Seed Soaking Using Methanol Kalanchoe pinnata Leaf Extracts Induces Rice Resistance against Bacterial Leaf Blight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of K. pinnata Extracts
2.3. Effects of K. pinnata Leaf Extracts on Rice Seed Germination and Seedling Growth
2.4. Effects of K. pinnata Leaf Extracts Using Seed Soaking on BLB Lesion Lengths under Greenhouse Conditions
2.4.1. Inoculum Preparation
2.4.2. Pathogen Inoculation and Disease Assessment
2.5. Assays of Enzyme Activities
2.5.1. Tissue Collection and Enzyme Extraction
2.5.2. POX Assay
2.5.3. CAT Assay
2.5.4. PPO Assay
2.5.5. PAL Assay
2.6. Data Analyses
3. Results
3.1. Effects of K. pinnata Leaf Extracts on Rice Seed Germination and Seedling Growth
3.2. Effects of K. pinnata Leaf Extracts Using Seed Soaking on BLB Lesion Lengths under Greenhouse Conditions
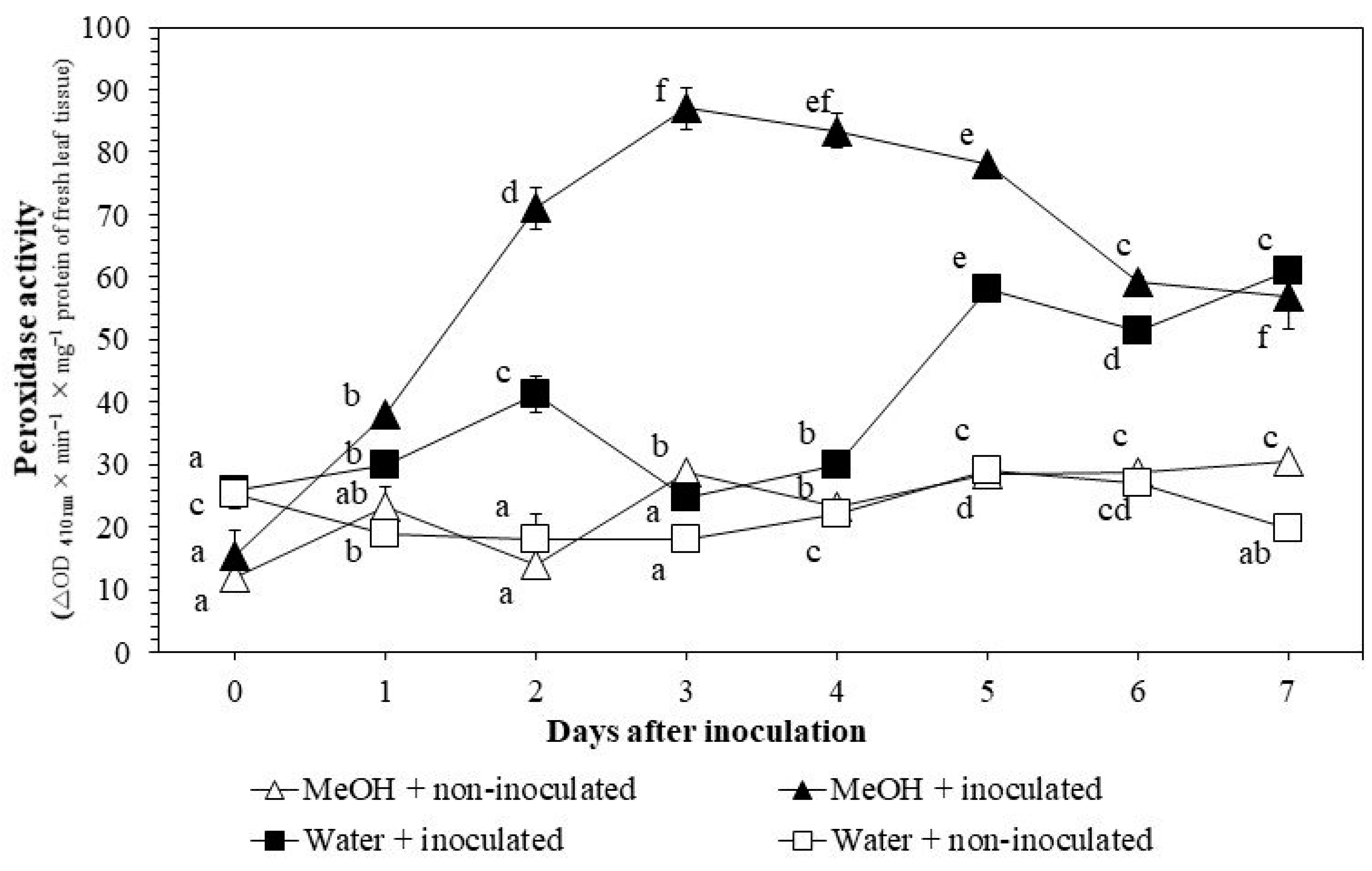
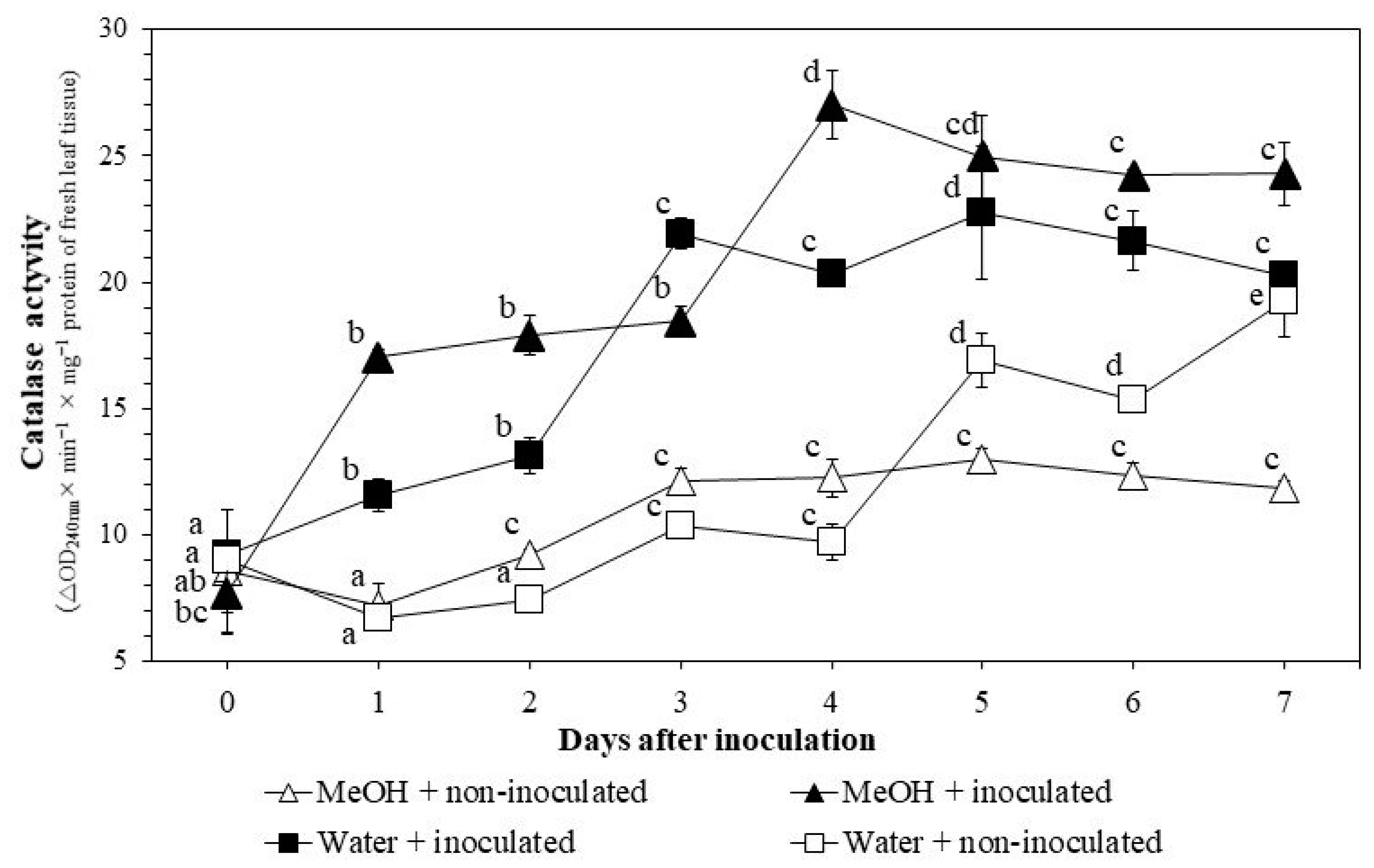
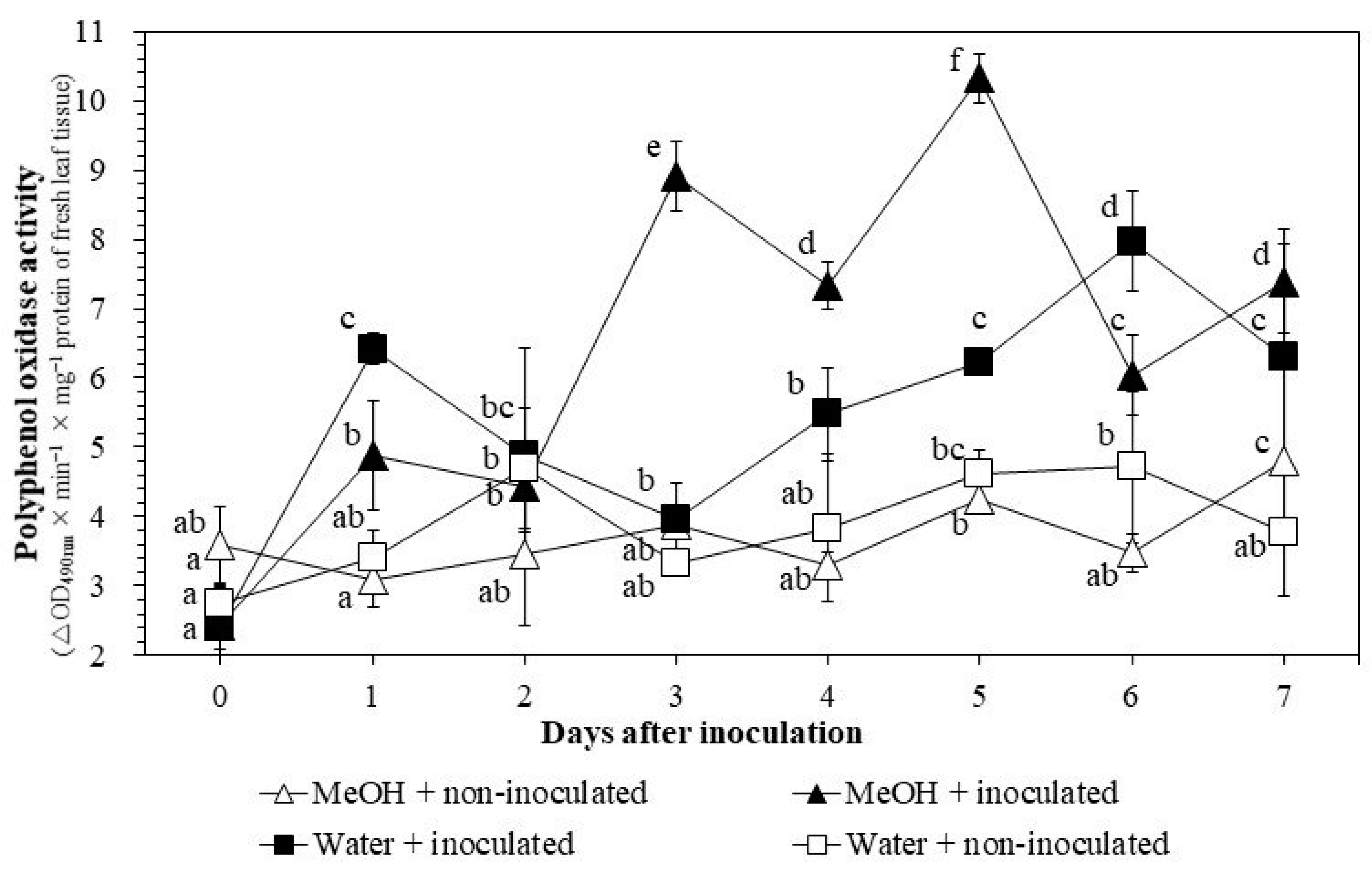
3.3. Enzyme Activities
3.3.1. POX
3.3.2. CAT
3.3.3. PPO
3.3.4. PAL
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mew, T.W. Focus on Bacterial Blight of Rice. Plant Disease 1993, 77, 5–12. [Google Scholar] [CrossRef]
- Khoa, N.Đ. Rice bacterial leaf blight. In Plant Diseases in Vietnam; Mân, V.T., Tuyến, B.C., Tuất, N.V., Kim, P.V., Eds.; Publishing House of the Vietnam National University of Agriculture: Ha Noi, Vietnam, 2018; pp. 107–113. [Google Scholar]
- Khan, J.A.; Siddiq, R.; Arshad, H.M.I.; Anwar, H.S.; Saleem, K.; Jamil, F.F. Chemical control of bacterial leaf blight of rice caused by Xanthomonas oryzae pv. oryzae. Pak. J. Phytopathol. 2012, 24, 97–100. [Google Scholar]
- Syed-Ab-Rahman, S.F.; Sijam, K.; Omar, D. Chemical composition of Piper sarmentosum extracts and antibacterial activity against the plant pathogenic bacteria Pseudomonas fuscovaginae and Xanthomonas oryzae pv. oryzae. J. Plant Dis. Prot. 2014, 121, 237–242. [Google Scholar] [CrossRef]
- Khoa, N.Đ.; Giàu, N.Đ.N.; Tuấn, T.Q. Effects of Serratia nematodiphila CT-78 on rice bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae. Biol. Control 2016, 103, 1–10. [Google Scholar] [CrossRef]
- Niño-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, R.; Sengupta, D.; Das, S.N.; Pandey, M.K.; Bohra, A.; Sharma, N.K.; Sinha, P.; Sok, H.; Ghazi, I.A.; et al. Deployment of genetic and genomic tools toward gaining a better understanding of rice Xanthomonas oryzae pv. oryzae interactions for development of durable bacterial blight resistant rice. Front. Plant Sci. 2020, 11, 1152. [Google Scholar]
- Zhang, F.; Zhuo, D.L.; Zhang, F.; Huang, L.Y.; Wang, W.S.; Xu, J.L.; Vera Cruz, C.; Li, Z.K.; Zhou, Y.L. Xa39, a novel dominant gene conferring broad-spectrum resistance to Xanthomonas oryzae pv. oryzae in rice. Plant Pathol. 2015, 64, 568–575. [Google Scholar] [CrossRef]
- Kagale, S.; Marimuthu, T.; Thayumanavan, B.; Nandakumar, R.; Samiyappan, R. Antimicrobial activity and induction of systemic resistance in rice by leaf extract of Datura metel against Rhizoctonia solani and Xanthomonas oryzae pv. oryzae. Physiol. Mol. Plant Pathol. 2004, 65, 91–100. [Google Scholar] [CrossRef]
- Khoa, N.Đ.; Xạ, T.V.; Hào, L.T. Disease-reducing effects of aqueous leaf extract of Kalanchoe pinnata on rice bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae involve induced resistance. Physiol. Mol. Plant Pathol. 2017, 100, 57–66. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Tuzun, S.; Kuć, J.A. Proposed definitions related to induced disease resistance. Biocontrol Sci. Technol. 1992, 2, 349–351. [Google Scholar] [CrossRef]
- Kang, Z.; Huang, L.; Buchenauer, H. Ultrastructural changes and localization of lignin and callose in compatible and incompatible interactions between wheat and Puccinia striiformis. J. Plant Dis. Prot. 2002, 109, 25–37. [Google Scholar]
- Latz, M.A.C.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J.L. Endophytic fungi as biocontrol agents: Elucidating mechanisms in disease suppression. Plant Ecol. Divers. 2018, 11, 555–567. [Google Scholar] [CrossRef]
- Khan, J.A.; Afroz, S.; Arshad, H.M.I.; Sarwar, N.; Anwar, H.S.; Saleem, K.; Jamil, F.F. Biochemical basis of resistance in rice against Bacterial leaf blight disease caused by Xanthomonas oryzae pv. oryzae. Adv. Life Sci. 2014, 1, 181–190. [Google Scholar]
- Fan, S.; Tian, F.; Li, J.; Hutchins, W.; Chen, H.; Yang, F.; Yuan, X.; Cui, Z.; Yang, C.; He, C. Identification of phenolic compounds that suppress the virulence of Xanthomonas oryzae on rice via the type III secretion system. Mol. Plant Pathol. 2017, 18, 555–568. [Google Scholar] [CrossRef]
- Cho, M.H.; Lee, S.W. Phenolic Phytoalexins in Rice: Biological Functions and Biosynthesis. Int. J. Mol. Sci. 2015, 16, 29120–29133. [Google Scholar] [CrossRef]
- Singh, R.; Chandrawat, K.S. Role of phytoalexins in plant disease resistance. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 125–129. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A large family of class III plant peroxidases. Plant Cell Physiol. 2001, 42, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.P.; Jørgensen, H.J.L.; Jensen, J.D.; Collinge, D.B.; Shetty, H.S. Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 2008, 121, 267–280. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Yu, C.; Wang, N.; Wu, M.; Tian, F.; Chen, H.; Yang, F.; Yuan, X.; Yang, C.H.; He, C. OxyR-regulated catalase CatB promotes the virulence in rice via detoxifying hydrogen peroxide in Xanthomonas oryzae pv. oryzae. BMC Microbiol. 2016, 16, 269. [Google Scholar] [CrossRef]
- Li, L.; Steffens, J.C. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 2002, 215, 239–247. [Google Scholar] [CrossRef]
- Solekha, R.; Susanto, F.A.; Joko, T.; Nuringtyas, T.R.; Purwestri, Y.A. Phenylalanine ammonia lyase (PAL) contributes to the resistance of black rice against Xanthomonas oryzae pv. oryzae. J. Plant Pathol. 2020, 102, 359–365. [Google Scholar] [CrossRef]
- Govindappa, M.; Umesha, S.; Lokesh, S. Adhatoda vasica leaf extract induces resistance in rice against bacterial leaf blight disease (Xanthomonas oryzae pv. oryzae). Int. J. Plant Physiol. Biochem. 2011, 3, 6–14. [Google Scholar]
- Nisha, S.; Revathi, K.; Chandrasekaran, R.; Kirubakaran, S.A.; Sathish-Narayanan, S.; Stout, M.J.; Senthil-Nathan, S. Effect of plant compounds on induced activities of defense-related enzymes and pathogenesis related protein in bacterial blight disease susceptible rice plant. Physiol. Mol. Plant Pathol. 2012, 80, 1–9. [Google Scholar] [CrossRef]
- Mohan Babu, R.; Sajeena, A.; Vijaya, S.A.; Sreedhar, A.; Vidhyasekaran, P.; Seetharaman, K.; Reddy, M.S. Induction of systemic resistance to Xanthomonas oryzae pv. oryzae by salicylic acid in Oryza sativa (L.). J. Plant Dis. Prot. 2003, 110, 419–431. [Google Scholar]
- Ahn, I.P.; Kim, S.; Lee, Y.H. Vitamin B1 functions as an activator of plant disease resistance. Plant Physiol. 2005, 138, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Chithrashree, U.; Udayashankar, A.C.; Nayaka, S.C.; Reddy, M.S.; Srinivas, C. Plant growth-promoting rhizobacteria mediate induced systemic resistance in rice against bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae. Biol. Control 2011, 59, 114–122. [Google Scholar] [CrossRef]
- Lingaiah, S.; Umesha, S. Pseudomonas fluorescens inhibits the Xanthomonas oryzae pv. oryzae the bacterial leaf blight pathogen in rice. Can. J. Plant Prot. 2013, 1, 147–153. [Google Scholar]
- Hương, N.T.T.; Hào, L.T.; Khoa, N.Đ. Effects of foliar spraying of Kalanchoe pinnata leaf extract on rice bacterial leaf blight involve phenylalanine ammonia-lyase and polyphenol oxidase activities in induced resistance. Can. Tho Univ. J. Sci. 2018, 54, 13. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Rajauria, G.; Abu-Ghannam, N.; Gupta, S. Effect of different solvents on polyphenolic content, antioxidant capacity and antibacterial activity of irish york cabbage. J. Food Biochem. 2012, 36, 344–358. [Google Scholar] [CrossRef]
- Singh, R.A.; Rao, M.H.S. A simple technique for detection of Xanthomonas oryzae in rice seeds. Seed Sci. Technol. 1997, 5, 123–127. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Teixeira, S.B.; Pires, S.N.; Asvila, G.E.; Silva, B.E.P.; Schmitz, V.N.; Deuner, C.; Silva Armesto, R.; Silva Moura, D.; Deuner, S. Application of vigor indexes to evaluate the cold tolerance in rice seeds germination conditioned in plant extract. Sci. Rep. 2021, 11, 11038. [Google Scholar] [CrossRef] [PubMed]
- Kleitman, F.; Shtienberg, D.; Blachinsky, D.; Oppenheim, D.; Zilberstaine, M.; Dror, O.; Manulis, S. Erwinia amylovora populations resistant to oxolinic acid in Israel: Prevalence, persistence and fitness. Plant Pathol. 2005, 54, 108–115. [Google Scholar] [CrossRef]
- Karganilla, A. A comparative study of culture media for Xanthomonas oryzae. Philipp. Agric. 1973, 57, 141–152. [Google Scholar]
- Kauffman, H.E. An improved technique for evaluating resistance to rice varieties of Xanthomonas oryzae. Plant Dis. Rep. 1973, 57, 537–541. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Beers, R.D.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, D.P.; Pascholati, S.F.; Hagerman, A.E.; Butler, L.G.; Nicholson, R.L. Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol. Plant Pathol. 1984, 25, 111–123. [Google Scholar] [CrossRef]
- Pal, T.K.; Bhattacharya, S.; Chakraborty, K.; Highway, O.B.; Bengal, W.; Road, B.C. Induction of systemic resistance in rice by leaf extract of Cymbopogan citrus and Ocimum sanctum against sheath blight disease. Science 2011, 3, 392–400. [Google Scholar]
- Araji, S.; Grammer, T.A.; Gertzen, R.; Anderson, S.D.; Mikulic-Petkovsek, M.; Veberic, R.; Phu, M.L.; Solar, A.; Leslie, C.A.; Dandekar, A.M. Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut. Plant Physiol. 2014, 164, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Yoruk, R.; Marshall, M.R. Physicochemical properties and function of plant polyphenol oxidase: A review. J. Food Biochem. 2003, 27, 361–422. [Google Scholar] [CrossRef]
- Constabel, C.P.; Barbehenn, R. Defensive roles of polyphenol oxidase in plants. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 253–270. [Google Scholar]



| Extracts | The Vigor Index | |||
|---|---|---|---|---|
| Concentrations (w/v) | Untreated Control (Distilled Water) | |||
| 1% | 1.5% | 2% | ||
| MeOH | 10,555 ± 1427 bc | 10,917 ± 953 bc | 12,038 ± 629 b | 10,240 ± 873 c |
| CH2Cl2 | 11,296 ± 163 bc | 11,083 ± 898 bc | 15,033 ± 481 a | |
| H2O | 9966 ± 706 c | 11,328 ± 935 bc | 12,048 ± 890 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xạ, T.V.; Thoa, T.K.; Độ, N.Đ.; Khoa, N.Đ. Seed Soaking Using Methanol Kalanchoe pinnata Leaf Extracts Induces Rice Resistance against Bacterial Leaf Blight. Int. J. Plant Biol. 2023, 14, 1155-1166. https://doi.org/10.3390/ijpb14040084
Xạ TV, Thoa TK, Độ NĐ, Khoa NĐ. Seed Soaking Using Methanol Kalanchoe pinnata Leaf Extracts Induces Rice Resistance against Bacterial Leaf Blight. International Journal of Plant Biology. 2023; 14(4):1155-1166. https://doi.org/10.3390/ijpb14040084
Chicago/Turabian StyleXạ, Trương Văn, Trần Kim Thoa, Nguyễn Đức Độ, and Nguyễn Đắc Khoa. 2023. "Seed Soaking Using Methanol Kalanchoe pinnata Leaf Extracts Induces Rice Resistance against Bacterial Leaf Blight" International Journal of Plant Biology 14, no. 4: 1155-1166. https://doi.org/10.3390/ijpb14040084
APA StyleXạ, T. V., Thoa, T. K., Độ, N. Đ., & Khoa, N. Đ. (2023). Seed Soaking Using Methanol Kalanchoe pinnata Leaf Extracts Induces Rice Resistance against Bacterial Leaf Blight. International Journal of Plant Biology, 14(4), 1155-1166. https://doi.org/10.3390/ijpb14040084






