar] [CrossRef]
- Tekaya, M.; Dahmen, S.; Mansour, M.B.; Ferhout, H.; Chehab, H.; Hammami, M.; Attia, F.; Mechri, B. Foliar application of fertilizers and biostimulant has a strong impact on the olive (Olea europaea) rhizosphere microbial community profile and the abundance of arbuscular mycorrhizal fungi. Rhizosphere 2021, 19, 100402. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Tzanakakis, V.A.; Papaioannou, A.; Giannakoula, A. Comparative Study between Urea and Biogas Digestate Application towards Enhancing Sustainable Fertilization Management in Olive (Olea europaea L., cv. ‘Koroneiki’) Plants. Sustainability 2022, 14, 4785. [Google Scholar] [CrossRef]
- Chatzistathis, T.A.; Papadakis, I.E.; Therios, I.N.; Giannakoula, A.; Dimassi, K. Is chlorophyll fluorescence technique a useful tool to assess manganese deficiency and toxicity stress in olive plants? J. Plant Nutr. 2010, 34, 98–114. [Google Scholar] [CrossRef]
- Chouliaras, V.; Tasioula, M.; Chatzissavvidis, C.; Therios, I.; Tsabolatidou, E. The effects of a seaweed extract in addition to nitrogen and boron fertilization on productivity, fruit maturation, leaf nutritional status and oil quality of the olive (Olea europaea L.) cultivar Koroneiki. J. Sci. Food Agric. 2009, 89, 984–988. [Google Scholar] [CrossRef]
- Chatzissavvidis, C.A.; Therios, I.N.; Molassiotis, A.N. Seasonal Variation of Nutritional Status of Olive Plants as Affected by Boron Concentration in Nutrient Solution. J. Plant Nutr. 2005, 28, 309–321. [Google Scholar] [CrossRef]
- Barouchas, P.E.; Vatista, P.; Kalantzis, E.; Moustakas, N.K. Seasonal changes of macronutrients concentration in olive trees grown in acid and in alkaline soils. Notulae Botanicae Horti Agrobotanici 2021, 49, 12498. [Google Scholar] [CrossRef]
- Martínez-Navarro, M.E.; Cebrián-Tarancón, C.; Alonso, G.L.; Salinas, M.R. Determination of the Variability of Bioactive Compounds and Minerals in Olive Leaf along an Agronomic Cycle. Agronomy 2021, 11, 2447. [Google Scholar] [CrossRef]
- Pasković, I.; Lukić, I.; Žurga, P.; Germek, V.M.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D.; et al. Temporal Variation of Phenolic and Mineral Composition in Olive Leaves Is Cultivar Dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Melgar, J.C.; Mohamed, Y.; Navarro, C.; Parra, M.A.; Benlloch, M.; Fernández-Escobar, R. Long-term growth and yield responses of olive trees to different irrigation regimes. Agric. Water Manag. 2008, 95, 968–972. [Google Scholar] [CrossRef]
- International Olive Council. Production techniques in olive growing. Madrid, Spain. 2017. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/12/Olivicultura_eng.pdf (accessed on 18 March 2022).
- Fernández-Escobar, R.; Guerreiro, M.; Benlloch, M.; Benlloch-González, M. Symptoms of nutrient deficiencies in young olive trees and leaf nutrient concentration at which such symptoms appear. Sci. Hortic. 2016, 209, 279–285. [Google Scholar] [CrossRef]
- Bechara, E.; Papafilippaki, A.; Doupis, G.; Sofo, A.; Koubouris, G. Nutrient dynamics, soil properties and microbiological aspects in an irrigated olive orchard managed with five different management systems involving soil tillage, cover crops and compost. J. Water Clim. Change 2018, 9, 736–747. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Antonaya-Baena, F.; Almeida-Lavado, S. Nitrogen Uptake Efficiency of Olive Cultivars. Horticulturae 2021, 7, 136. [Google Scholar] [CrossRef]
- Haberman, A.; Dag, A.; Erel, R.; Zipori, I.; Shtern, N.; Ben-Gal, A.; Yermiyahu, U. Long-Term Impact of Phosphorous Fertilization on Yield and Alternate Bearing in Intensive Irrigated Olive Cultivation. Plants 2021, 10, 1821. [Google Scholar] [CrossRef]
- Haberman, A.; Dag, A.; Shtern, N.; Zipori, I.; Erel, R.; Ben-Gal, A.; Yermiyahu, U. Long-Term Impact of Potassium Fertilization on Soil and Productivity in Intensive Olive Cultivation. Agronomy 2019, 9, 525. [Google Scholar] [CrossRef] [Green Version]
- Coco, L.D.; Migoni, D.; Girelli, C.R.; Angilè, F.; Scortichini, M.; Fanizzi, F.P. Soil and Leaf Ionome Heterogeneity in Xylella fastidiosa Subsp. Pauca-Infected, Non-Infected and Treated Olive Groves in Apulia, Italy. Plants 2020, 9, 760. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Therios, I.; Alifragis, D.; Dimassi, K. Effect of sampling time and soil type on Mn, Fe, Zn, Ca, Mg, K and P concentrations of olive (Olea europaea L., cv. ‘Koroneiki’) leaves. Sci. Hortic. 2010, 126, 291–296. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Therios, I.; Alifragis, D. Differential Uptake, Distribution within Tissues, and Use Efficiency of Manganese, Iron, and Zinc by Olive Cultivars Kothreiki and Koroneiki. Hort. Sci. 2009, 44, 1994–1999. [Google Scholar] [CrossRef] [Green Version]



| F Value | ||||
|---|---|---|---|---|
| Source 1 | df | Ca (%) | Fe (ppm) | Mn (ppm) |
| Variety | 5 | 79.966 *** | 12.667 *** | 90.200 *** |
| Time | 11 | 50.641 *** | 11.344 *** | 20.554 *** |
| Variety × Time | 55 | 2.743 *** | 1.286 ns | 1.717 ** |
| Mean values ± SE 2 | ||||
| Main factor | Ca (%) | Fe (ppm) | Mn (ppm) | |
| Variety | Amfissis | 1.11 ± 0.06 c | 62.9 ± 3.9 a | 30.6 ± 1.6 b |
| Chondrolia Chalkidikis | 0.94 ± 0.03 b | 67.5 ± 2.7 a | 35.8 ± 1.5 c | |
| Kalamon | 0.88 ± 0.04 ab | 63.4 ± 3.1 a | 47.8 ± 3.1 d | |
| Koroneiki | 1.38 ± 0.07 e | 68.9 ± 3.9 a | 21.5 ± 0.9 a | |
| Mastoidis | 0.81 ± 0.02 a | 95.9 ± 8.0 b | 16.9 ± 0.6 a | |
| Picual | 1.24 ± 0.05 d | 70.8 ± 2.3 a | 28.9 ± 1.5 b | |
| Time | May 2019 | 0.62 ± 0.06 a | 87.6 ± 2.9 c | 20.2 ± 1.7 ab |
| June 2019 | 0.64 ± 0.02 a | 53.0 ± 3.1 a | 17.5 ± 1.1 a | |
| July 2019 | 0.89 ± 0.04 b | 78.0 ± 4.5 bc | 25.6 ± 1.6 b–d | |
| August 2019 | 1.08 ± 0.05 cd | 78.0 ± 7.9 bc | 30.8 ± 2.8 c–e | |
| September 2019 | 1.12 ± 0.07 cd | 64.7 ± 4.4 a–c | 31.9 ± 3.3 de | |
| October 2019 | 0.96 ± 0.06 bc | 58.5 ± 3.9 ab | 24.1 ± 2.0 a–c | |
| November 2019 | 1.11 ± 0.07 cd | 69.1 ± 4.9 a–c | 33.2 ± 3.6 de | |
| December 2019 | 1.13 ± 0.06 d | 59.0 ± 3.4 ab | 32.8 ± 3.1 de | |
| January 2020 | 1.22 ± 0.08 d | 66.0 ± 2.7 a–c | 34.4 ± 3.2 e | |
| February 2020 | 1.20 ± 0.07 d | 66.7 ± 3.9 a–c | 34.0 ± 3.7 e | |
| March 2020 | 1.19 ± 0.06 d | 63.2 ± 4.6 ab | 34.0 ± 3.0 e | |
| April 2020 | 1.51 ± 0.09 e | 114.2 ± 13.4 d | 44.7 ± 4.8 f | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolikaki, I.; Digalaki, N.; Psarras, G.; Tzerakis, C.; Sergentani, C.; Papamanolioudaki, A.; Tul, S.; Koubouris, G. Seasonal Variation of Leaf Ca, Fe, and Mn Concentration in Six Olive Varieties. Int. J. Plant Biol. 2022, 13, 95-105. https://doi.org/10.3390/ijpb13020010
Manolikaki I, Digalaki N, Psarras G, Tzerakis C, Sergentani C, Papamanolioudaki A, Tul S, Koubouris G. Seasonal Variation of Leaf Ca, Fe, and Mn Concentration in Six Olive Varieties. International Journal of Plant Biology. 2022; 13(2):95-105. https://doi.org/10.3390/ijpb13020010
Chicago/Turabian StyleManolikaki, Ioanna, Nektaria Digalaki, Georgios Psarras, Constantinos Tzerakis, Chrysi Sergentani, Anastasia Papamanolioudaki, Safiye Tul, and Georgios Koubouris. 2022. "Seasonal Variation of Leaf Ca, Fe, and Mn Concentration in Six Olive Varieties" International Journal of Plant Biology 13, no. 2: 95-105. https://doi.org/10.3390/ijpb13020010
APA StyleManolikaki, I., Digalaki, N., Psarras, G., Tzerakis, C., Sergentani, C., Papamanolioudaki, A., Tul, S., & Koubouris, G. (2022). Seasonal Variation of Leaf Ca, Fe, and Mn Concentration in Six Olive Varieties. International Journal of Plant Biology, 13(2), 95-105. https://doi.org/10.3390/ijpb13020010







