The tgd5 Mutation Affects Plastid Structure and Causes Giant Lipid Droplet Formation in Trichomes of Arabidopsis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation
2.2. Microscopy and Lipid Staining
3. Results
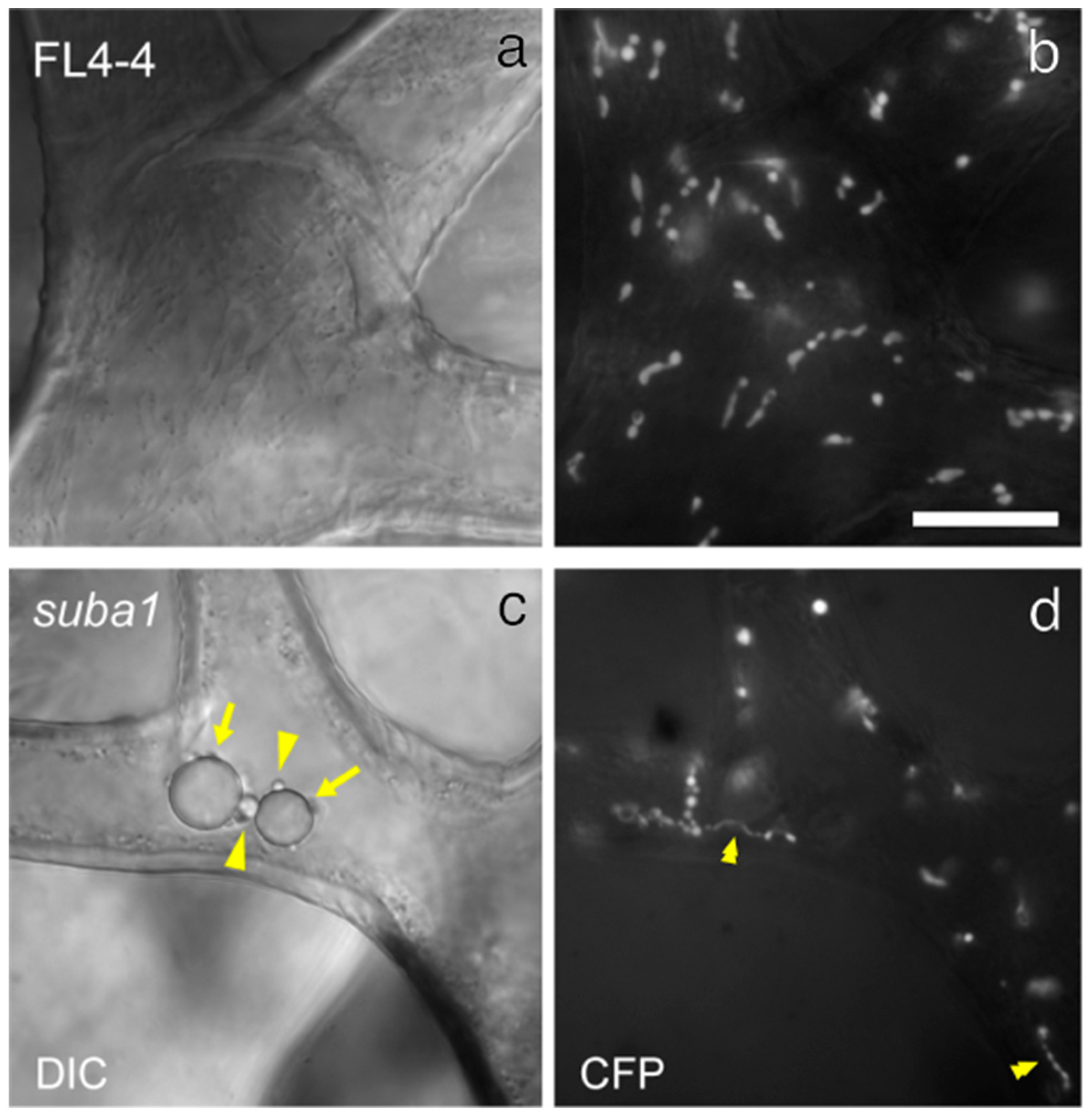
3.1. Morphology and Ultrastructure of Trichome Plastids in the Arabidopsis tgd5 Mutants
3.2. Formation of Giant Lipid Droplets in Trichomes of the Arabidopsis tgd5 Mutants
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Mo, Y.; Wang, N.; Xing, L.; Qu, Y.; Chen, Y.; Yuan, Z.; Ali, A.; Qi, J.; Fernández, V.; et al. The Overlooked Functions of Trichomes: Water Absorption and Metal Detoxication. Plant Cell Environ. 2023, 46, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Hülskamp, M. Trichomes. Curr. Biol. 2019, 29, R273–R274. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Li, Y.; Yang, Z.; Wang, C.; Zhang, Y.; Wang, B. Molecular Mechanisms of Plant Trichome Development. Front. Plant Sci. 2022, 13, 910228. [Google Scholar] [CrossRef] [PubMed]
- Livingston, S.J.; Rensing, K.H.; Page, J.E.; Samuels, A.L. A Polarized Supercell Produces Specialized Metabolites in Cannabis Trichomes. Curr. Biol. 2022, 32, 4040–4047.e4. [Google Scholar] [CrossRef] [PubMed]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D.; Bresta, P. Protective and Defensive Roles of Non-Glandular Trichomes against Multiple Stresses: Structure–Function Coordination. J. For. Res. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Ishikawa, H.; Yasuzawa, M.; Koike, N.; Sanjaya, A.; Moriyama, S.; Nishizawa, A.; Matsuoka, K.; Sasaki, S.; Kazama, Y.; Hayashi, Y.; et al. Arabidopsis PARC6 Is Critical for Plastid Morphogenesis in Pavement, Trichome, and Guard Cells in Leaf Epidermis. Front. Plant Sci. 2020, 10, 1665. [Google Scholar] [CrossRef]
- Glynn, J.M.; Yang, Y.; Vitha, S.; Schmitz, A.J.; Hemmes, M.; Miyagishima, S.; Osteryoung, K.W. PARC6, a Novel Chloroplast Division Factor, Influences FtsZ Assembly and Is Required for Recruitment of PDV1 during Chloroplast Division in Arabidopsis. Plant J. 2009, 59, 700–711. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Y.; Jia, J.; Li, D.; Zhang, R.; Gao, H.; He, Y. CDP1, a Novel Component of Chloroplast Division Site Positioning System in Arabidopsis. Cell Res. 2009, 19, 877–886. [Google Scholar] [CrossRef]
- Ottesen, E.; Zhong, R.; Lamppa, G.K. Identification of a Chloroplast Division Mutant Coding for ARC6H, an ARC6 Homolog That Plays a Nonredundant Role. Plant Sci. 2010, 178, 114–122. [Google Scholar] [CrossRef]
- Itoh, R.D.; Ishikawa, H.; Nakajima, K.P.; Moriyama, S.; Fujiwara, M.T. Isolation and Analysis of a Stromule-Overproducing Arabidopsis Mutant Suggest the Role of PARC6 in Plastid Morphology Maintenance in the Leaf Epidermis. Physiol. Plant. 2018, 162, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Itoh, R.D.; Nakajima, K.P.; Sasaki, S.; Ishikawa, H.; Kazama, Y.; Abe, T.; Fujiwara, M.T. TGD5 Is Required for Normal Morphogenesis of Non-mesophyll Plastids, but Not Mesophyll Chloroplasts, in Arabidopsis. Plant J. 2021, 107, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhai, Z.; Yan, C.; Xu, C. Arabidopsis TRIGALACTOSYLDIACYLGLYCEROL5 Interacts with TGD1, TGD2, and TGD4 to Facilitate Lipid Transfer from the Endoplasmic Reticulum to Plastids. Plant Cell 2015, 27, 2941–2955. [Google Scholar] [CrossRef] [PubMed]
- Leterme, S.; Michaud, M. Non-Vesicular Glycerolipids Transport in Plant Cells. Adv. Bot. Res. 2021, 101, 121–189. [Google Scholar] [CrossRef]
- Bowman, J.; Callos, J.D.; Behringer, F.J.; Vasinda, J.; Stewart, D.; Link, B.M.; Medford, J.I.; Griffith, M.; Pyke, K.A.; Marrison, J.L.; et al. Vegetative Development. In Arabidopsis, an Atlas of Morphology and Development; Bowman, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 1–89. [Google Scholar] [CrossRef]
- Negi, J.; Munemasa, S.; Song, B.; Tadakuma, R.; Fujita, M.; Azoulay-Shemer, T.; Engineer, C.B.; Kusumi, K.; Nishida, I.; Schroeder, J.I.; et al. Eukaryotic Lipid Metabolic Pathway Is Essential for Functional Chloroplasts and CO2 and Light Responses in Arabidopsis Guard Cells. Proc. Natl. Acad. Sci. USA 2018, 115, 9038–9043. [Google Scholar] [CrossRef] [PubMed]
- Shanmugabalaji, V.; Zita, W.; Collombat, J.; Kessler, F. Plastoglobules: A Hub of Lipid Metabolism in the Chloroplast. Adv. Bot. Res. 2021, 101, 91–119. [Google Scholar] [CrossRef]
- Matsumura, M.; Nomoto, M.; Itaya, T.; Aratani, Y.; Iwamoto, M.; Matsuura, T.; Hayashi, Y.; Mori, T.; Skelly, M.J.; Yamamoto, Y.Y.; et al. Mechanosensory Trichome Cells Evoke a Mechanical Stimuli–Induced Immune Response in Arabidopsis thaliana. Nat. Commun. 2022, 13, 1216. [Google Scholar] [CrossRef] [PubMed]




| Plant Line | Number of Examined Trichome Cells (Number of Plants) | Number of Trichome Cells with at Least One Sphere | Frequency of Sphere Occurrence (%) | Significant Difference from Wild Type (p < 0.001) |
|---|---|---|---|---|
| Wild type | 191 (5) | 0 | 0 | – |
| suba1 | 171 (6) | 93 | 54 | yes |
| tgd5-3 | 168 (6) | 96 | 57 | yes |
| Staining Dye | Plant Line | Number of Examined Trichome Cells (Number of Plants) | Number of Trichome Cells Possessing at Least One Dye-Positive Sphere | Frequency of Dye-Positive Sphere Occurrence (%) | Significant Difference from Wild Type (p < 0.001) |
|---|---|---|---|---|---|
| Sudan Black B | Wild type | 209 (6) | 0 | 0 | – |
| suba1 | 206 (7) | 95 | 46 | yes | |
| tgd5-3 | 200 (6) | 88 | 44 | yes | |
| Sudan III | Wild type | 212 (6) | 0 | 0 | – |
| suba1 | 192 (6) | 70 | 36 | yes | |
| tgd5-3 | 198 (6) | 72 | 36 | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuoka, K.; Kubotera, H.; Miyazaki, R.; Moriyama, S.; Fujiwara, M.T.; Itoh, R.D. The tgd5 Mutation Affects Plastid Structure and Causes Giant Lipid Droplet Formation in Trichomes of Arabidopsis. Int. J. Plant Biol. 2024, 15, 46-53. https://doi.org/10.3390/ijpb15010004
Matsuoka K, Kubotera H, Miyazaki R, Moriyama S, Fujiwara MT, Itoh RD. The tgd5 Mutation Affects Plastid Structure and Causes Giant Lipid Droplet Formation in Trichomes of Arabidopsis. International Journal of Plant Biology. 2024; 15(1):46-53. https://doi.org/10.3390/ijpb15010004
Chicago/Turabian StyleMatsuoka, Kanae, Hiroko Kubotera, Rina Miyazaki, Shota Moriyama, Makoto T. Fujiwara, and Ryuuichi D. Itoh. 2024. "The tgd5 Mutation Affects Plastid Structure and Causes Giant Lipid Droplet Formation in Trichomes of Arabidopsis" International Journal of Plant Biology 15, no. 1: 46-53. https://doi.org/10.3390/ijpb15010004
APA StyleMatsuoka, K., Kubotera, H., Miyazaki, R., Moriyama, S., Fujiwara, M. T., & Itoh, R. D. (2024). The tgd5 Mutation Affects Plastid Structure and Causes Giant Lipid Droplet Formation in Trichomes of Arabidopsis. International Journal of Plant Biology, 15(1), 46-53. https://doi.org/10.3390/ijpb15010004






