Effect of Different Cytokinins on Shoot Outgrowth and Bioactive Compounds Profile of Lemograss Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Germination
2.2. In Vitro Shoot Culture Establishment and Propagation Protocol
2.3. Effect of Sucrose and Gelrite™ Concentrations on Shoot Propagation
2.4. Effect of Culture Media and Cytokinin Type and Concentration on Shoot Propagation
2.5. Experimental Design
2.6. Root Induction
2.7. Acclimatization of In Vitro Rooted Shoots
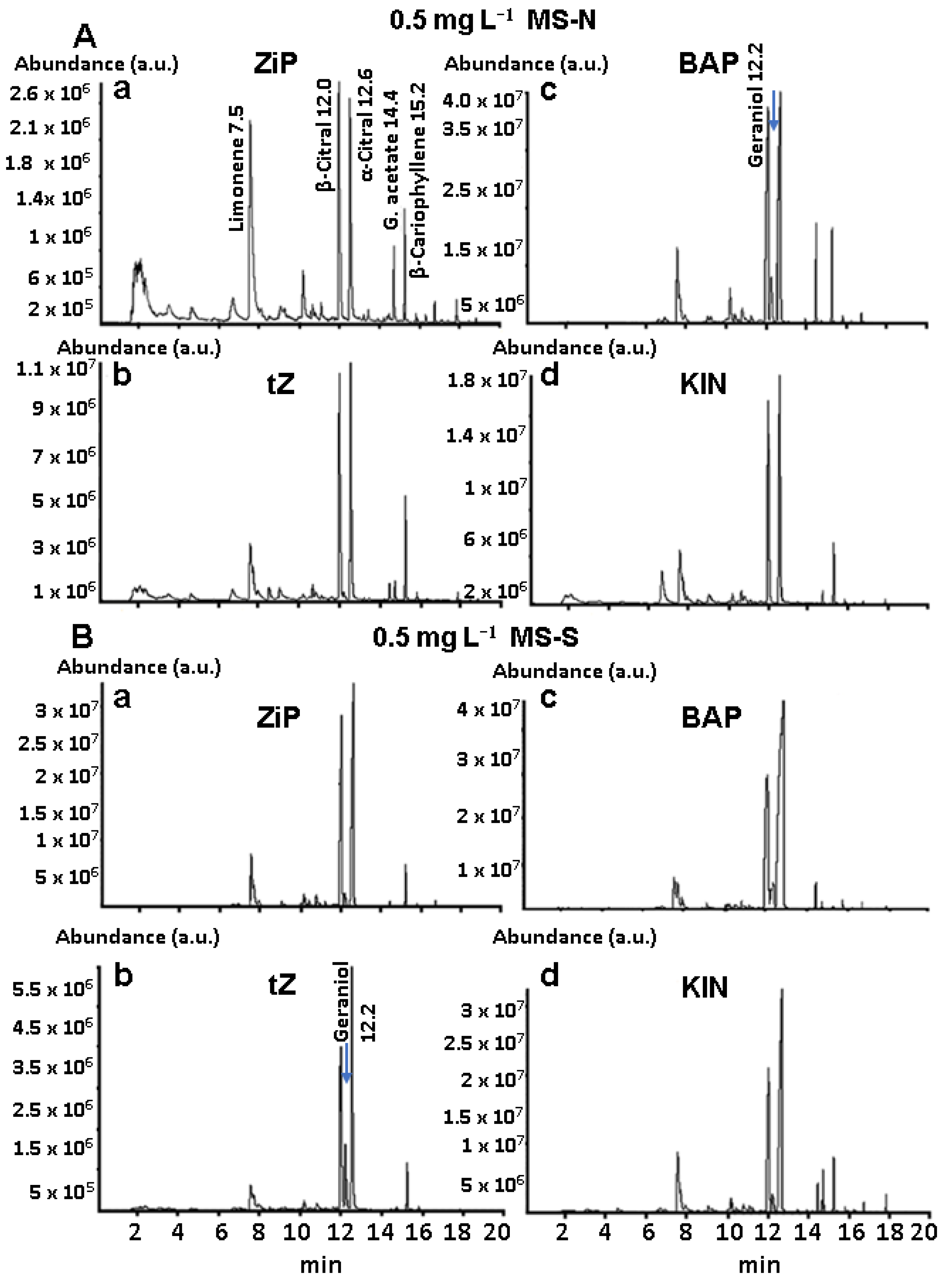
2.8. SPME/Gas Chromatography-Mass Spectrometry Analysis
3. Results
3.1. Effect of Sucrose and Gelrite™ Concentrantions on Shoot Development
3.2. Effect of Cytokinin Type and Concentration on Shoot Propagation and Shoot Morphology
3.3. Direct Root Formation Was Induced in Lemongrass Shoots during Clonal Propagation
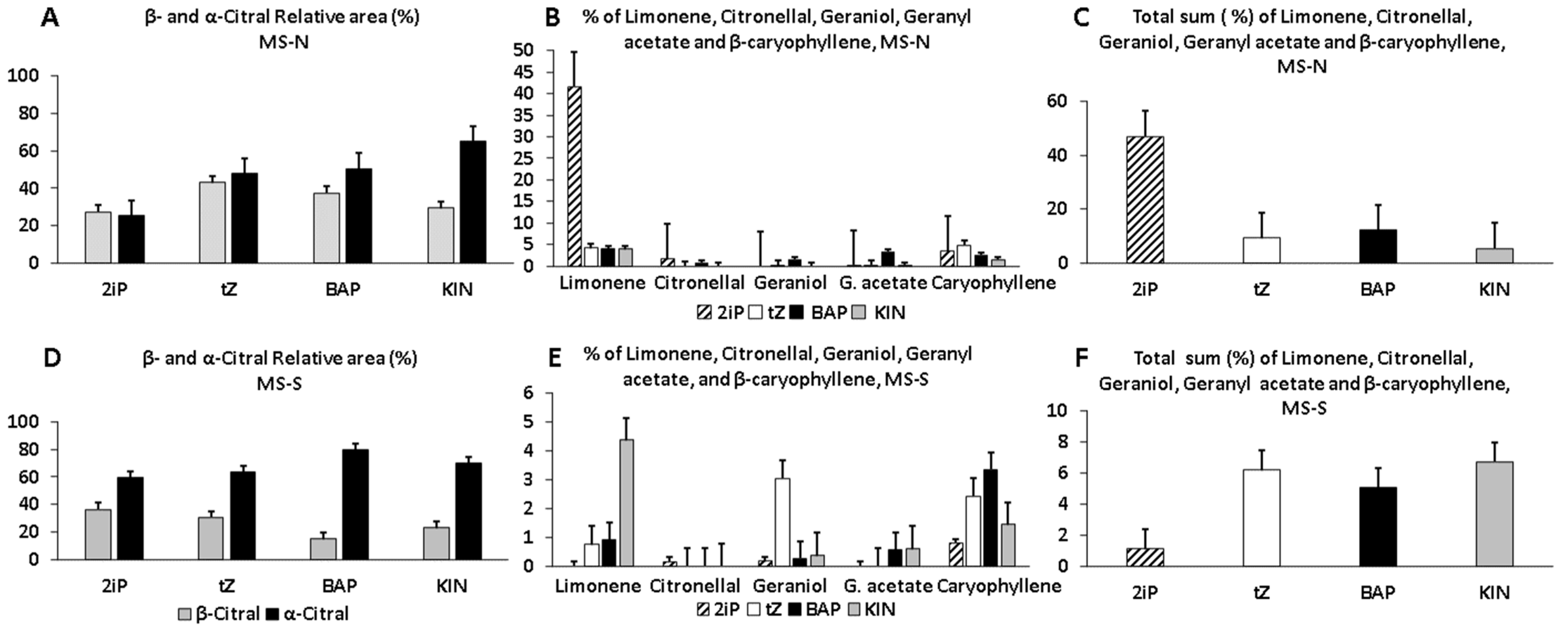
3.4. Main Constituents of BC Profiles of Plants in Pots
3.5. BC Profiles of Lyophilized Leaves of Established Plants in Pots
3.6. Quantitative Analysis of the Main Constituents of BC Profiles of Established Plants in Pots
4. Discussion
4.1. CK Treatments Affected Shoot Number and Shoot Branching Morphology
4.2. Direct Root Formation and Development Are not Related to Essential Oil Production
4.3. Water Stress Medium (MS-S) Altered the Number and Morphology of In Vitro Shoots
4.4. CK Treatments Caused Qualitative and Quantitative Changes in BC Profiles
4.5. Variations in BC Profiles Related to Shoot Number and Shoot Branching Morphology Could Be Explained Because Plants Sense Distinct CK Types Differently
4.6. BC of Lemongrass Might Regulate Axillary Meristem Growth and Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganjewala, D.; Luthra, R. Essential Oil Biosynthesis and Regulation in the Genus Cymbopogon. Nat. Prod. Commun. 2010, 5, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ganjewala, D. Cymbopogon essential oils: Chemical compositions and bioactivities. Int. J. Essent. Oil Ther. 2009, 3, 56–65. [Google Scholar]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass essential oil components with antimicrobial and anticancer activities. Antioxidants 2022, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K. Anticancer effect of lemongrass oil and citral on cervical cancer cell lines. Pharmacogn. Commun. 2013, 3, 41–48. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Ramani, S.; Natarajan, S.; Kim, Y.J.; Perumalsamy, H. Integrated transcriptome and in vitro analysis revealed anti-proliferative effect of citral in human stomach cancer through apoptosis. Sci. Rep. 2019, 9, 4883. [Google Scholar] [CrossRef] [PubMed]
- Maczka, W.; Winska, K.; Grabarczyk, M. One hundred faces of geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef]
- Gonçalves, M.J.; Cruz, M.T.; Tavares, A.C.; Cavaleiro, C.; Lopes, M.C.; Canhoto, J. LígiaSalgueiro Composition and biological activity of the essential oil from Thapsia minor, a new source of geranyl acetate. Ind. Crops Prod. 2012, 35, 166–171. [Google Scholar] [CrossRef]
- Teixeira, R.R.; Da Silva, A.M.; Siqueira, R.P.; Gonçalves, V.H.S.; Pereira, H.S.; Ferreira, R.S.; Costa, A.V.; de Melo, E.B.; Paula, F.R.; Ferreira, M.M.C. Synthesis of nerol derivatives containing a 1, 2, 3-triazole moiety and evaluation of their activities against cancer cell lines. J. Braz. Chem. Soc. 2019, 30, 541–561. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; Torre, C.L.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-caryophyllene: A sesquiterpene with countless biological properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Motolinía-Alcántara, E.A.; Castillo-Araiza, C.O.; Rodríguez-Monroy, M.; Román-Guerrero, A.; Cruz-sosa, F. Engineering considerations to produce bioactive compounds from plant cell suspension culture in bioreactors. Plants 2021, 10, 2762. [Google Scholar] [CrossRef] [PubMed]
- Koshima, F.A.T.; Ming, L.C.; Marques, M.O.M. Cymbopogon citratus (DC.) Stapf, com cobertura morta nas estações do ano. Rev. Bras. PI Med. 2006, 8, 112–116. [Google Scholar]
- Khan, Z.H.; Mohammad, F.; Khan, M.M.A. Enhancing the growth, yield and production of essential oil and citral in lemongrass by the application of Triacontanol. Int. J. Agric. Sci. Res. 2014, 4, 113–122. [Google Scholar]
- Prins, C.L.; Freitas, S.P.; Gomes, M.M.A.; Vieira, I.J.C.; Gravina, G.A. Citral accumulation in Cymbopogon citratus plant as influenced by N6-benzylaminopurine and light intensity. Theor. Exp. Plant Physiol. 2013, 25, 159–165. [Google Scholar] [CrossRef]
- Dutta, S.; Munda, S.; Chikkaputtaiah, C.; Lal, M. Assessment of selection criteria for development of high yielding genoptypes using variability parameters in Lemongrass Cymbopogon flexuosus L. J. Essent. Oil-Bearing Plants 2017, 20, 1450–1460. [Google Scholar] [CrossRef]
- Stoeva, T.; Iliev, L. Influence of some phenylurea cytokinins. Bulg. J. Plant Pysiol. 1997, 23, 66–71. [Google Scholar]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Radwan, A.; Kleinwacter, M.; Selmar, D. Impact of drought stress on specialised meristems: Biosynthesis and the expression of monoterpene synthases in sage (Salvia officinalis). Phytochemistry 2017, 141, 20–26. [Google Scholar] [CrossRef]
- Yadav, R.K.; Sangwan, R.S.; Sabir, F.; Srivastava, A.K.; Sangwan, N.S. Effect of prolonged water stress on specialized secondary metabolites, peltate glandular trichomes, and pathway gene expression in Artemisia annua L. Plant Physiol Biochem. 2014, 74, 70–83. [Google Scholar] [CrossRef]
- Camas-Reyes, A.; Ramírez-Laguna, R.; Jofre-Garfias, A.E.; Cardozo-Martínez, F.; Hernández-Orihuela, A.L.; Molina-Torres, J.; Martínez-Antonio, A. E. coli cultures expressing a synthetic sequence of ptz gene (stz) promoted in vitro direct organogenesis in Nicotiana tabacum L. Pant Cell Tissue Organ. Cult. 2019, 137, 87–100. [Google Scholar] [CrossRef]
- Stermer, B.A.; Bianchini, G.M.; Korth, K.L. Regulation of HMG-CoA reductase activity in plants. J. Lipid Res. 1994, 35, 1133–1140. [Google Scholar] [CrossRef]
- Scavroni, J.; Vasconcellos, M.C.; Valmorbida, J.; Ferri, A.F.; Marques, M.O.M.; Ono, E.O.; Rodrigues, J.D. Rendimento e composição química do óleo essencial de Mentha piperita L. submetida a aplicações de giberelina e citocinina. Rev. Bras. Pl Med. 2006, 510, 40–43. [Google Scholar]
- Craveiro, A.; Barreira, E.S.; Rabi, J.D.D. Estudo sobre o efeito de citocininas na biossintese de monoterpenos. In Proceedings of the 41st Annual SBPC Meeting, Fortaleza, Brazil, 9–15 July 1989; p. 531. [Google Scholar]
- Kamínek, M.; Březinov, A.; Gaudinová, A.; Motyka, V.; Vaňková, R.; Zaăímalová, E. Purine cytokinins: A proposal of abbreviations. Plant Growth Regul. 2000, 32, 253–256. [Google Scholar] [CrossRef]
- da Silva, J. Is BA (6-benzyladenine) BAP (6-benzylaminopurine)? J. Plant Sci Biotechnol. 2012, 6(SI1), 121–124. [Google Scholar]
- Kieber, J.J.; Schaller, G.E. Cytokinins. Arab. B 2014, 14, e0168. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with Tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mcsteen, P.; Leyser, O. Shoot Branching. Annu. Rev. Plant Biol. 2005, 56, 353–374. [Google Scholar] [CrossRef]
- Komatsu, K.; Maekawa, M.; Ujiie, S.; Satake, Y.; Furutani, I.; Okamoto, H. LAX and SPA : Major regulators of shoot branching in rice. Proc. Natl. Acad. Sci. USA 2003, 100, 11765–11770. [Google Scholar] [CrossRef]
- Shaaf, S.; Bretani, G.; Biswas, A.; Fontana, I.M.; Rossini, L. Genetics of barley tiller and leaf development. J. Integr. Plant Biol. 2019, 61, 226–256. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. β-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.V.; Ribeiro, B.H.; Salgueiro, L.C.L.; Sato, A. Influence of growth regulators in biomass production and volatile profile of in vitro plantlets of Thymus vulgaris L. J. Agric. Food. Chem. 2009, 57, 6392–6395. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Haider, I.; Jamil, M.; Fiorilli, V.; Saito, Y.; Mi, J.; Baz, L.; Kountche, B.A.; Jia, K.P.; Guo, X. Growth and strigolactone biosynthesis in rice. Nat. Commun. 2018, 2019, 810. [Google Scholar] [CrossRef]
- Mizukami, H.; Okada, Y.; Ohashi, H. Clonal propagation of Lemon Grass (Cymbopogon citratus Stapf) through shoot tip culture. Plant Tissue Cult. Lett. 1989, 6, 22–24. [Google Scholar] [CrossRef]
- Quiala, E.; Barbón, R.; Capote, A.; Pérez-Alonso, N. Scaling-up the biomass production of Cymbopogon citratus L. in temporary immersion system. Biotecnol. Veg. 2014, 14, 67–71. [Google Scholar]
- Licea Moreno, R.J.; Fernandez Moreno, M.; Alvarado Ruffo, K.; Gómez-kosky, R. Influencia de la concentración de agar sobre la multiplicación in vitro de Cymbopogon citratus (D.C.) Stapf. Biotecnología Vegetal. 2001, 1, 77–81. [Google Scholar]
- Ribeiro, A.V.; Ribeiro, B.H.; Santos, L.S.; Apparecida, E.M.; Sato, A. Solid phase microextraction (SPME) analysis of volatile compounds produced by in vitro shoots of Lantana camara L. under the influence of auxins and cytokinins. J. Braz. Chem. Soc. 2007, 18, 1504–1508. [Google Scholar]
- Zalabák, D.; Pospíšilová, H.; Šmehilová, M.; Mrízová, K.; Frébort, I.; Galuszka, P. Genetic engineering of cytokinin metabolism: Prospective way to improve agricultural traits of crop plants. Biotechnol. Adv. 2013, 31, 97–117. [Google Scholar] [CrossRef]
- Kamenicka, A.; Valka, J.; Vzarova, G. A comparative study of different cytokinins on the formation of Rhododendron forrestii Bait’. f. ex Diels. axillary shoots in vitro. Acta Phisiologyae Plant. 1998, 20, 167–171. [Google Scholar] [CrossRef]
- Stojičić, D.; Tošić, S.; Stojanović, G.; Slatković, B.; Jovanović, S.; Budimir, S.; Uzelac, B. Volatile organic compound composition and glandular trichome characteristics of in vitro propagated Clinopodium pulegium (Rochel) Bräuchler: Effect of Carbon Source. Plants 2022, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Kacem, N.S.; Delporte, F.; Muhovski, Y.; Djekoun, A.; Watillon, B. In vitro screening of durum wheat against water-stress mediated through polyethylene glycol. J. Gen. Engin. Biotech. 2017, 15, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Teyssier, C.; Bonhomme, L.; Lomenech, A.; Vallance, M.; Morabito, D.; Label, P. Increased gelling agent concentration promotes somatic embryo maturation in hybrid larch (Larix × eurolepsis): A 2-DE proteomic analysis. Physiol. Plant. 2011, 141, 152–165. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Bernier-Cardou, M.; Cyr, D.R.; Sutton, B.C.S. Influence of gelling agents on culture medium gel strength, water availability, tissue water potential, and maturation response in embryogenic cultures of Pinus strobus L. Cell Develop. Biol.-Plant. 2000, 36, 279–286. [Google Scholar] [CrossRef]
- Seingre, D.; O’Rourke, J.; Gavillet, S.; Moncousin, C. Influence of gelling agent and carbon source on the in vitro proliferation rate of apple rootstock EM IX. Acta Hortic. 1991, 289, 151–156. [Google Scholar] [CrossRef]
- Letham, D.S.; Gollnow, B.I. Regulators of Cell division in plant tissues.XXX. Cytokinin metabolism in relation to radish cotyledon expansion and senescence. J. Plant Growth Regul. 1985, 4, 129–145. [Google Scholar] [CrossRef]
- Balibrea Lara, M.E.; Garcia Gonzalez, M.-C.; Fatima, T.; Ehness, R.; Lee, T.K.; Proels, R.; Tanner, W.; Roitsch, T. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 2004, 16, 1276–1287. [Google Scholar] [CrossRef]
- Albacete, A.; Cantero-Navarro, E.; Großkinsky, D.K.; Arias, C.L.; Balibrea, M.E.; Bru, R.; Fragner, L.; Ghanem, M.E.; González, M.d.L.C.; Hernández, J.A.; et al. Ectopic overexpression of the cell wall invertase gene CIN1 leads to dehydration avoidance in tomato. J. Exp. Bot. 2015, 66, 863–878. [Google Scholar] [CrossRef]
- Barbier, F.F.; Dun, E.A.; Kerr, S.C.; Chabikwa, T.G.; Beveridge, C.A. An update on the signals controlling shoot branching. Trends Plant Sci. 2019, 24, 220–236. [Google Scholar] [CrossRef]
- Wang, M.; Gourrierec, L.; Jiao, F.; Demotes-Mainard, S.; Perez-Garcia, M.D.; Ogé, L.; Hamama, L.; Crespel, L.; Bertheloot, J.; Chen, J.; et al. Convergence and divergence of sugar and cytokinin signaling in plant development. Int. J. Mol. Sci. 2021, 1, 1282. [Google Scholar] [CrossRef] [PubMed]
- Trang, D.T.; Van, H.T.K.; Minh, N.T.T.; Van, C.P.; Dang, N.H.; Dang, H.D.; Quang, T.N.; Tien, D.N. Essential oils of lemongrass (Cymbopogon citratus Stapf) induces apoptosis and cell cyclle arrst in A549 lung cancer cells. BioMed. Res. Int. 2020, 2020, 5925856. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.H.T.; Minh, Q.N.; Vinh, H.D.T.; Hai, N.T.; Ly, H.T.; Hien, N.T.; Trang, D.T.; Dang, N.H.; Dat, N.T. Chemical composition and cytotoxic activity of the essential oils of Cymbopogon citratus L. grown in Phu Tho province. J. Biotech. 2016, 14, 683–687. [Google Scholar]
- Stolz, A.; Riefler, M.; Lomin, S.N.; Achazi, K.; Romanov, G.A.; Schmülling, T. The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors. Plant J. 2011, 67, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Pischke, M.S.; Mahonen, A.P.; Miyawaki, K.; Hashimoto, Y.; Seki, M.; Kobayashi, M.; Shinozaki, K.; Kato, T.; Tabata, S.; et al. In planta functions of the Arabidopsis cytokinin. Proc. Natl. Acad. Sci. USA 2004, 101, 8821–8826. [Google Scholar] [CrossRef] [PubMed]
- Hare, B.; van Staden, J. Minireview Cytokinin oxidase : Biochemical features and physiological significance. Physiol. Plant. 1994, 91, 128–136. [Google Scholar] [CrossRef]
- Werner, T.; Motika, V.; Strnad, M.S.T. Regulation of plant growth by cytokinin. Proc. Nat. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H. Cytokinin-deficient transgenic arabidopsis plants show functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003, 15, 2532–2550. [Google Scholar] [CrossRef]
- Werner, T.; Köllmer, I.; Bartrina, I.; Holst, K.; Schmülling, T. New insights into the biology of cytokinin degradation. Plant Biol. 2006, 8, 371–381. [Google Scholar] [CrossRef]
- Galuszka, P.; Popelkova, H.; Werner, T.; Frebortova, J.; Pospisilova, H.; Vaclav, M.; Kollmer, I.; Schmülling, I.F. Biochemical characterization of Cytokinin Oxidases / Dehydrogenases from Arabidopsis thaliana expressed in Nicotiana tabacum L. J. Plant Growth Regul. 2007, 26, 255–267. [Google Scholar] [CrossRef]
- Cárdenas-Aquino, M.R.; Sarria-Guzmán, Y.; Martínez-Antonio, A. Review: Isoprenoid and aromatic cytokinins in shoot branching. Plant Sci. 2022, 319, 111240. [Google Scholar] [CrossRef] [PubMed]
- Papon, N.; Bremer, J.; Rideau, M. Cytokinin and ethylene control indole alkaloid production at the level of the MEP/Terpenoid pathway in Catharanthus roseus suspension cells. Planta Med. 2005, 71, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Phatak, S.V.; Heble, M.R. Organogenesis and terpenoid synthesis in Mentha arvensis. Fitoterapia 2002, 73, 32–39. [Google Scholar] [CrossRef]
- Danova, K.; Todorova, M.; Trendafilova, A.; Evstatieva, L. Cytokinin and auxin effect on the terpenoid profile of the essential oil and morphological characteristics of shoot cultures of Artimisia alba. Nat. Prod. Commun. 2012, 7, 1075–1076. [Google Scholar] [PubMed]
- Krumova, S.; Motyka, V.; Dobrev, P.; Trendafilova, A.; Evstatieva, L.; Danova, K. Terpenoid profile of Artemisia alba is related to endogenous cytokinins in vitro. Bulg. J. Agric. Sci. 2013, 19 (Suppl. 2), 26–30. [Google Scholar]
- Danova, K.; Motyka, V.; Todorova, M.; Trendafilova, A.; Krumova, S.; Dobrev, P.; Andreeva, T.; Oreshkova, T.; Taneva, S.; Evstatieva, L. Effect of cytokinin and auxin treatments on morphogenesis, terpenoid biosynthesis, photosystem structural organization, and endogenous isoprenoid cytokinin profile in Artemisia alba Turra in vitro. J. Plant Growth Regul. 2018, 37, 403–418. [Google Scholar] [CrossRef]
- Wanke, M.; Skorupinska-Tudek, K.; Swiezewska, E. Isoprenoid biosynthesis via 1-deoxy-D-xylulose5-phosphate/2-C-methyl-erythritol 4-phosphate (DOXP/MEP) pathway. Acta Biochim. Pol. 2001, 48, 663–672. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Non-mevalonate isoprenoide biosynthesis: Enzymes, genes, and inhibitors. Biochem. Soc. Trans. 2000, 28, 785–789. [Google Scholar] [CrossRef]
- Silva, G.D.S.E.; Jesus Márques, J.N.; Moreira Linhares, E.P.; Martinez Bonora, C.; Tosoni Costa, E.; Frota, S.M. Review of anticancer activity of monoterpenoids: Geraniol, nerol, geranial and neral. Chem. Biol. Interact. 2022, 1, 109994. [Google Scholar] [CrossRef]
- Yu, S.G.; Hildebrandt, L.A.; Elson, C.E. Geraniol, an ihibitor of mevalonate biosynthesis, suppresses the growth of hepatomas and melanomas trasplanted to rats and mice. J. Nutr. 1995, 125, 2763–2767. [Google Scholar]
- Pattanayak, M.; Seth, P.K.; Smita, S.; Gupta, S.K. Geraniol and limonene interaction with 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase for their role as cancer chemo-preventive agents. J. Proteomics Bioinform. 2009, 2, 466–474. [Google Scholar] [CrossRef]
- Izumi, S.; Takashima, O.; Hirata, T. Geraniol is a potent inducer of apoptosis-like cell death in the cultured shoot primordia of Matricaria chamomilla. Biochem. Biophys. Res. Comm. 1999, 259, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Kumar, S.U.; Meli, V.S.; Kumar, V.M.; Kumar, A.; Irfan, M.; Chacraborty, N.; Chakraborty, S.; Datta, A. Induction of senescence and identification of differentially expressed genes in tomato in response to monoterpene. PLoS ONE 2013, 8, e76029. [Google Scholar] [CrossRef] [PubMed]
- Chaimovitsh, D.; Abu-Abied, M.; Belausov, E.; Rubin, B.; Dudai, N.; Sadot, E. Microtubules are an intracellular target of the plant terpene citral. Plant J. 2010, 61, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.J.; Lehner, K.; Mi, J.; Jia, K.; Mijar, M.; Dinneny, J. β -Cyclocitral is a conserved root growth regulator. Proc. Nat. Acad. Sci. USA 2019, 116, 10563–10567. [Google Scholar] [CrossRef] [PubMed]

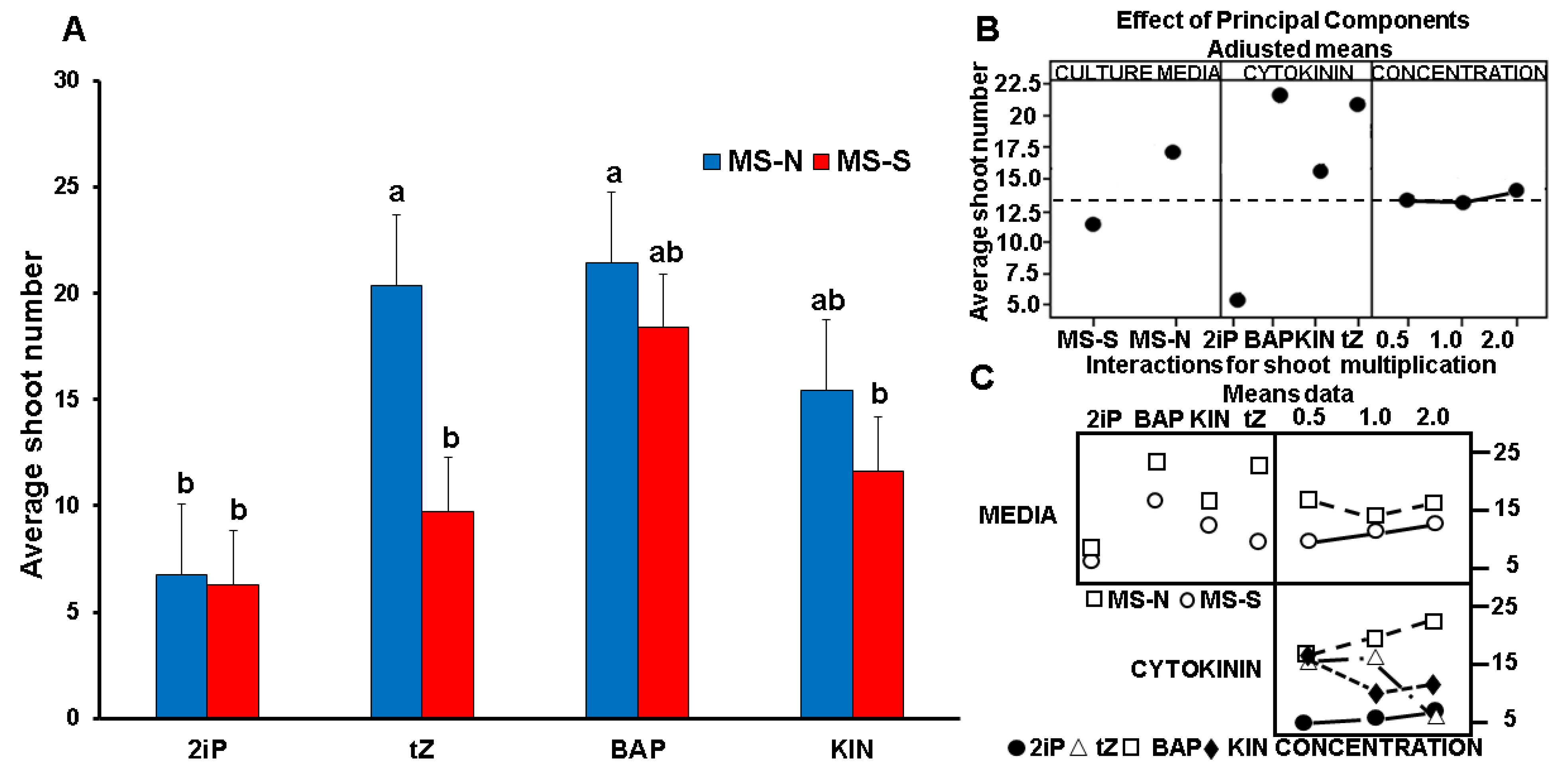

| Culture Medium | CK | Average Shoot Number Per Flask | Multiplication Index 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mg L−1 | mg L−1 | ||||||||
| 0.5 | 1 | 2 | 0.5 | 1 | 2 | ||||
| MS-N | 2iP | 6.2 | 6.5 | 7.4 | b | 2.0 | 2.1 | 2.5 | b |
| KIN | 20.1 | 12.0 | 14.1 | a | 6.7 | 4.0 | 4.7 | a | |
| BAP | 21.3 | 21.0 | 21.9 | a | 7.1 | 7.0 | 7.3 | a | |
| tZ | 20.9 | 19.7 | 20.4 | a | 6.9 | 5.9 | 6.8 | a | |
| MS-S | 2iP | 4.4 | 5.7 | 8.8 | b | 1.5 | 1.9 | 2.9 | b |
| KIN | 15.0 | 12.0 | 7.9 | b | 5.0 | 4.1 | 2.2 | bc | |
| BAP | 13.9 | 17.9 | 23.3 | a | 4.6 | 5.9 | 7.8 | ac | |
| tZ | 7.9 | 12.0 | 9.3 | b | 2.6 | 3.4 | 2.1 | b | |
| C. citratus Essential Oil Composition Obtained by SPME/GC-MS | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RT | Main Constituents | MS-N (%) | MS-S (%) | ||||||
| 2iP | tZ | BAP | KIN | 2iP | tZ | BAP | KIN | ||
| 7.50 | Limonene | 41.61 | 4.20 | 3.94 | 3.89 | 4.26 | 1.14 | 1.08 | 2.61 |
| 10.20 | Citronellal | 0.04 | - | - | - | 0.28 | - | - | - |
| 12.00 | β-citral (Neral) | 27.52 | 42.99 | 37.33 | 29.56 | 36.28 | 30.57 | 14.89 | 23.04 |
| 12.20 | Geraniol | - | 0.10 | 1.57 | - | 1.04 | 7.49 | 0.09 | 1.54 |
| 12.50 | α-citral (Geranial) | 25.62 | 47.71 | 50.56 | 64.96 | 59.48 | 66.23 | 80.04 | 70.23 |
| 14.40 | Geranyl acetate | 0.23 | 0.20 | 3.34 | 0.16 | 25.79 | 0.58 | 0.31 | 1.22 |
| 15.20 | β-caryophyllene | 3.42 | 4.80 | 2.53 | 1.43 | 2.16 | 1.99 | 2.89 | 1.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camas-Reyes, A.; Vuelvas-Nolasco, R.; Cabrera-Ponce, J.L.; Pereyra-Alférez, B.; Molina-Torres, J.; Martínez-Antonio, A. Effect of Different Cytokinins on Shoot Outgrowth and Bioactive Compounds Profile of Lemograss Essential Oil. Int. J. Plant Biol. 2022, 13, 298-314. https://doi.org/10.3390/ijpb13030025
Camas-Reyes A, Vuelvas-Nolasco R, Cabrera-Ponce JL, Pereyra-Alférez B, Molina-Torres J, Martínez-Antonio A. Effect of Different Cytokinins on Shoot Outgrowth and Bioactive Compounds Profile of Lemograss Essential Oil. International Journal of Plant Biology. 2022; 13(3):298-314. https://doi.org/10.3390/ijpb13030025
Chicago/Turabian StyleCamas-Reyes, Alberto, Rosalía Vuelvas-Nolasco, José Luis Cabrera-Ponce, Benito Pereyra-Alférez, Jorge Molina-Torres, and Agustino Martínez-Antonio. 2022. "Effect of Different Cytokinins on Shoot Outgrowth and Bioactive Compounds Profile of Lemograss Essential Oil" International Journal of Plant Biology 13, no. 3: 298-314. https://doi.org/10.3390/ijpb13030025
APA StyleCamas-Reyes, A., Vuelvas-Nolasco, R., Cabrera-Ponce, J. L., Pereyra-Alférez, B., Molina-Torres, J., & Martínez-Antonio, A. (2022). Effect of Different Cytokinins on Shoot Outgrowth and Bioactive Compounds Profile of Lemograss Essential Oil. International Journal of Plant Biology, 13(3), 298-314. https://doi.org/10.3390/ijpb13030025







