Pollen Variability of Alnus glutinosa (L.) Gaerth. (Betulaceae) from Southern Range Edge Populations in Northern Morocco
Abstract
:1. Introduction
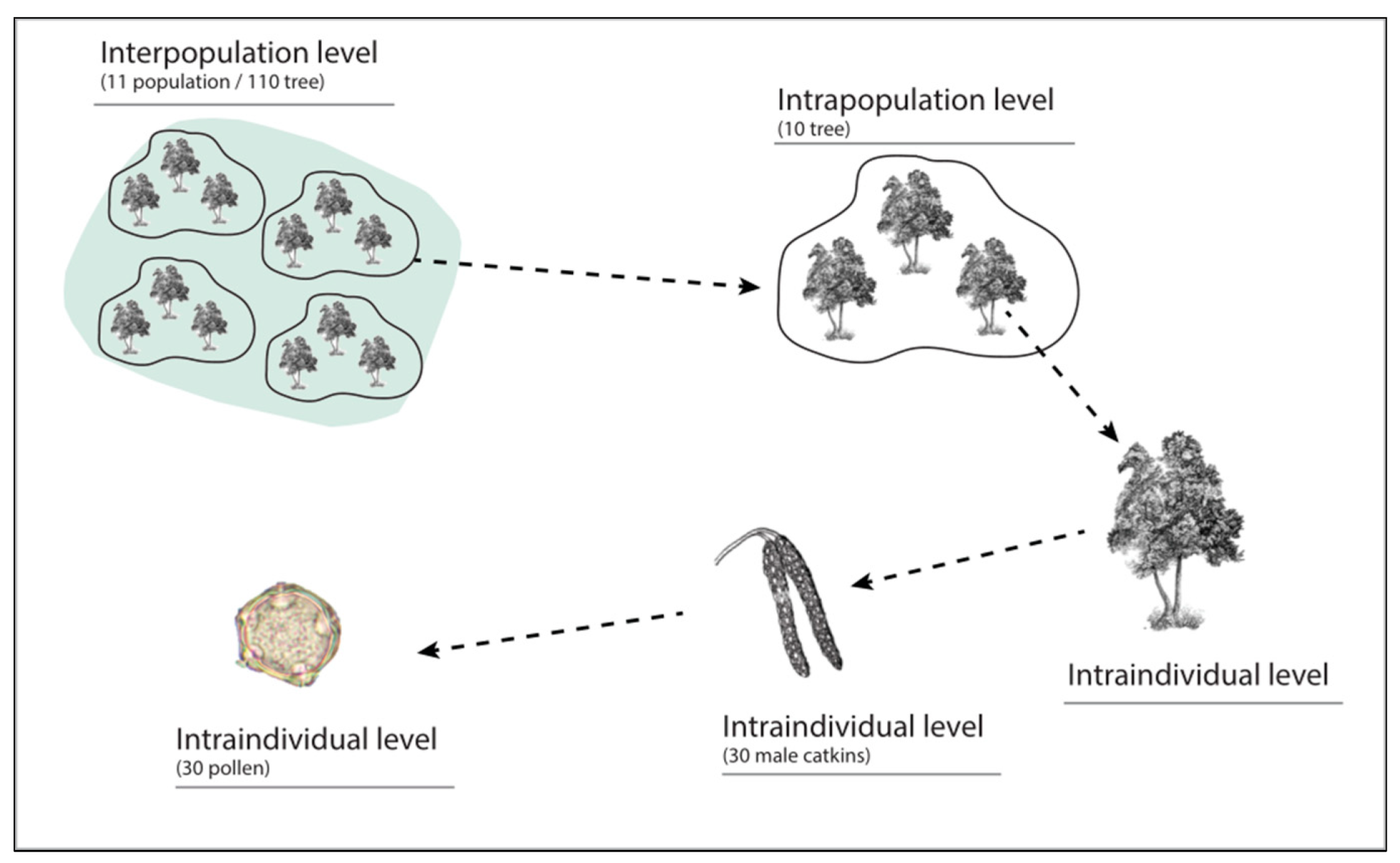
2. Materials and Methods
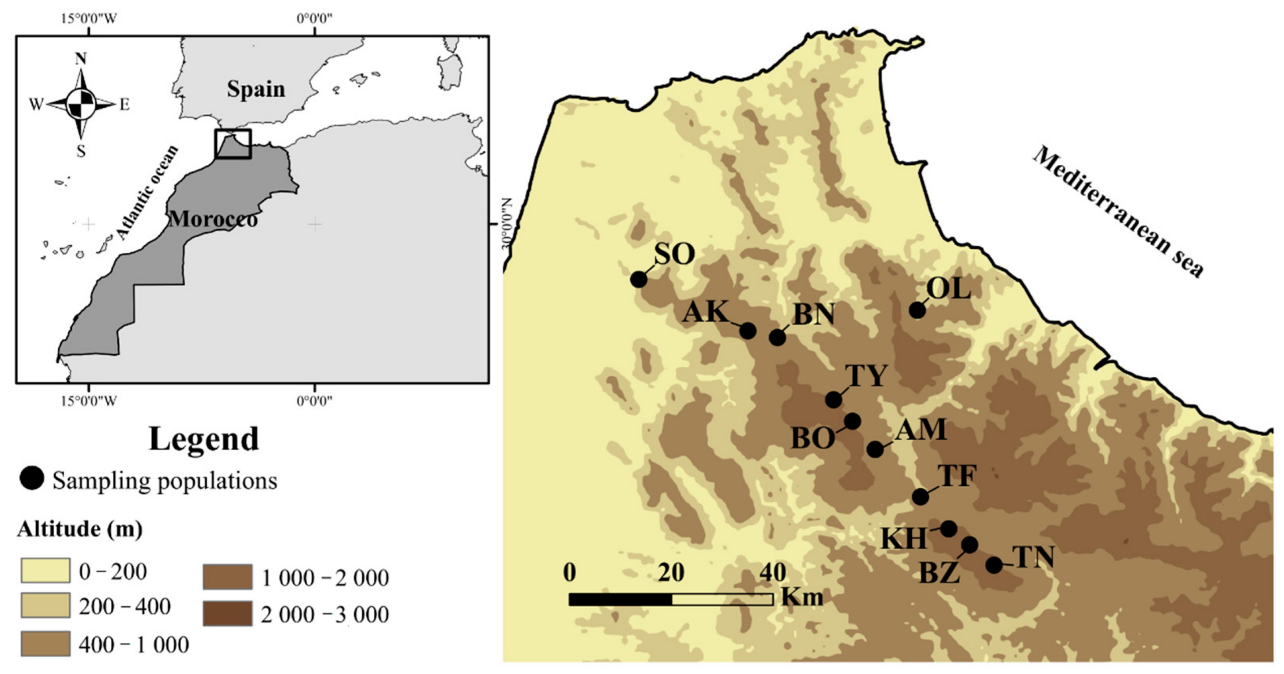
2.1. Study Area and Sampling
2.2. Photonic Microscope Observations and Measurements
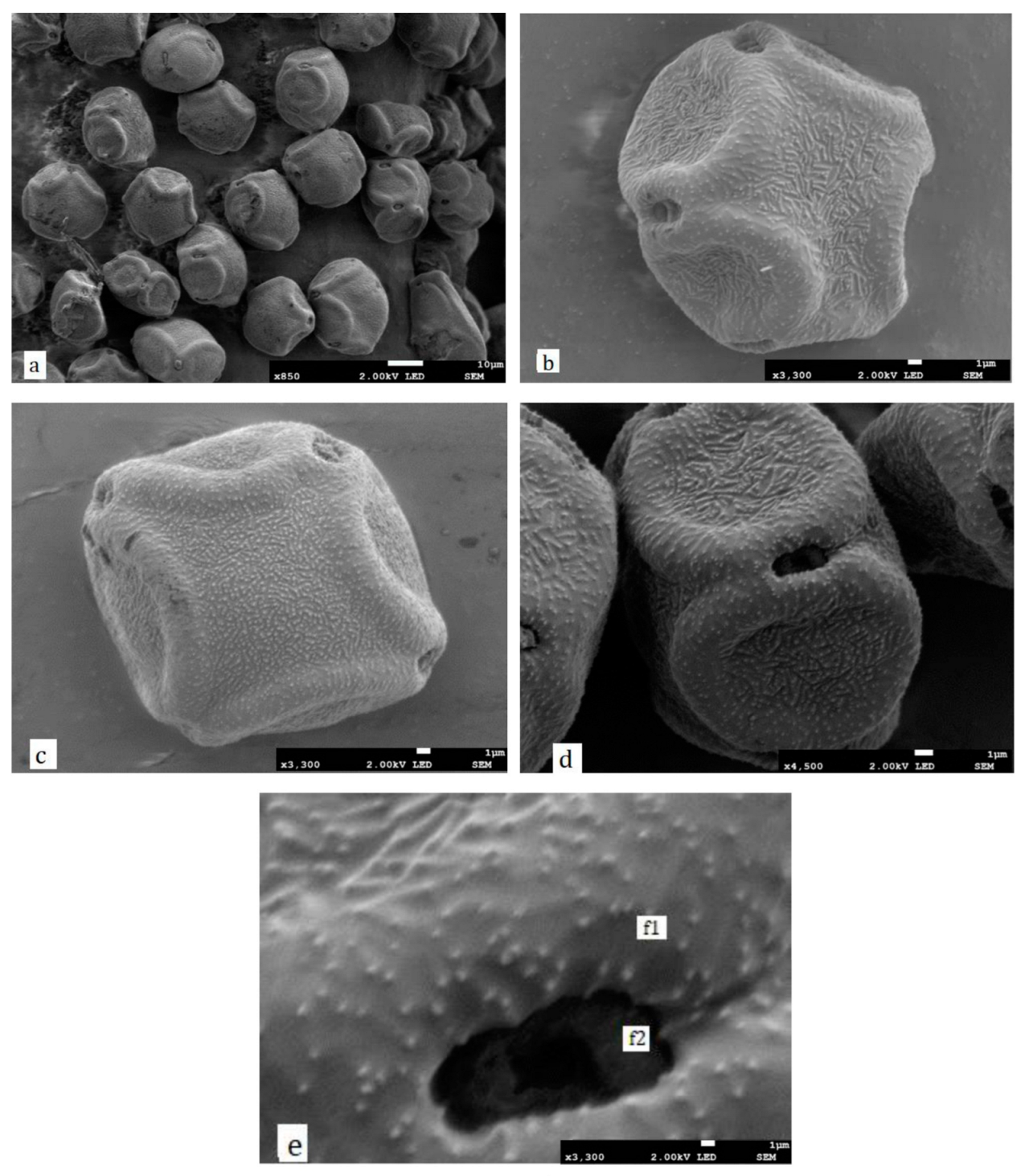
2.3. Scanning Electron Microscope Observations
2.4. Data Analysis
3. Results
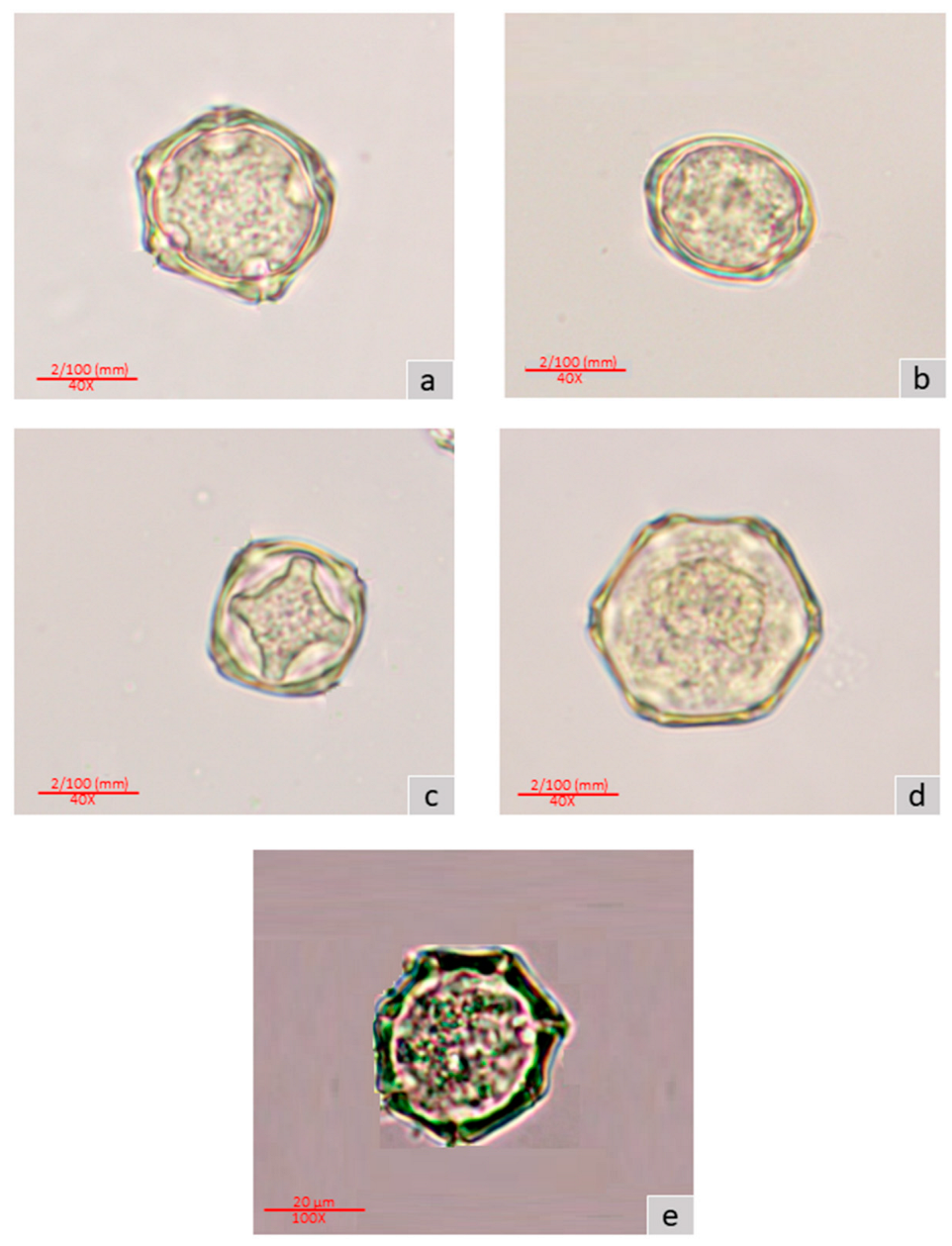
3.1. Pollen Morphology
3.2. Variability of Pollen Diameters
3.3. Variability in the Number of Apertures
4. Discussion
4.1. Pollen Morphology
4.2. Pollen Size Variability
4.3. Variability in the Number of Apertures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Population | Code P | Alt-Min (m) | Alt-Max (m) | Latitude (°) | Longitude (°) | Bioclimates |
|---|---|---|---|---|---|---|
| Ain Korra | AK | 240 | 1377 | 35.16546 | −5.31642 | PH-F |
| Amlay | AM | 285 | 1412 | 35.10789 | −5.22525 | H-T |
| BeniIdder | BN | 200 | 1377 | 35.22106 | −5.323 | H-T |
| Boubiyine | BO | 285 | 1412 | 35.12983 | −5.23099 | H-T |
| Bouztat | BZ | 985 | 1505 | 35.00803 | −5.11503 | H-F |
| Khezana | KH | 654 | 1562 | 35.03445 | −5.14031 | H-F |
| Oued Lakhmis | OL | 78 | 240 | 35.28634 | −5.15291 | SH-C |
| Siouana | SO | 64 | 596 | 35.28816 | −5.46806 | H-T |
| Tanghaya | TN | 846 | 1477 | 34.972778 | −5.15222 | H-F |
| Tayenza | TY | 514 | 1603 | 35.16082 | −5.25836 | PH-F |
| Tifouzal | TF | 385 | 390 | 35.06139 | −5.16553 | H-T |
Appendix B
| Populations | Code P | P1 | P2 | P3 | P4 | P5 | P6 | P7 |
|---|---|---|---|---|---|---|---|---|
| Ain Korra | AK | - | - | 1.14 ± 0.62 | 9.20 ± 4.45 a | 89.50 ± 4.98 a | 3.15 ± 1.79 ab | - |
| Amlay | AM | - | - | - | 9.32 ± 3.72 a | 76.32 ± 5.29 a | 13.57 ± 6.08 ab | 0.08 ± 0.00 |
| Ben Idder | BN | - | - | - | 9.87 ± 2.34 a | 80.13 ± 1.62 a | 10.00 ± 2.21 ab | - |
| Bobiyine | BO | 1.23 ± 0.21 | 1.18 ± 0.25 | 1.77 ± 0.38 | 8.64 ± 1.95 a | 83.83 ± 3.23 a | 3.362 ± 0.56 a | - |
| Bouztate | BZ | - | 0.73 ± 0.01 | 2.24 ± 0.04 | 13.16 ± 3.32 a | 81.16 ± 2.79 a | 2.71 ± 0.86 ab | - |
| Khezana | KH | - | - | - | 12.80 ± 6.85 a | 71.00 ± 4.12 a | 16.20 ± 4.92 ab | - |
| Oued Lakhemis | OL | 1.42 ± 0.00 | 0.71 ± 0.01 | 0.66 ± 0.01 | 3.16 ± 3.15 a | 79.82 ± 5.02 a | 14.22 ± 5.22 ab | - |
| Siouana | SO | 1.67 ± 0.16 | 1.26 ± 0.02 | 2.63 ± 0.66 | 7.64 ± 0.72 a | 83.60 ± 0.96 a | 3.20 ± 0.77 ab | - |
| Tanghaya | TN | - | - | - | 11.20 ± 4.04 a | 81.31 ± 3.56 a | 7.49 ± 2.37 ab | - |
| Tayenza | TY | - | - | 1.74 ± 0.31 | 14.66 ± 3.42 a | 78.32 ± 2.71 a | 5.29 ± 1.92 ab | - |
| Tifouzal | TF | 0.72 ± 0.01 | 0.72 ± 0.01 | 1.65 ± 0.47 | 6.10 ± 2.39 a | 70.60 ± 4.59 a | 20.21 ± 6.04 c | - |
| Mean | 0.46 ± 0.29 | 0.42 ± 0.22 | 1.08 ± 0.46 | 9.61 ± 3.71 | 79.33 ± 4.08 | 9.04 ± 3.98 | 0.08 ± 0.00 | |
| ANOVAinter F10, 99 | - | - | - | 0.04 n.s. | 0.27 n.s. | 0.01 * | - |
References
- Mcvean, D.N. Alnus glutinsa (L.) Graertn. Biological Flora of the British Isles. J. Ecol. 1953, 41, 447–466. [Google Scholar] [CrossRef]
- Walters, S.M. Alnus mill. In Flora Europaea, 2nd ed.; Tutin, T.G., Burges, N.A., Chater, A.O., Edmonson, J.R., Heywood, V.H., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1993; pp. 68–70. [Google Scholar]
- Claessens, H.; Oosterbaan, A.; Savill, P.; Rondeux, J. A Review of the Characteristics of Black Alder (Alnus glutinosa (L.) Gaertn.) and Their Implications for Silvicultural Practices. Forestry 2010, 83, 163–175. [Google Scholar] [CrossRef]
- Lepais, O.; Muller, S.D.; Ben Saad-Limam, S.; Benslama, M.; Rhazi, L.; Belouahem-Abed, D.; Daoud-Bouattour, A.; Gammar, A.M.; Ghrabi-Gammar, Z.; Bacles, C.F.E. High Genetic Diversity and Distinctiveness of Rear-Edge Climate Relicts Maintained by Ancient Tetraploidisation for Alnus glutinosa. PLoS ONE 2013, 8, e75029. [Google Scholar] [CrossRef] [PubMed]
- Houston Durrant, T.; de Rigo, D.; Caudullo, G. Alnus glutinosa in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 64–65. ISBN 978-92-79-52833-0. [Google Scholar]
- Erdtman, G. An Introduction to Pollen Analysis; The Cnronica Botanica Company: Leyden, The Netherlands, 1943; ISBN 978-1410209351. [Google Scholar]
- Blackmore, S.; Steinmann, J.A.J.; Hoen, P.P.; Punt, W. Betulaceae and Corylaceae. Rev. Palaeobot. Palynol. 2003, 123, 71–98. [Google Scholar] [CrossRef]
- Leopold, E.B.; Birkebak, J.; Reinink-Smith, L.; Jayachandar, A.P.; Narváez, P.; Zaborac-Reed, S. Pollen Morphology of the Three Subgenera of Alnus. Palynology 2012, 36, 131–151. [Google Scholar] [CrossRef]
- Ennouni, H.; Sahli, A.; Ater, M. Contribution à l’étude Des Paramètres Dendrométriques et à La Cartographie Des Peuplements Relictuels d’une Espèce Septentrionale Rare Au Maroc: Alnus glutinosa (L.) Gaertn. Bois Forets Des Trop. 2021, 349, 25–39. [Google Scholar] [CrossRef]
- Petit, R.J.; Brewer, S.; Bordács, S.; Burg, K.; Cheddadi, R.; Coart, E.; Cottrell, J.; Csaikl, U.M.; Van Dam, B.; Deans, J.D.; et al. Identification of Refugia and Post-Glacial Colonisation Routes of European White Oaks Based on Chloroplast DNA and Fossil Pollen Evidence. For. Ecol. Manag. 2002, 156, 49–74. [Google Scholar] [CrossRef]
- Quézel, P.; Médail, F. Valeur Phytoécologique et Biologique Des Ripisylves Méditerranéennes. Méditerranéenne 2003, XXIV, 231–248. [Google Scholar]
- Hampe, A.; Petit, R.J. Conserving Biodiversity under Climate Change: The Rear Edge Matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef]
- Douda, J.; Doudová, J.; Drašnarová, A.; Kuneš, P.; Hadincová, V.; Krak, K.; Zákravský, P.; Mandák, B. Migration Patterns of Subgenus Alnus in Europe since the Last Glacial Maximum: A Systematic Review. PLoS ONE 2014, 9, e88709. [Google Scholar] [CrossRef]
- Mandák, B.; Vít, P.; Krak, K.; Trávníček, P.; Havrdová, A.; Hadincová, V.; Zákravský, P.; Jarolímová, V.; Bacles, C.F.E.; Douda, J. Flow Cytometry, Microsatellites and Niche Models Reveal the Origins and Geographical Structure of Alnus glutinosa Populations in Europe. Ann. Bot. 2016, 117, 107–120. [Google Scholar] [CrossRef]
- Stebbins, G.L. Chromosomal Evolution in Higher Plants, 1st ed.; Barrington, E.J.W., Willis, A.J., Eds.; Edward Arnold Ltd.: London, UK, 1971; ISBN 07131 2287 0. [Google Scholar]
- Jiao, F.; Wijaya, N.; Zhang, L.; Ninomiya, Y.; Hocking, R. Synchrotron-Based XANES Speciation of Chromium in the Oxy-Fuel Fly Ash Collected from Lab-Scale Drop-Tube Furnace. Environ. Sci. Technol. 2011, 45, 6640–6646. [Google Scholar] [CrossRef]
- Comai, L. The Advantages and Disadvantages of Being Polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Alix, K.; Gérard, P.R.; Schwarzacher, T.; Heslop-Harrison, J.S.P. Polyploidy and Interspecific Hybridization: Partners for Adaptation, Speciation and Evolution in Plants. Ann. Bot. 2017, 120, 183–194. [Google Scholar] [CrossRef]
- Vít, P.; Douda, J.; Krak, K.; Havrdová, A.; Mandák, B. Two New Polyploid Species Closely Related to Alnus glutinosa in Europe and North Africa—An Analysis Based on Morphometry, Karyology, Flow Cytometry and Microsatellites. Taxon 2017, 66, 567–583. [Google Scholar] [CrossRef]
- Muller, J.A.N. Form and Function in Angiosperm Pollen. Ann. Mo. Bot. Gard. 1979, 66, 593–632. [Google Scholar] [CrossRef]
- Harder, L.D. Pollinated Angiosperms with Different Pollination Characteristics. Biol. J. Linneam Soc. 1998, 980235, 513–525. [Google Scholar] [CrossRef]
- Bennett, M. Parental Genome Separation in F1 Hybrids between Grass Species; Kew chromo; HMSO: London, UK, 1988; Volume 306. [Google Scholar]
- Kim, K.H.; Zsuffa, L.; Kenney, A.; Mosseler, A. Interspecific and Intraspecific Variation in Pollen Morphology in Four Species of Salix. Can. J. Bot. 1990, 68, 1497–1501. [Google Scholar] [CrossRef]
- Morinho, R.C.; Mendez-Rodriguez, C.; Bonetti, A.M.; Oliveira, P.E. Pollen and Stomata Mormetrics and Ploidy in Eriotheca (Malvaceae-Bonibacoidae). Plant Biol. 2014, 16, 508–511. [Google Scholar] [CrossRef]
- Amer, W.M.; Amany, S.A. Infra-Specific Pollen Diversity of Atriplex halimus L. in Egyptian Flora. Int. J. Res. Stud. Biosci. 2014, 2, 36–48. [Google Scholar]
- Singhal, V.K.; Tantray, Y.R.; Kaur, D.; Gupta, R.C. First Report of Intraspecific Polyploidy (2x, 4x) in Physochlaina praealta (Decne.) Miers. (Family: Solanaceae). Cytologia 2017, 82, 245–250. [Google Scholar] [CrossRef]
- De Freitas, J.M.B.; Kuhn, A.W.; Frescura, V.D.-S.; Essi, L.; Tedesco, S.B. Differences in Stomatal and Pollen Grain Dimensions and Pollen Viability between Paspalum rawitscheri Populations. Ciência Nat. 2020, 42, e46. [Google Scholar] [CrossRef]
- Knight, C.A.; Clancy, R.B.; Götzenberger, L.; Dann, L.; Beaulieu, J.M. On the Relationship between Pollen Size and Genome Size. J. Bot. 2010, 2010, 612017 . [Google Scholar] [CrossRef]
- Möller, M. Nuclear DNA C-Values Are Correlated with Pollen Size at Tetraploid but Not Diploid Level and Linked to Phylogenetic Descent in Streptocarpus (Gesneriaceae). S. Afr. J. Bot. 2018, 114, 323–344. [Google Scholar] [CrossRef]
- Wodehouse, R.P. Preparation of Pollen for Microscopic Examination. Bull. Torrey Bot. Club 1933, 60, 417–421. [Google Scholar] [CrossRef]
- Cushing, E.J. Size Increase in Pollen Grains Mounted in Thin Slides. Pollen Spores 1961, 3, 264–274. [Google Scholar]
- Punt, W.; Hoen, P.P.; Blackmore, S.; Nilsson, S.; Le Thomas, A. Glossary of Pollen and Spore Terminology. Rev. Palaeobot. Palynol. 2007, 143, 1–81. [Google Scholar] [CrossRef]
- Hesse, M.; Halbritter, H.; Zetter, R.; Weber, M.; Buchner, R.; Frosch-Radivo, A.; Ulrich, S. Pollen Terminology an Illustrated Handbook, 1st ed.; Springer: Vienna, Austria, 2009; ISBN 9783211798935. [Google Scholar]
- R Development Core Team R. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 6 August 2018).
- Kassout, J.; Terral, J.F.; Hodgson, J.G.; Ater, M. Trait-Based Plant Ecology a Flawed Tool in Climate Studies? The Leaf Traits of Wild Olive That Pattern with Climate Are Not Those Routinely Measured. PLoS ONE 2019, 14, e0219908. [Google Scholar] [CrossRef]
- Kassout, J.; Ater, M.; Ivorra, S.; Barbara, H.; Limier, B.; Ros, J.; Girard, V.; Paradis, L.; Terral, J.F. Resisting Aridification: Adaptation of Sap Conduction Performance in Moroccan Wild Olive Subspecies Distributed over an Aridity Gradient. Front. Plant Sci. 2021, 12, 663721. [Google Scholar] [CrossRef]
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R.; Svojtka, M.; Frosch-Radivo, A. Illustrated Pollen Terminology, 2nd ed.; Springer: Vienna, Austria, 2018; ISBN 9783319713649. [Google Scholar]
- Temizer, I.K.; Turkmen, Z. Pollen Morphology of A. glutinosa (L.) Gaertner subsp. glutinosa (Betulaceae). Kast. Univ. J. For. Fac. 2016, 16, 423–426. [Google Scholar] [CrossRef]
- Shayanmehr, F.; Jalali, S.H.; Hosseinzadeh Colagar, A.; Yousefzadeh, H.; Zare, H. Pollen Morphology of the Genus Alnus Mill. In Hyrcanian Forests, North of Iran. Appl. Environ. Res. 2014, 13, 819–831. [Google Scholar] [CrossRef]
- Faegri, K.; Van Der Pijl, L. Principles of Pollination Ecolog, 3rd ed.; Pergamon Press: Oxford, UK, 1979. [Google Scholar]
- Lacourse, T.; May, L. Morphological Differentiation of Alnus (Alder) Pollen from Western North America Morphological Differentiation of Alnus (Alder) Pollen from Western North America. Rev. Palaeobot. Palynol. 2012, 180, 15–24. [Google Scholar] [CrossRef]
- Karlsdóttir, L.; Hallsdóttir, M.; Thórsson, A.T.; Anamthawat-Jónsson, K. Characteristics of Pollen from Natural Triploid Betula Hybrids. Grana 2008, 47, 52–59. [Google Scholar] [CrossRef]
- Cramer, C.S. Laboratory Techniques for Determining Ploidy in Plants. Horttechnology 1999, 9, 594–596. [Google Scholar] [CrossRef]
- Ejsmond, M.J.; Wrońska-Pilarek, D.; Ejsmond, A.; Dragosz-Kluska, D.; Karpińska-Kołaczek, M.; Kołaczek, P.; Kozłowski, J.A.N. Does Climate Affect Pollen Morphology? Optimal Size and Shape of Pollen Grains under Various Desiccation Intensity. Ecosphere 2011, 2, 1–15. [Google Scholar] [CrossRef]
- Bell, B.A.; Fletcher, W.J.; Ryan, P.; Seddon, A.W.R.; Wogelius, R.A.; Ilmen, R. UV-B-Absorbing Compounds in Modern Cedrus Atlantica Pollen: The Potential for a Summer UV-B Proxy for Northwest Africa. Holocene 2018, 28, 1382–1394. [Google Scholar] [CrossRef]
- Wronska-Pilarek, D.; Bocianowski, J.; Lechowicz, K.; Wiatrowska, B.M.; Janyszek-Sołtysiak, M.; Beker, C. How Do Pollen Grains of Convallaria majalis L. Respond to Different Habitat Conditions? Diversity 2023, 15, 501. [Google Scholar] [CrossRef]
- Havrdová, A.; Douda, J.; Krak, K.; Vít, P.; Hadincová, V.; Zákravský, P.; Mandák, B. Higher Genetic Diversity in Recolonized Areas than in Refugia of Alnus glutinosa Triggered by Continent-Wide Lineage Admixture. Mol. Ecol. 2015, 24, 4759–4777. [Google Scholar] [CrossRef]
- Flenley, J. Some Prospects for Lake Sediment Analysis in the 21st Century. Quat. Int. 2003, 104, 77–80. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J. Phylogenetics and Biogeography of Alnus (Betulaceae) Inferred from Sequences of Nuclear Ribosomal DNA ITS Region. Int. J. Plant Sci. 2004, 165, 325–335. [Google Scholar] [CrossRef]
- Reinink-Smith, L.M. Variations in Alder Pollen Pore Numbers-A Possible New Correlation Tool for the Neogene Kenai Lowland, Alaska. Palynology 2010, 34, 180–194. [Google Scholar] [CrossRef]
- Edlund, A.F.; Swanson, R.; Preuss, D. Pollen and Stigma Structure and Function: The Role of Diversity in Pollination. Plant Cell 2004, 16, S84–S97. [Google Scholar] [CrossRef] [PubMed]
- Ressayre, A.; Godelle, B.; Raquin, C.; Gouyon, P.H. Aperture Pattern Ontogeny in Angiosperms. J. Exp. Zool. 2002, 294, 122–135. [Google Scholar] [CrossRef]
- Mignot, A.; Hoss, C.; Dajoz, I.; Leuret, C.; Henry, J.P.; Dreuillaux, J.M.; Heberle-Bors, E.; Till-Bottraud, I. Pollen Aperture Polymorphism in the Angiosperms: Importance, Possible Causes and Consequences. Acta Bot. Gall. 1994, 141, 109–122. [Google Scholar] [CrossRef]
- Dzyuba, O.F. Pollen from Surface Samples as an Environmental Indicator. Paleontol. J. 2006, 40, 584–589. [Google Scholar] [CrossRef]
- Lee, S. A Factor Analysis Study of the Functional Significance of Angiosperm Pollen. Syst. Bot. 1978, 3, 1–19. [Google Scholar] [CrossRef]


| Populations (P) | Code P | Polar Diameter (P) (µm) | Equatorial Diameter (E) (µm) | P/E | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± CI | CV (%) | ANOVAIntra F9290 | Mean ± CI | CV (%) | ANOVAIntra F9290 | Mean ± CI | CV (%) | ||
| Ain Korra | AK | 19.09 ± 0.62 d,e | 9.13 | 20.36 *** | 26.64 ± 0.73 c | 7.68 | 13.83 *** | 0.71 ± 0.01 a | 4.64 |
| Amlay | AM | 19.66 ± 0.69 f | 9.76 | 31.81 *** | 26.76 ± 0.69 d,e | 7.18 | 10.06 *** | 0.73 ± 0.01 a | 4.54 |
| Beni Idder | BN | 18.26 ± 0.36 a,b | 9.53 | 9.22 *** | 26.41 ± 0.76 c,d | 8.09 | 26.22 *** | 0.69 ± 0.01 a | 4.8 |
| Bobiyine | BO | 18.95 ± 0.69 d | 10.19 | 17.41 *** | 27.31 ± 0.71 e,f | 7.29 | 9.45 *** | 0.69 ± 0.01 a | 5 |
| Bouztate | BZ | 18.16 ± 0.67 a | 10.34 | 23.24 *** | 25.75 ± 0.69 b,c,d | 7.54 | 22.62 *** | 0.70 ± 0.01 a | 4.98 |
| Khezana | KH | 18.08 ± 0.62 a | 9.61 | 28.25 *** | 24.77 ± 0.70 a | 7.91 | 9.47 *** | 0.73 ± 0.01 a | 4.55 |
| Oued Lakhemis | OL | 18.69 ± 0.75 b,c,d | 11.28 | 19.31 *** | 26.54 ± 1.32 c | 13.94 | 7.85 *** | 0.70 ± 0.01 a | 3.94 |
| Siouana | SO | 18.45 ± 0.65 a,b,c | 9.88 | 4.47 *** | 26.30 ± 0.65 b,c,d | 6.89 | 13.32 *** | 0.70 ± 0.01 a | 4.74 |
| Tanghaya | TN | 18.80 ± 0.61 c,d | 9 | 11.42 *** | 26.17 ± 0.71 b,c,d | 7.67 | 9.88 *** | 0.72 ± 0.01 a | 4.63 |
| Tayenza | TY | 18.75 ± 0.63 b,c,d | 9.36 | 12.27 *** | 26.60 ± 0.70 c,d | 7.33 | 7.88 *** | 0.70 ± 0.01 a | 4.72 |
| Tifouzal | TF | 19.46 ± 0.62 e,f | 8.85 | 13.24 *** | 27.37 ± 0.71 f | 7.28 | 8.877 *** | 0.71 ± 0.01 a | 4.68 |
| Mean | 18.75 ± 0.62 | 9.72 | 26.42 ± 0.76 | 8.07 | 0.73 ± 0.01 | 4.2 | |||
| ANOVAInter F10, 3239 | 23.29 *** | 31.22 *** | 1.23 n.s. | ||||||
| Polar Diameter (P) | Equatorial Diameter (E) | |
|---|---|---|
| Variance Decomposition (%) | ||
| Interpopulation variance | 6.92 | 3.85 |
| Intrapopulation variance | 24.36 | 31.49 |
| Intraindividual variance | 59.16 | 55.53 |
| Residual | 9.53 | 9.14 |
| Nested ANOVA | ||
| Interpopulation F10,99 | 2.16 *** | 2.36 *** |
| Intrapopulation F99, 3131 | 15.31 *** | 11.39 *** |
| P4 | P5 | P6 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Populations | Code p | Mean | Min-Max | CV% | Mean | Min-Max | CV% | Mean | Min-Max | CV% |
| Ain Korra | AK | 9.20 ± 4.45 a | 1.6–22.01 | 69.27 | 89.50 ± 4.98 a | 77.06–97.6 | 7.43 | 3.15 ± 1.79 ab | 0.76–15.26 | 145.44 |
| Amlay | AM | 9.32 ± 3.72 a | 0–30.92 | 122.00 | 76.32 ± 5.29 a | 55.92–97.84 | 18.42 | 13.57 ± 6.08 ab | 0–42.10 | 135.77 |
| Ben Idder | BN | 9.87 ± 2.34 a | 0 -18.97 | 137.41 | 80.13 ± 1.62 a | 81.02 -100 | 6.36 | 10.00 ± 2.21 ab | 0–14.49 | 119.95 |
| Bobiyine | BO | 8.64 ± 1.95 a | 2.94–19.01 | 62.03 | 83.83 ± 3.23 a | 76.05-93.07 | 6.60 | 3.362 ± 0.56 a | 0–5.15 | 79.13 |
| Bouztate | BZ | 13.16 ± 3.32 a | 5.64–30.11 | 63.00 | 81.16 ± 2.79 a | 66.94–90.64 | 10.50 | 2.71 ± 0.86 ab | 0–7.34 | 86.10 |
| Khezana | KH | 12.80 ± 6.85 a | 0–45.34 | 123.50 | 71.00 ± 4.12 a | 54.65–98.33 | 19.75 | 16.20 ± 4.92 ab | 0–36.84 | 138.70 |
| Oued Lakhemis | OL | 3.16 ± 3.15 a | 0 -7.09 | 179.67 | 79.82 ± 5.02 a | 49.69–94.02 | 16.24 | 14.22 ± 5.22 ab | 2.58–50.30 | 100.24 |
| Siouana | SO | 7.64 ± 0.72 a | 0–42.85 | 185.39 | 83.60 ± 0.96 a | 46.42–97.1 | 16.86 | 3.20 ± 0.77 ab | 0–6.28 | 72.17 |
| Tanghaya | TN | 11.20 ± 4.04 a | 2.08–39.13 | 100.73 | 81.31 ± 3.56 a | 60.86–92.96 | 12.00 | 7.49 ± 2.37 ab | 0–21.18 | 110.93 |
| Tayenza | TY | 14.66 ± 3.42 a | 0.71–30.66 | 69.46 | 78.32 ± 2.71 a | 68.66–94 | 10.13 | 5.29 ± 1.92 ab | 0–16.54 | 147.15 |
| Tifouzal | TF | 6.10 ± 2.39 a | 0–17.51 | 131.13 | 70.60 ± 4.59 a | 54.26–96.55 | 17.51 | 20.21 ± 6.04 c | 0–44.96 | 105.26 |
| Mean | 9.61 ± 3.71 | 0–45.34 | 114.38 | 79.33 ± 4.08 | 46.42–100 | 13.71 | 9.04 ± 3.98 | 0–50.30 | 146.97 | |
| ANOVAinter F10, 99 | 0.04 n.s | 0.27 n.s | 0.01 * | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahli, A.; Kassout, J.; Boselli, V.A.; Ennouni, H.; Chakkour, S.; Kadaoui, K.; Houssni, M.; Ater, M. Pollen Variability of Alnus glutinosa (L.) Gaerth. (Betulaceae) from Southern Range Edge Populations in Northern Morocco. Int. J. Plant Biol. 2023, 14, 797-810. https://doi.org/10.3390/ijpb14030059
Sahli A, Kassout J, Boselli VA, Ennouni H, Chakkour S, Kadaoui K, Houssni M, Ater M. Pollen Variability of Alnus glutinosa (L.) Gaerth. (Betulaceae) from Southern Range Edge Populations in Northern Morocco. International Journal of Plant Biology. 2023; 14(3):797-810. https://doi.org/10.3390/ijpb14030059
Chicago/Turabian StyleSahli, Abdelouahab, Jalal Kassout, Vladimiro Andrea Boselli, Hassan Ennouni, Soufian Chakkour, Khalil Kadaoui, Mhammad Houssni, and Mohammed Ater. 2023. "Pollen Variability of Alnus glutinosa (L.) Gaerth. (Betulaceae) from Southern Range Edge Populations in Northern Morocco" International Journal of Plant Biology 14, no. 3: 797-810. https://doi.org/10.3390/ijpb14030059
APA StyleSahli, A., Kassout, J., Boselli, V. A., Ennouni, H., Chakkour, S., Kadaoui, K., Houssni, M., & Ater, M. (2023). Pollen Variability of Alnus glutinosa (L.) Gaerth. (Betulaceae) from Southern Range Edge Populations in Northern Morocco. International Journal of Plant Biology, 14(3), 797-810. https://doi.org/10.3390/ijpb14030059







