Abstract
Barley stripe mosaic virus (BSMV)-based vectors are frequently used in virus-induced gene silencing (VIGS) and, more recently, viral overexpression (VOX) studies in wheat. Two general strategies are employed to initiate infection in wheat plants with BSMV in VIGS and VOX studies. One method involves the direct infection of wheat using viral RNA produced via the in vitro transcription of BSMV constructs. The second class utilizes viral replication in an intermediate host plant to produce large amounts of BSMV viral particles that are then used to inoculate wheat plants. This study was designed to examine the potential for BSMV-VIGS constructs to rearrange during replication in the intermediate host and result in initiating the VIGS studies with a virus that is significantly different from the original experimental construct. It is shown that in the case of BSMV-VIGS constructs harboring a PDS-silencing fragment, significant rearrangement can occur during replication in the intermediate host that has the potential to introduce artifactual experimental outcomes.
1. Introduction
Engineered derivatives of Barley stripe mosaic virus (BSMV) are frequently employed for virus-induced gene silencing (VIGS) as well as virus-induced overexpression (VOX) studies in wheat [,,]. As wheat is very recalcitrant to transformation, VIGS and VOX offer significant advantages by avoiding the need to generate transgenic plants. BSMV is positive-sense single-strand RNA virus. Its tripartite genome is composed of the α, β, and γ genomic RNAs. The region of the γRNA just downstream from the γb gene is the site where BSMV has been engineered to either carry plant gene fragments for VIGS or coding sequences for VOX experiments.
The first protocols for performing BSMV VIGS experiments employed the direct inoculation of wheat with in vitro transcribed 5′ capped viral transcripts [,]. However, in vitro transcription is relatively costly, particularly because large amounts of transcripts must be produced to inoculate the number of plants needed for robust VIGS experiments. Subsequently, other methods were devised to circumvent the need for large-scale in vitro transcription. The alternative strategies utilize the inoculation of an intermediate host plant, most often Nicotiana benthamiana, which will support the replication of BSMV and produce large amounts of infective BSMV viral particles. Viral particles are harvested from the intermediate host and then rub inoculated onto large numbers of wheat plants. Variations in the indirect inoculation protocols involve the inoculation of the intermediate host with in vitro transcripts via rub or biolistic inoculation [] or the expression of the engineered BSMV genomic RNA from Agrobacterium tumefaciens T-DNA vectors [].
Genomic instability is a general characteristic of viruses, particularly in single-strand RNA viruses [,]. Having short replication cycles, recombination, error-prone replication, and strong selection drive genome instability in RNA viruses []. This study was conducted to examine the possibility that the BSMV VIGS produced in protocols employing an intermediate replication step may result in an inoculum that has undergone significant genome changes prior to the inoculation of the experimental plants. Here, the retention of a 185 bp fragment of wheat PDS inserted into BSMV γRNAs was examined after replication in N. benthamina inoculated with either in vitro transcribed RNAs or Agrobacterium-harboring BSMV T-DNA constructs.
2. Materials and Methods
2.1. In Vitro Transcription to Produce Inoculum and to Recover Virus from N. benthiana Plants
The genome of BSMV is tripartite, comprising α, β, and γRNAs. Each RNA has been cloned in pBlueScript []. Infection with BSMV:TaPDS is accomplished using a mixture of the α, β, and γ:TaPDS in vitro transcribed RNAs. The RNAs were produced via the in vitro transcription of each plasmid using a Message Machine T7 kit (Ambion, Austin, TX, USA) following the manufacturer’s instructions and the protocol reported by Scofield et al. []. Separate reactions were performed for each of the three DNA plasmids carrying the α, β, and γ:TaPDS genomic RNAs. All three DNA plasmids were linearized through the application of MluI digestion prior to in vitro transcription. These 10 μL reactions typically yielded RNA at final concentrations of 1–1.5 μg/uL. One μg of each RNA was combined and diluted with 22.5 μL FES buffer []. This mixture was rub inoculated onto the 2nd leaves of fully expanded N. benthamiana plants using a gloved hand. The N. bethamiana plants were grown for 10 days in a Percival growth chamber at 20 °C with a 14/10 h light/dark cycle.
2.2. Inoculation of N. benthamiana with BSMV:TaPDS by T-DNA Vectors
Binary T-DNA plasmids carrying α, β, and γ:TaPDS were constructed and designated pCaBS-α, pCaBS-β, and pCa-γb:TaPDS []. Each of these T-DNA binary plasmids was transformed in Agrobacterium strain EHA105 []. Single Agrobacterium colonies carrying pCaBS-α, pCaBS-β, and pCa-γb:TaPDS were selected and confirmed through the use of PCR. Each transformant was grown overnight in LB containing rifampicin (25 mg/mL) and kanamycin (100 mg/mL) at 28 °C, with constant shaking, and then pelleted and resuspended at a concentration of 0.7 OD600 in infiltration buffer consisting of 10 mM MgCl2 and 1 mM morpholino ethanesulfonic acid (MES), with a pH 5.2 plus 0.1 mM acetosyringone, as described in Yuan et al. []. Equal amounts of each of the 3 cultures were combined. This mixture was then infiltrated with a needless 1 mL syringe into the second leaf above the cotyledon of five N. benthamiana plants. These plants were grown in a Percival growth chamber for 10 days.
2.3. Preparation of Viral RNA and cDNA Synthesis
The infected leaves were frozen in liquid nitrogen and then ground with mortar and pestle. The RNA was extracted using TRIzol (Sigma, Milwaukee, WI, USA), as described by the manufacturer. Each RNA sample was treated with DNase I prior to cDNA synthesis using the TURBO DNA-free kit (Ambion, Austin, TX, USA). First-strand DNA was synthesized using an I-Script kit (Bio-Rad, Hercules, CA, USA) following the manufacturer’s directions.
2.4. PCR Amplification for Observing the PDS Insert in BSMV γRNA
First-strand cDNA was PCR amplified using the γ Forward primer TGATGATTCTTCTTCCGTTGC and the γ Reverse primer TGGTTTCCATTCAGGCATCT. This primer pair amplified a 354 bp fragment when the PDS fragment was inserted and an 182 bp product when PDS was absent. The PCR products were observed after being electrophoresed on a 1.2% agarose gel.
The description of trade names or commercial products is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
3. Results and Discussion
N. bethamiana plants inoculated with either BSMV α, β, and γ:ΤaPDS in vitro transcripts or infiltrated with a mixture of A. tumefaciens strains carrying pCaBS-α, pCaBS-β, and pCa-γb:TaPDS, were grown for 10 days. The inoculated plants showed leaf crinkling, which is characteristic of BSMV infection, but no photobleaching was observed due to insufficient sequence complementarity of 67% between the wheat PDS sequence (GenBank ID: DQ270236.1) and N. benthamina PDS sequences. The third and fourth leaves above the cotyledon were harvested for RNA extraction and PCR analysis. To provide size standards PCR, was also performed on the DNA plasmids BSMV:00 and BSMV:PDS using the primers γ Forward and γ Reverse, which produce a 354 bp fragment when PDS is present and 182 bp if it is absent (see Figure 1).
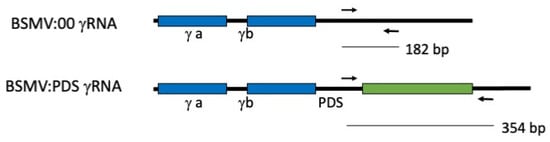
Figure 1.
Schematic of the PCR detection of PDS inserts in the BSMV γRNA of in vitro transcribed RNAs or Agrobacterium T-DNA infections.
The PCR reactions were electrophoresed on a 1.2% agarose gel (see Figure 2). Lanes 1–6 were loaded with PCR products representing six independent plants that had been infected with Agroinfiltration, while lanes 8–13 were inoculated with in vitro transcripts. Comparing the gel band intensity of the 354 and 182 bp products, Figure 2 clearly indicates that after 10 days of replication, the majority of the γRNAs from either infection method have lost the PDS insert. A few intermediate-sized fragments are evident, suggesting that there is strong pressure driving the loss of the PDS4 sequence. It has been observed that fragment loss is not precisely complete and leaves heterogeneous sequences at the junction point []. Such heterogeneity is evident in this study as not all deletion fragments are precisely 182 bp.
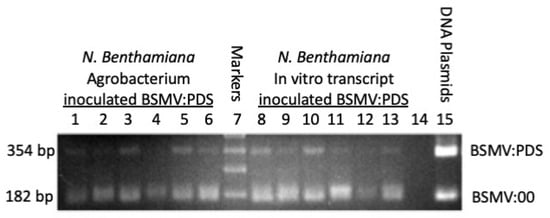
Figure 2.
PCR analysis shows that after 10 days of replication in N. benthamiana most BSMV γRNAs have undergone rearrangements, removing the PDS insert. PCR products were amplified from N. benthamiana plants inoculated with BSMV:PDS and electrophoresed on a 1.2% agarose gel. Lanes 1-6: Six N. benthamiana plants inoculated with BSMV:PDS by Agrobacterium. Lanes 8–13: Six plants inoculated with BSMV:PDS in vitro transcripts. Lane 7 was loaded with DNA size standards, lane 14 was not loaded, and lane 15 was loaded with PCR products amplified from the BSMV:00 and BSMV:PDS DNA plasmids to provide size standards for intact and rearranged BSMV:PDS γRNA.
Yuan et al. [] performed a similar analysis concerning the fragment stability in N. benthamiana infected with BSMV T-DNA constructs. They observed little fragment loss at 10 days post-inoculation, but significant instability was evident at 20 days. It is unclear why the appearance of significant instability was delayed in their analysis, although the wheat PDS insert fragment in their study was amplified from a different PDS sequence (GenBank ID: FJ517553) and was 400 bp in length.
The instability of viral genomes is a general property of viruses of all classes, whether composed of DNA or RNA and whether double- or single-stranded []. Inserted sequences are known to be even more unstable. The reasons for this are thought to be mostly due to the fitness costs of replication in comparison to wildtype viruses. The factors affecting the loss of fitness may be related to the location of the inserted fragment within the virus, its length, or sequence []. In this study, the insert sequences and locations are identical. The critical aspect being compared is the method used to inoculate the BSMV-VIGS constructs in the intermediate host, N. benthamiana.
4. Conclusions
Although this study is quite limited and only examines a single BSMV-VIGS construct, it nonetheless indicates that the passage of BSMV-VIGS constructs through an intermediate host can produce an inoculum that differs significantly from the original experimental vector. The rearranged inoculum is likely to result in a lower frequency of silenced plants, but the possibility exists that some rearranged sequences could result in unintended effects, such as the silencing of off-target genes. It clearly establishes that BSMV-VIGS studies employing indirect inoculation may have the potential for artifactual outcomes that are less likely in studies employing direct inoculation.
Funding
This work was supported by USDA-ARS project 5020-21220-014-000D. The USDA is an equal opportunity employer.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is available from the corresponding author upon request.
Acknowledgments
The author greatly appreciates the technical assistance of Amanda Brandt.
Conflicts of Interest
The author declares no conflict of interest.
References
- Bennypaul, H.; Gill, U.S. Barley Stripe Mosaic Virus (BSMV)-Based Virus-Induced Gene Silencing to Functionally Characterize Genes in Wheat and Barley. Methods Mol. Biol. 2022, 2408, 85–93. [Google Scholar] [PubMed]
- Lee, W.S.; Hammond-Kosack, K.E.; Kanyuka, K. Barley stripe mosaic virus-mediated tools for investigating gene function in cereal plants and their pathogens: Virus-induced gene silencing, host-mediated gene silencing, and virus-mediated overexpression of heterologous protein. Plant Physiol. 2012, 160, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Kanyuka, K. Virus-Induced Gene Silencing in Wheat and Related Monocot Species. Methods Mol. Biol. 2022, 2408, 95–107. [Google Scholar] [PubMed]
- Holzberg, S.; Brosio, P.; Gross, C.; Pogue, G.P. Barley stripe mosaic virus-induced gene silencing in a monocot plant. Plant J. 2002, 30, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Scofield, S.R.; Huang, L.; Brandt, A.S.; Gill, B.S. Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21-mediated leaf rust resistance pathway. Plant Physiol. 2005, 138, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Moscou, M.; Wise, R.P. Bluefinsin1 negatively impacts basal defense in response to barley powdery mildew. Plant Physiol. 2008, 149, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, C.; Yan, L.; Jackson, A.O.; Liu, Z.; Han, C.; Yu, J.; Li, D. A high throughput barley stripe mosaic virus vector for virus induced gene silencing in monocots and dicots. PLoS ONE 2011, 6, e26468. [Google Scholar] [CrossRef]
- Kosakovsky, S.L.; Murrel, B.; Poon, A.F.Y. Evolution of viral genomes: Interplay between selection, recombination, and other forces. Evol. Genet. 2012, 856, 239–272. [Google Scholar]
- Lucaci, A.G.; Zehr, J.D.; Enard, D.; Thornton, J.W.; Kosakovsky Pond, S.L. Evolutionary Shortcuts via Multinucleotide Substitutions and Their Impact on Natural Selection Analyses. Mol. Biol. Evol. 2023, 40, msad150. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, R.; Domingo-Calap, P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 2016, 73, 4433–4448. [Google Scholar] [CrossRef] [PubMed]
- Petty, I.T.; Hunter, B.G.; Wei, N.; Jackson, A.O. Infectious barley stripe mosaic virus RNA transcribed in vitro from full-length genomic cDNA clones. Virology 1989, 171, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Pogue, G.P.; Lindbo, J.A.; Dawson, W.O.; Turpen, T.H. Tobamovirus Transient Expression Vectors: Tools for Plant Biology and High-Level Expression of Foreign Proteins in Plants; Gelvin, S.B., Schilperoot, R.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 1–27. [Google Scholar]
- Ding, X.S.; Mannas, S.W.; Bishop, B.A.; Rao, X.; Lecoultre, M.; Kwon, S.; Nelson, R.S. An Improved Brome mosaic virus Silencing Vector: Greater Insert Stability and More Extensive VIGS. Plant Physiol. 2018, 176, 496–510. [Google Scholar] [CrossRef]
- Willemsen, A.; Zwart, M.P. On the stability of sequences inserted into viral genomes. Virus Evol. 2019, 5, vez045. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.N.; Canto, T.; Palukaitis, P. Stability of recombinant plant viruses containing genes of unrelated plant viruses. J. Gen. Virol. 2007, 88 Pt 4, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).