From Signaling to Stress: How Does Plant Redox Homeostasis Behave under Phytophagous Mite Infestation?
Abstract
:1. Introduction
2. Mite–Plant Relationship: General Aspects
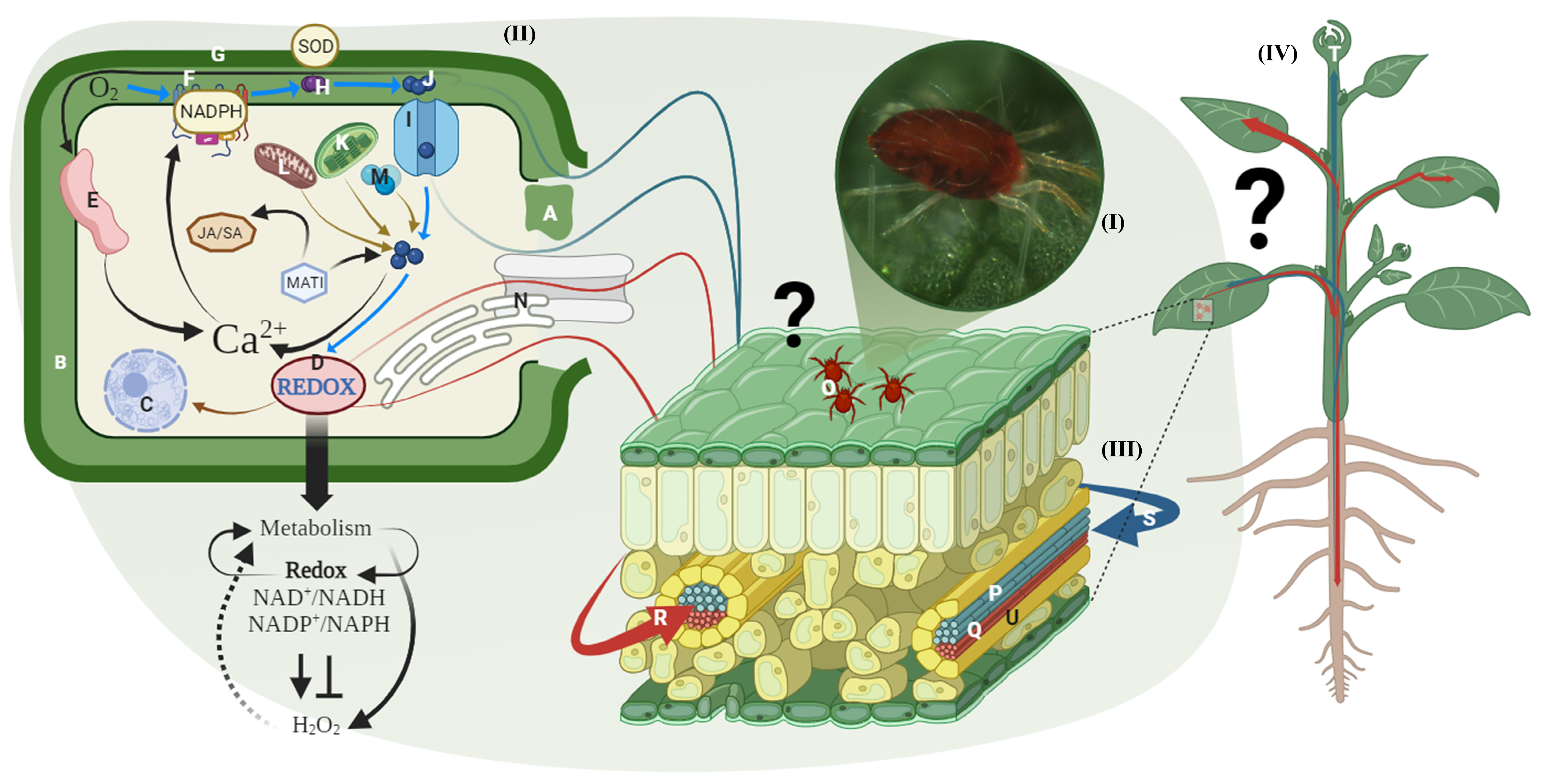
3. Phytophagous Mite Infestation Alters Plant Redox Homeostasis
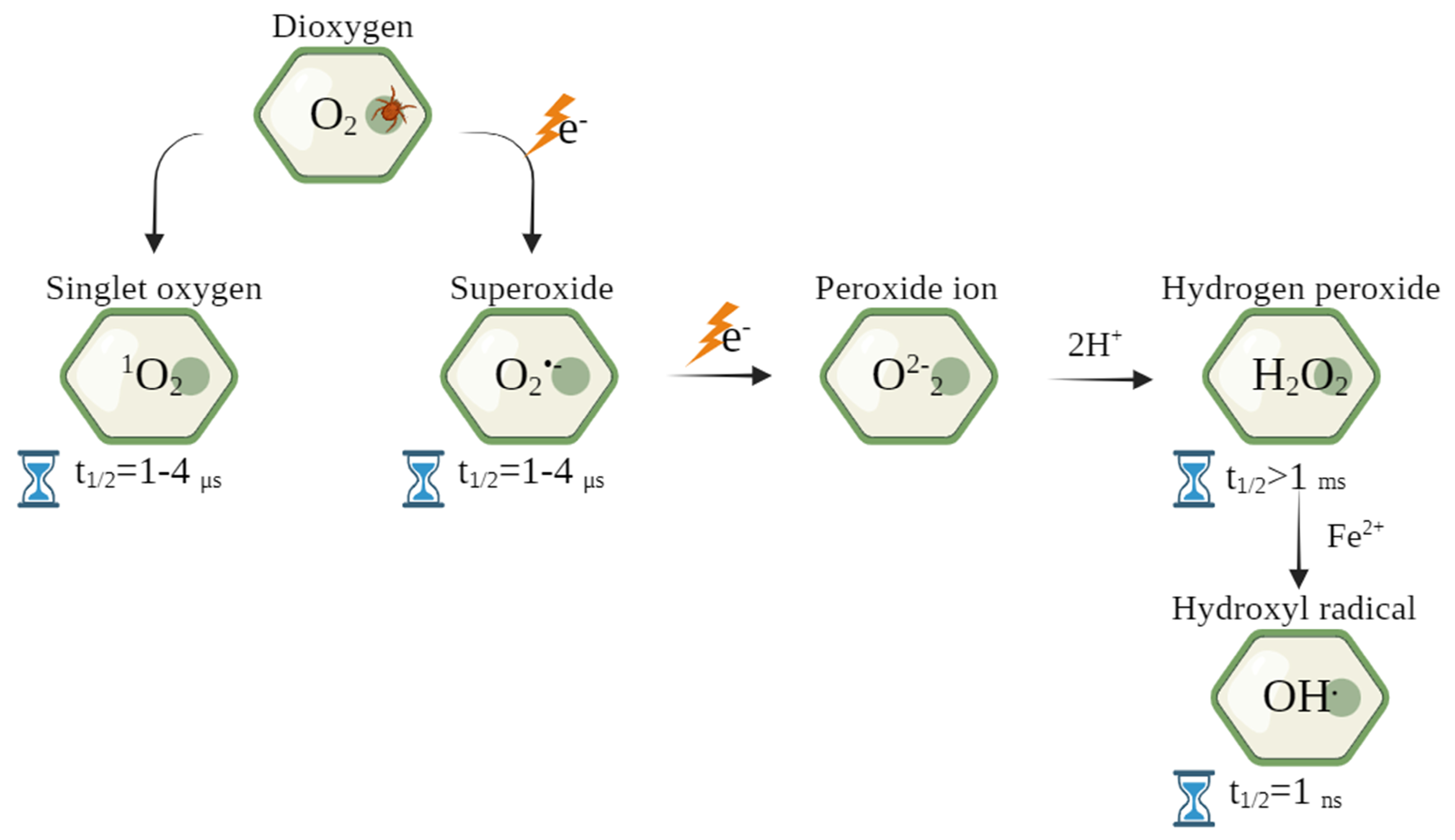
3.1. Production of Reactive Oxygen Species in Infested Plants

| Mite Family | Species | Plant | Cultivar | Time of Infestation | Levels of ROS (%) | References |
|---|---|---|---|---|---|---|
| Eriophyidae | Aceria tosichella | Hordeum vulgare | Airway | 18 * | ↑680 H2O2 ↓25 O2•− | [34] |
| Eriophyes tiliae | Tilia platyphyllos | − | − | in situ H2O2 | [25] | |
| Colomerus vitis | Vitis vinifera | Ghalati (Su) | 7 * | ↑3 H2O2 | [32] | |
| 14 * | ↑20 H2O2 | |||||
| 28 * | ↑32 H2O2 | |||||
| Rishbaba (Su) | 7 * | ↑9 H2O2 | ||||
| 14 * | ↑24 H2O2 | |||||
| 28 * | ↑56 H2O2 | |||||
| Neyshaboori (Su) | 7 * | ↑7 H2O2 | ||||
| 14 * | ↑15 H2O2 | |||||
| 28 * | ↑46 H2O2 | |||||
| Muscat (Su) | 7 * | ↑7 H2O2 | ||||
| 14 * | ↑48 H2O2 | |||||
| 28 * | ↑84 H2O2 | |||||
| White Thompson (Re) | 7 * | ↑25 H2O2 | ||||
| 14 * | ↑50 H2O2 | |||||
| 28 * | ↑104 H2O2 | |||||
| Sahebi (Re) | 7 * | ↑23 H2O2 | ||||
| 14 * | ↑53 H2O2 | |||||
| 28 * | ↑78 H2O2 | |||||
| Koladari (Re) | 7 * | ↑28 H2O2 | ||||
| 14 * | ↑62 H2O2 | |||||
| 28 * | ↑101 H2O2 | |||||
| Atabaki (Re) | 7 * | ↑9 H2O2 | ||||
| 14 * | ↑24 H2O2 | |||||
| 28 * | ↑105 H2O2 | |||||
| Tenuipalpidae | Brevipalpus yothersi | Arabidopsis thaliana | genotypes + | 6 ** | in situ H2O2 | [26] |
| 12 ** | in situ H2O2 | |||||
| 24 ** | in situ H2O2 | |||||
| 8 * | in situ H2O2 | |||||
| Tetranychidae | Schizotetranychus oryzae | Oryza sativa | IRGA 424 | 60 * | in situ H2O2 and O2•− | [21] |
| Tetranychidae | Tetranychus macfarlanei | Plumbago zeylanica | − | 0 * | ↑2 H2O2 | [39] |
| 15 * | ↑19 H2O2 | |||||
| 30 * | ↑60 H2O2 | |||||
| 60 * | ↑92 H2O2 | |||||
| 90 * | ↑134 H2O2 | |||||
| 120 * | ↑92 H2O2 | |||||
| Tetranychus urticae | Phaseolus vulgaris | Bronco | 55 * | ↑110 H2O2 | [31] | |
| Arabidopsis thaliana | genotypes + | 24 ** | ↑119 H2O2 | [40] | ||
| Medicago truncatula | ecotype + | 1 * | ↑100 H2O2 | [41] | ||
| Ocimum basilicum | − | 1 * | ↑300 H2O2 | [42] | ||
| 7 * | ↑444 H2O2 | |||||
| 14 * | ↑233 H2O2 | |||||
| Melissa officinalis | − | 1 * | ↑32 H2O2 | |||
| 7 * | ↑8 H2O2 | |||||
| 14 * | ↑12 H2O2 | |||||
| Salvia officinalis | − | 1 * | ↑82 H2O2 | |||
| 7 * | ↑12 H2O2 | |||||
| 14 * | ↑106 H2O2 | |||||
| Ocimum basilicum | Sweet basil (Su) | 1 * | ↑280 H2O2 | [33] | ||
| 7 * | ↑390 H2O2 | |||||
| 14 * | ↑190 H2O2 | |||||
| Purpurascens (Su) | 1 * | ↑200 H2O2 | ||||
| 7 * | ↑210 H2O2 | |||||
| 14 * | ↑180 H2O2 | |||||
| Fino Verde (Su) | 1 * | ↑25 H2O2 | ||||
| 7 * | ↓10 H2O2 | |||||
| 14 * | ↓5 H2O2 |
3.2. Action of Antioxidant Enzymes as a Defense Mechanism on Infested Plants
| Mite Family | Species | Plant | Cultivar | Time of Infestation | Antioxidant Enzyme Levels (%) | References |
|---|---|---|---|---|---|---|
| Eriophyidae | Aceria cladophthirus | Solanum dulcamara | − | 3 * | ↑850 POD | [66] |
| Aculops lycopersici | Lycopersicon esculentum | Castlemart | 4 * | ↑239 POD, ↑100 LOX, ↑57 PPO | [67] | |
| Colomerus vitis | Vitis vinifera | Ghalati (Su) | 7 * | ↑24 SOD, ↑5 CAT, 0 POD, ↓2 PAL, ↑8 PPO | [32] | |
| 14 * | ↓15 SOD, ↓6 CAT, ↑16 POD, ↓0 PAL, ↑2 PPO | |||||
| 28 * | ↑36 SOD, ↓9 CAT, ↑25 POD, ↑13 PAL, ↑36 PPO | |||||
| Rishbaba (Su) | 7 * | 0 SOD, ↑4 CAT, ↑1 POD, ↑2 PAL, ↓3 PPO | ||||
| 14 * | ↑66 SOD, ↑8 CAT, ↑27 POD, ↑3 PAL, ↑31 PPO | |||||
| 28 * | ↑61 SOD, ↑7 CAT, ↑80 POD, ↑3 PAL, ↑50 PPO | |||||
| Neyshaboori (Su) | 7 * | ↓2 SOD, ↑6 CAT, ↓16 POD, ↑6 PAL, ↓5 PPO | ||||
| 14 * | ↑4 SOD, ↓5 CAT, ↑13 POD, ↑28 PAL, ↑8 PPO | |||||
| 28 * | ↑1 SOD, ↓10 CAT, ↑41 POD, ↑23 PAL, ↑25 PPO | |||||
| Muscat (Su) | 7 * | ↑24 SOD, ↑7 CAT, 0 POD, ↑12 PAL, ↑5 PPO | ||||
| 14 * | ↑68 SOD, ↑2 CAT, ↓2 POD, ↑8 PAL, ↑10 PPO | |||||
| 28 * | ↑56 SOD, ↑15 CAT, ↑81 POD, 0 PAL, ↑54 PPO | |||||
| Eriophyidae | Colomerus vitis | Vitis vinifera | White Thompson (Re) | 7 * | ↑27 SOD, ↑9 CAT, ↑37 POD, ↑3 PAL, ↑24 PPO | [32] |
| 14 * | ↑152 SOD, ↑17 CAT, ↑74 POD, ↑3 PAL, ↑58 PPO | |||||
| 28 * | ↑61 SOD, ↑23 CAT, ↑71 POD, ↑13 PAL, ↑80 PPO | |||||
| Sahebi (Re) | 7 * | ↑46 SOD, ↑68 CAT, ↑33 POD, ↑12 PAL, ↑43 PPO | ||||
| 14 * | ↑20 SOD, ↑80 CAT, ↑1 POD, ↑4 PAL, ↑167 PPO | |||||
| 28 * | ↑53 SOD, ↑75 CAT, ↑19 POD, ↑25 PAL, ↑178 PPO | |||||
| Koladari (Re) | 7 * | ↑69 SOD, ↑13 CAT, ↑63 POD, ↑14 PAL, ↑31 PPO | ||||
| 14 * | ↑61 SOD, ↑19 CAT, ↑73 POD, ↑12 PAL, ↑42 PPO | |||||
| 28 * | ↑126 SOD, ↑14 CAT, ↑101 POD, ↑18 PAL, ↑78 PPO | |||||
| Atabaki (Re) | 7 * | ↑84 SOD, ↑8 CAT, ↑41 POD, ↑12 PAL, ↑32 PPO | ||||
| 14 * | ↑162 SOD, ↑14 CAT, ↑66 POD, ↑7 PAL, ↑49 PPO | |||||
| 28 * | ↑131 SOD, ↑21 CAT, ↑81 POD, ↑10 PAL, ↑74 PPO | |||||
| Cabernet Sauvignon | 15 * | ↑168 SOD, ↑2167 CAT, ↓27 POD, ↑82 PPO | [56] | |||
| Aceria tristriata | Juglans regia | Chandler | 15 * | ↑113 POD, ↑66 PPO | [68] | |
| Hartly | 15 * | ↑4 POD, ↑32 PPO | ||||
| Pedro | 15 * | ↑81 POD, ↑57 PPO | ||||
| Jamal | 15 * | ↑38 POD, ↑76 PPO | ||||
| Franquette | 15 * | ↑33 POD, ↑24 PPO | ||||
| Lara | 15 * | ↑8 POD, ↑15 PPO | ||||
| genotypes + | 15 * | ↑45 POD, ↑53 PPO | ||||
| Eriophyidae | Aceria tosichella | Hordeum vulgare | Airway | 18* | ↓30 SOD, ↓39 CAT, ↓21 POD, ↓36 APX, ↓22 DHAR, ↓29 GR, ↑27 GSNOR, ↑20 ARG | [34] |
| Tetranychidae | Schizotetranychus oryzae | Oryza sativa | IRGA 424 | 60* | ↑57 POD, ↑18 GST | [21] |
| − | ↑100 GST | [69] | ||||
| Tetranychus macfarlanei | Plumbago zeylanica | − | 0 * | 0 SOD, ↑5 CAT | [39] | |
| 15 * | ↓11 SOD, ↓17 CAT | |||||
| 30 * | ↓38 SOD, ↓32 CAT | |||||
| 60 * | ↓47 SOD, ↓42 CAT | |||||
| 90 * | ↓54 SOD, ↓46 CAT | |||||
| 120 * | ↓64 SOD, ↓53 CAT | |||||
| Tetranychus evansi | Solanum lycopersicum | Moneymaker | 4 * | ↑152 POD, ↑127 PPO | [70] | |
| 10 * | ↑88 POD, ↑58 PPO | |||||
| Tetranychus urticae | Phaseolus vulgaris | Bronco | 55 * | ↓37 CAT, ↑43 POD | [31] | |
| Cucumis melo | genotypes + | 2 * | ↑50 POD, 0 PPO | [71] | ||
| 4 * | ↑50 POD, 0 PPO | |||||
| 6 * | 0 POD, ↑33 PPO | |||||
| 8 * | 0 POD, ↑33 PPO | |||||
| Arabidopsis thaliana | genotypes + | 24 ** | ↓5 CAT, ↑24 APX, ↑14 DHAR, ↓17 GR | [40] | ||
| Ocimum basilicum | − | 1 * | ↓40 CAT, ↑131 GPOX | [42] | ||
| 7 * | ↓20 CAT, ↑275 GPOX | |||||
| 14 * | ↓60 CAT, ↑525 GPOX | |||||
| Melissa officinalis | − | 1 * | ↑8 CAT, ↑1900 GPOX | |||
| 7 * | ↑8 CAT, ↑3400 GPOX | |||||
| 14 * | ↑31 CAT, ↑1400 GPOX | |||||
| Tetranychidae | Tetranychus urticae | Salvia officinalis | − | 1 * | ↑6 CAT, ↑1346 GPOX | [42] |
| 7 * | ↓67 CAT, ↑1247 GPOX | |||||
| 14 * | ↓72 CAT, ↑573 GPOX | |||||
| Humulus lupulus | Hallertauer Mittelfruh | 10* | ↑100 POD | [72] | ||
| Glycine max | Williams (Re) | 20 * | ↑96 SOD, ↓25 CAT, ↑13 POD, ↑920 LOX | [73] | ||
| 34 * | ↑17 SOD, ↑14 CAT, ↑31 POD, ↑270 LOX | |||||
| Bonus (Su) | 20 * | ↓30 SOD, ↑17 CAT, ↑36 POD, ↑73 LOX | ||||
| 34 * | ↓44 SOD, ↑107 CAT, ↑72 POD | |||||
| Ocimum basilicum | Sweet basil (Su) | 1 * | ↓67 CAT, ↑250 GPOX | [33] | ||
| 7 * | ↓17 CAT, ↑120 GPOX | |||||
| 14 * | ↓67 CAT, ↑900 GPOX | |||||
| Purpurascens (Su) | 1 * | ↑76 CAT, ↑100 GPOX | ||||
| 7 * | ↓60 CAT, ↑120 GPOX | |||||
| 14 * | ↓16 CAT, ↑380 GPOX | |||||
| Fino Verde (Su) | 1 * | ↓50 CAT, ↑1081 GPOX | ||||
| 7 * | ↓100 CAT, ↑1869 GPOX | |||||
| 14 * | 0 CAT, ↑1869 GPOX | |||||
| Zea mays | Bosman | 6 * | ↑4 SOD, ↓67 CAT, ↓6 APX, ↑67 GR, ↑7 POD, ↓8 PPO | [74] |
4. Lipid Peroxidation and Physiological Responses
4.1. Infested Plants and Oxidative Stress
4.2. Mites: Dangerous or Promising?
5. Molecular Responses Produced by Oxidative Stress: Transcriptomic Responses and Transcription Factors Engaged in Redox State Regulation in Mite-Infested Plants
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faucon, M.P.; Houben, D.; Lambers, H. Plant Functional Traits: Soil and Ecosystem Services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Czaja, M.; Kołton, A.; Muras, P. The Complex Issue of Urban Trees—Stress Factor Accumulation and Ecological Service Possibilities. Forests 2020, 11, 932. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Poswal, F.S.; Russell, G.; Mackonochie, M.; MacLennan, E.; Adukwu, E.C.; Rolfe, V. Herbal Teas and Their Health Benefits: A Scoping Review. Plant Foods Hum. Nutr. 2019, 74, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Parise, A.G.; Gagliano, M.; Souza, G.M. Extended Cognition in Plants: Is It Possible? Plant Signal. Behav. 2020, 15, 1710661. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Fichman, Y.; Devireddy, A.R.; Sengupta, S.; Azad, R.K.; Mittler, R. Systemic Signaling during Abiotic Stress Combination in Plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13810–13820. [Google Scholar] [CrossRef] [PubMed]
- Padilha, G.; Fiorin, R.A.; Filho, A.C.; Pozebon, H.; Rogers, J.; Marques, R.P.; Castilhos, L.B.; Donatti, A.; Stefanelo, L.; Burtet, L.M.; et al. Damage Assessment and Economic Injury Level of the Two-Spotted Spider Mite Tetranychus urticae in Soybean. Pesqui. Agropecu. Bras. 2020, 55, e01836. [Google Scholar] [CrossRef]
- Savary, S.; Ficke, A.; Aubertot, J.N.; Hollier, C. Crop Losses Due to Diseases and Their Implications for Global Food Production Losses and Food Security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- McDonald, B.A.; Stukenbrock, E.H. Rapid Emergence of Pathogens in Agro-Ecosystems: Global Threats to Agricultural Sustainability and Food Security. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160026. [Google Scholar] [CrossRef]
- Hirakuri, M.H. Perdas Econômicas Geradas Por Estresses Bióticos e Abióticos Na Produção Brasileira de Soja No Período 2016–2020; Circular Técnica (INFOTECA-E); Embrapa Soja: Londrina, Brazil, 2021. [Google Scholar]
- Santamaria, M.; Arnaiz, A.; Rosa-Diaz, I.; González-Melendi, P.; Romero-Hernandez, G.; Ojeda-Martinez, D.A.; Garcia, A.; Contreras, E.; Martinez, M.; Diaz, I. Plant Defenses Against Tetranychus urticae: Mind the Gaps. Plants 2020, 9, 464. [Google Scholar] [CrossRef]
- Song, J.; Yang, F.; Xun, M.; Xu, L.; Tian, X.; Zhang, W.; Yang, H. Genome-Wide Identification and Characterization of Vacuolar Processing Enzyme Gene Family and Diverse Expression Under Stress in Apple (Malus × Domestic). Front. Plant Sci. 2020, 11, 522810. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, G.J.; Flechtmann, C.H.W. Manual de Acarologia: Acarologia Básica e Ácaros de Plantas Cultivadas No Brasil; Holos: Ribeirão Preto, Brazil, 2008. [Google Scholar]
- Vacante, V. The Handbook of Mites of Economic Plants: Identification, Bio-Ecology and Control; CABI International: Wallingford, UK, 2016. [Google Scholar]
- Dicke, M.; Sabelis, M.W. How Plants Obtain Predatory Mites as Bodyguards. Neth. J. Zool. 1987, 38, 148–165. [Google Scholar] [CrossRef]
- Godinho, D.P.; Janssen, A.; Dias, T.; Cruz, C.; Magalhães, S. Down-Regulation of Plant Defence in a Resident Spider Mite Species and Its Effect upon Con- and Heterospecifics. Oecologia 2016, 180, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Stahl, E.; Hilfiker, O.; Reymond, P. Plant–Arthropod Interactions: Who Is the Winner? Plant J. 2018, 93, 703–728. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, S.; Fan, B.; Zhu, C.; Chen, Z. The Mediator Complex: A Central Coordinator of Plant Adaptive Responses to Environmental Stresses. Int. J. Mol. Sci. 2022, 23, 6170. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Janku, M.; Luhová, L.; Petrivalský, M. On the Origin and Fate of Reactive Oxygen Species in Plant Cell Compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef]
- Blasi, É.A.R.; Buffon, G.; Rativa, A.G.S.; Lopes, M.C.B.; Berger, M.; Santi, L.; Lavallée-Adam, M.; Yates, J.R.; Schwambach, J.; Beys-da-Silva, W.O.; et al. High Infestation Levels of Schizotetranychus Oryzae Severely Affects Rice Metabolism. J. Plant Physiol. 2017, 219, 100–111. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- de Lillo, E.; Pozzebon, A.; Valenzano, D.; Duso, C. An Intimate Relationship between Eriophyoid Mites and Their Host Plants—A Review. Front. Plant Sci. 2018, 9, 418308. [Google Scholar] [CrossRef]
- Malagnini, V.; de Lillo, E.; Saldarelli, P.; Beber, R.; Duso, C.; Raiola, A.; Zanotelli, L.; Valenzano, D.; Giampetruzzi, A.; Morelli, M.; et al. Transmission of Grapevine Pinot Gris Virus by Colomerus Vitis (Acari: Eriophyidae) to Grapevine. Arch. Virol. 2016, 161, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- Guedes, L.M.; Sanhueza, C.; Torres, S.; Figueroa, C.; Gavilán, E.; Pérez, C.I.; Aguilera, N. Gall-Inducing Eriophyes iliae Stimulates the Metabolism of Tilia platyphyllos Leaves towards Oxidative Protection. Plant Physiol. Biochem. 2023, 195, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Arena, G.D.; Ramos-González, P.L.; Nunes, M.A.; Ribeiro-Alves, M.; Camargo, L.E.A.; Kitajima, E.W.; Machado, M.A.; Freitas-Astúa, J. Citrus Leprosis Virus C Infection Results in Hypersensitive-like Response, Suppression of the JA/ET Plant Defense Pathway and Promotion of the Colonization of Its Mite Vector. Front. Plant Sci. 2016, 7, 1757. [Google Scholar] [CrossRef] [PubMed]
- Agut, B.; Pastor, V.; Jaques, J.A.; Flors, V. Can Plant Defence Mechanisms Provide New Approaches for the Sustainable Control of the Two-Spotted Spider Mite Tetranychus urticae? Int. J. Mol. Sci. 2018, 19, 614. [Google Scholar] [CrossRef] [PubMed]
- Migeon, A.; Dorkeld, F. Spider Mites Web: A Comprehensive Database for Tetranychidae. Available online: https://www1.montpellier.inra.fr/CBGP/spmweb (accessed on 22 March 2024).
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide Resistance Mechanisms in the Two-Spotted Spider Mite Tetranychus urticae and Other Important Acari: A Review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Noggle, G.R. The Organization of Plants. In Introductory Plant Physiology; Noggle, G.R., Fritz, G.J., Eds.; Prentice Hall: Englewood Cliffs, NJ, USA, 1983; pp. 9–38. [Google Scholar]
- Farouk, S.; Osman, M.A. Alleviation of Oxidative Stress Induced by Spider Mite Invasion through Application of Elicitors in Bean Plants. Egypt. J. Biol. 2012, 14, 1–13. [Google Scholar] [CrossRef]
- Javadi Khederi, S.; Khanjani, M.; Gholami, M.; Panzarino, O.; de Lillo, E. Influence of the Erineum Strain of Colomerus vitis (Acari: Eriophyidae) on Grape (Vitis vinifera) Defense Mechanisms. Exp. Appl. Acarol. 2018, 75, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Golan, K.; Kot, I.; Górska-Drabik, E.; Jurado, I.G.; Kmieć, K.; Åagowska, B. Physiological Response of Basil Plants to Twospotted Spider Mite (Acari: Tetranychidae) Infestation. J. Econ. Entomol. 2019, 112, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Labudda, M.; Tokarz, K.; Tokarz, B.; Muszyńska, E.; Gietler, M.; Górecka, M.; Różańska, E.; Rybarczyk-Płońska, A.; Fidler, J.; Prabucka, B.; et al. Reactive Oxygen Species Metabolism and Photosynthetic Performance in Leaves of Hordeum vulgare Plants Co-Infested with Heterodera filipjevi and Aceria tosichella. Plant Cell Rep. 2020, 39, 1719–1741. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive Oxygen Species (ROS): Beneficial Companions of Plants’ Developmental Processes. Front. Plant Sci. 2016, 7, 186069. [Google Scholar] [CrossRef]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-Induced Reactive Oxygen Species Compartmentalization, Perception and Signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Kolupaev, Y.E.; Yastreb, T.O.; Ryabchun, N.I.; Kokorev, A.I.; Kolomatska, V.P.; Dmitriev, A.P. Redox Homeostasis of Cereals during Acclimation to Drought. Theor. Exp. Plant Physiol. 2023, 35, 133–168. [Google Scholar] [CrossRef]
- Perkel, J.M. The Software That Powers Scientific Illustration. Nature 2020, 582, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, M.; Dewanjee, S.; Chakraborty, K.; Ali, M.N.; Gupta, S.K. Continued Foliar Herbivory by the Red Spider Mite Tetranychus macfarlenei on Plumbago zeylanica Severely Reduces the Levels of a Medicinally Important Metabolite in the Roots. J. Plant Interact. 2014, 9, 529–538. [Google Scholar] [CrossRef]
- Santamaría, M.E.; Arnaiz, A.; Velasco-Arroyo, B.; Grbic, V.; Diaz, I.; Martinez, M. Arabidopsis Response to the Spider Mite Tetranychus urticae Depends on the Regulation of Reactive Oxygen Species Homeostasis. Sci. Rep. 2018, 8, 9432. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Fragkoudi, I.; Martinou, A.; Stavrinides, M.C.; Fotopoulos, V. Spatial Response of Medicago truncatula Plants to Drought and Spider Mite Attack. Plant Physiol. Biochem. 2018, 130, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Golan, K.; Jurado, I.G.; Kot, I.; Górska-Drabik, E.; Kmieć, K.; Łagowska, B.; Skwaryło-Bednarz, B.; Kopacki, M.; Jamiołkowska, A. Defense Responses in the Interactions between Medicinal Plants from Lamiaceae Family and the Two-Spotted Spider Mite Tetranychus urticae Koch (Acari: Tetranychidae). Agronomy 2021, 11, 438. [Google Scholar] [CrossRef]
- Mittler, R.; Jones, D.P. The Redox Code of Plants. Plant Cell Environ. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H. How Plant Cells Sense the Outside World through Hydrogen Peroxide. Nature 2020, 578, 518–519. [Google Scholar] [CrossRef]
- Iida, J.; Desaki, Y.; Hata, K.; Uemura, T.; Yasuno, A.; Islam, M.; Maffei, M.E.; Ozawa, R.; Nakajima, T.; Galis, I.; et al. Tetranins: New Putative Spider Mite Elicitors of Host Plant Defense. New Phytol. 2019, 224, 875–885. [Google Scholar] [CrossRef]
- Galviz, Y.; Souza, G.M.; Lüttge, U. The Biological Concept of Stress Revisited: Relations of Stress and Memory of Plants as a Matter of Space–Time. Theor. Exp. Plant Physiol. 2022, 34, 239–264. [Google Scholar] [CrossRef]
- Altaf, F.; Parveen, S.; Farooq, S.; Lone, M.L.; Haq, A.U.; Tahir, I. Enigmas of Senescence: A Reappraisal on the Hormonal Crosstalk and the Molecular Mechanisms. Theor. Exp. Plant Physiol. 2024, 36, 51–81. [Google Scholar] [CrossRef]
- Silveira, J.A.G.; Sousa, R.H.V. Looking for a Systemic Concept and Physiological Diagnosis of a Plant Stress State. Theor. Exp. Plant Physiol. 2024. ahead of print. [Google Scholar] [CrossRef]
- Isaias, R.M.S.; Oliveira, D.C.; Moreira, A.S.F.P.; Soares, G.L.G.; Carneiro, R.G.S. The Imbalance of Redox Homeostasis in Arthropod-Induced Plant Galls: Mechanisms of Stress Generation and Dissipation. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.G.; Carneiro, R.G.S.; Isaias, R.M.S. Multivesicular Bodies Differentiate Exclusively in Nutritive Fast-Dividing Cells in Marcetia taxifolia Galls. Protoplasma 2015, 252, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Keifer, H.H.; Baker, E.W.; Kono, T.; Delfinado, M.; Styler, W.E. An Illustrated Guide to Plant Abnormalities Caused by Eriophyid Mites in North America; Agriculture Handbook; USDA: Washington, DC, USA, 1982. [Google Scholar]
- Ferraz, C.S.; Ataide, L.M.S.; Gondim, M.G.C.; Pallini, A. Arthropods Associated with the Lychee Erinose Mite, Aceria litchii (Acari: Eriophyidae) on Lychee Trees in Minas Gerais, Brazil. Exp. Appl. Acarol. 2022, 88, 289–300. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Muphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed Editora Ltda: Porto Alegre, RS, Brazil, 2017. [Google Scholar]
- Schneider, J.R.; Caverzan, A.; Chavarria, G. Water Deficit Stress, ROS Involvement, and Plant Performance. Arch. Agron. Soil. Sci. 2019, 65, 1160–1181. [Google Scholar] [CrossRef]
- Wünsche, H.; Baldwin, I.T.; Wu, J. S-Nitrosoglutathione Reductase (GSNOR) Mediates the Biosynthesis of Jasmonic Acid and Ethylene Induced by Feeding of the Insect Herbivore Manduca sexta and Is Important for Jasmonate-Elicited Responses in Nicotiana Attenuata. J. Exp. Bot. 2011, 62, 4605–4616. [Google Scholar] [CrossRef]
- Shi, W.; He, W.; Zhang, Z.; Sun, J.; Zhu, C.; Liu, Z.; Xu, Y.; Zhao, B. Study on the Resistance of ‘Cabernet Sauvignon’ Grapevine with Different Rootstocks to Colomerus vitis. Sustainability 2022, 14, 15193. [Google Scholar] [CrossRef]
- Schneider, J.R.; Müller, M.; Klein, V.A.; Rossato-Grando, L.G.; Barcelos, R.P.; Dalmago, G.A.; Chavarria, G. Soybean Plant Metabolism under Water Deficit and Xenobiotic and Antioxidant Agent Application. Biology 2020, 9, 266. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 121942. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide Dismutase—Mentor of Abiotic Stress Tolerance in Crop Plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, F.R.; Oliveira, J.T.A.; Martins-Miranda, A.S.; Viégas, R.A.; Silveira, J.A.G. Superoxide Dismutase, Catalase and Peroxidase Activities Do Not Confer Protection against Oxidative Damage in Salt-Stressed Cowpea Leaves. New Phytol. 2004, 163, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Godinho, D.P.; Janssen, A.; Li, D.; Cruz, C.; Magalhães, S. The Distribution of Herbivores between Leaves Matches Their Performance Only in the Absence of Competitors. Ecol. Evol. 2020, 10, 8405–8415. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Noctor, G.; Baker, A. Plant Catalases: Peroxisomal Redox Guardians. Arch. Biochem. Biophys. 2012, 525, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Teng, X.L.; Xiao, X.G. Subcellular Localization of a Plant Catalase-Phenol Oxidase, AcCATPO, from Amaranthus and Identification of a Non-Canonical Peroxisome Targeting Signal. Front. Plant Sci. 2017, 8, 269801. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.H.; Carvalho, F.E.; Daloso, D.M.; Lima-Melo, Y.; Margis-Pinheiro, M.; Komatsu, S.; Silveira, J.A. Impairment in Photosynthesis Induced by CAT Inhibition Depends on the Intensity of Photorespiration and Peroxisomal APX Expression in Rice. Plant Physiol. Biochem. 2023, 203, 108066. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Bronner, R.; Westphal, E.; Dreger, F. Enhanced Peroxidase Activity Associated with the Hypersensitive Response of Solanum dulcamara to the Gall Mite Aceria cladophthirus (Acari: Eriophyoidea). Can. J. Bot. 2011, 69, 2192–2196. [Google Scholar] [CrossRef]
- Stout, M.J.; Workman, K.V.; Duffey, S.S. Identity, Spatial Distribution, and Variability of Induced Chemical Responses in Tomato Plants. Entomol. Exp. Appl. 1996, 79, 255–271. [Google Scholar] [CrossRef]
- Ahmad–Hosseini, M.; Khanjani, M.; Karamian, R. Resistance of Some Commercial Walnut Cultivars and Genotypes to Aceria tristriata (Nalepa) (Acari: Eriophyidae) and Its Correlation with Some Plant Features. Pest. Manag. Sci. 2020, 76, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Buffon, G.; Blasi, É.A.R.; Adamski, J.M.; Ferla, N.J.; Berger, M.; Santi, L.; Lavallée-Adam, M.; Yates, J.R.; Beys-Da-Silva, W.O.; Sperotto, R.A. Physiological and Molecular Alterations Promoted by Schizotetranychus oryzae Mite Infestation in Rice Leaves. J. Proteome Res. 2016, 15, 431–446. [Google Scholar] [CrossRef]
- Ximénez-Embún, M.G.; Ortego, F.; Castañera, P. Drought-Stressed Tomato Plants Trigger Bottom–Up Effects on the Invasive Tetranychus evansi. PLoS ONE 2016, 11, e0145275. [Google Scholar] [CrossRef] [PubMed]
- Shoorooei, M.; Lotfi, M.; Nabipour, A.; Mansouri, A.I.; Kheradmand, K.; Zalom, F.G.; Madadkhah, E.; Parsafar, A. Antixenosis and Antibiosis of Some Melon (Cucumis melo) Genotypes to the Two-Spotted Spider Mite (Tetranychus urticae) and a Possible Mechanism for Resistance. J. Hortic. Sci. Biotechnol. 2013, 88, 73–78. [Google Scholar] [CrossRef]
- Trevisan, M.T.S.; Scheffer, J.J.C.; Verpoorte, R. Peroxidase Activity in Hop Plants after Infestation by Red Spider Mites. Crop Prot. 2003, 22, 423–424. [Google Scholar] [CrossRef]
- Hildebrand, D.F.; Rodriguez, J.G.; Brown, G.C.; Luu, K.T.; Volden, C.S. Peroxidative Responses of Leaves in Two Soybean Genotypes Injured by Twospotted Spider Mites (Acari: Tetranychidae). J. Econ. Entomol. 1986, 79, 1459–1465. [Google Scholar] [CrossRef]
- Dworak, A.; Nykiel, M.; Walczak, B.; Miazek, A.; Szworst-Łupina, D.; Zagdańska, B.; Kiełkiewicz, M. Maize Proteomic Responses to Separate or Overlapping Soil Drought and Two-Spotted Spider Mite Stresses. Planta 2016, 244, 939–960. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.E.L.; Silveira, J.A.G. H2O2-Retrograde Signaling as a Pivotal Mechanism to Understand Priming and Cross Stress Tolerance in Plants. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Academic Press: Cambridge, MA, USA, 2020; pp. 57–78. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants Facing Oxidative Challenges—A Little Help from the Antioxidant Networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Felton, G.W.; Donato, K.; Del Vecchio, R.J.; Duffey, S.S. Activation of Plant Foliar Oxidases by Insect Feeding Reduces Nutritive Quality of Foliage for Noctuid Herbivores. J. Chem. Ecol. 1989, 15, 2667–2694. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kazemi, H. Changes in Peroxidase and Polyphenol Oxidase Activities in Susceptible and Resistant Wheat Heads Inoculated with Fusarium graminearum and Induced Resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Gulsen, O.; Eickhoff, T.; Heng-Moss, T.; Shearman, R.; Baxendale, F.; Sarath, G.; Lee, D. Characterization of Peroxidase Changes in Resistant and Susceptible Warm-Season Turfgrasses Challenged by Blissus occiduus. Arthropod Plant Interact. 2010, 4, 45–55. [Google Scholar] [CrossRef]
- Aljbory, Z.; Chen, M.S. Indirect Plant Defense against Insect Herbivores: A Review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Radja Commare, R.; Nandakumar, R.; Kandan, A.; Suresh, S.; Bharathi, M.; Raguchander, T.; Samiyappan, R. Pseudomonas fluorescens Based Bio-Formulation for the Management of Sheath Blight Disease and Leaffolder Insect in Rice. Crop Prot. 2002, 21, 671–677. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, X.; Jiang, H.; Hansen, J.; Jørgensen, B.; Moore, J.P.; Fu, P.; Wu, W.; Yang, B.; Ye, W.; et al. Comprehensive Leaf Cell Wall Analysis Using Carbohydrate Microarrays Reveals Polysaccharide-Level Variation between Vitis Species with Differing Resistance to Downy Mildew. Polymers 2021, 13, 1379. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.M. Phenolics in Ecological Interactions: The Importance of Oxidation. J. Chem. Ecol. 1993, 19, 1521–1552. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, V.; Raguchander, T.; Samiyappan, R. Induction of Defense-Related Proteins in Tomato Roots Treated with Pseudomonas fluorescens Pf1 and Fusarium oxysporum f. Sp. Lycopersici. Plant Soil. 2002, 239, 55–68. [Google Scholar] [CrossRef]
- Sonmez, D.A.; Urun, I.; Alagoz, D.; Attar, S.H.; Dogu, Z.; Yesil, B.; Wozniak, A.; Labudda, M.; Zydlik, Z.; Zydlik, P.; et al. Phenylalanine Ammonialyase and Invertase Activities in Strawberry Fruit during Ripening Progress. Acta Hortic. 2021, 1309, 947–953. [Google Scholar] [CrossRef]
- Siedow, J.N. Plant Lipoxygenase: Structure and Function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 145–188. [Google Scholar] [CrossRef]
- Croft, K.P.C.; Jüttner, F.; Slusarenko, A.J. Volatile Products of the Lipoxygenase Pathway Evolved from Phaseolus vulgaris (L.) Leaves Inoculated with Pseudomonas syringae Pv Phaseolicola. Plant Physiol. 1993, 101, 13–24. [Google Scholar] [CrossRef]
- Paré, P.W.; Tumlinson, J.H. De Novo Biosynthesis of Volatiles Induced by Insect Herbivory in Cotton Plants. Plant Physiol. 1997, 114, 1161–1167. [Google Scholar] [CrossRef]
- Silva, F.B.; De, M.G.; Oliveira, A.; Batista, R.B.; Pires, C.V.; Xavier, L.P.; Piovesan, N.D.; De Oliveira, J.A.; José, I.C.; Moreira, M.A. Função Fisiológica de Lipoxigenases de Folhas de Soja Submetidas Ao Ataque de Lagarta (Anticarsia Gemmatalis Hübner). Arq. Inst. Biol. 2002, 69, 67–74. [Google Scholar]
- Batista, R.B.; Oliveira, M.G.D.A.; Pires, C.V.; Piovesan, N.D.; De Rezende, S.T.; Moreira, M.A. Biochemical and Kinetic Characterization of Lipoxygenases of Two Soybean Genotypes Submitted to Leaf Spraying of Polyunsaturated Fatty Acids. Pesqui. Agropecu. Bras. 2002, 37, 1517–1524. [Google Scholar] [CrossRef]
- Grinberg, M.; Perl-Treves, R.; Palevsky, E.; Shomer, I.; Soroker, V. Interaction between Cucumber Plants and the Broad Mite, Polyphagotarsonemus Latus: From Damage to Defense Gene Expression. Entomol. Exp. Appl. 2005, 115, 135–144. [Google Scholar] [CrossRef]
- Saravitz, D.M.; Siedow, J.N. The Differential Expression of Wound-Inducible Lipoxygenase Genes in Soybean Leaves. Plant Physiol. 1996, 110, 287–299. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in Plants: An Integrated Overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Labudda, M. Lipid Peroxidation as a Biochemical Marker for Oxidative Stress During Drought. An Effective Tool for Plant Breeding; E-Wydawnictwo: Poznan, Poland, 2013. [Google Scholar]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and Proteins—Major Targets of Oxidative Modifications in Abiotic Stressed Plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, MS, USA, 2015. [Google Scholar]
- Banerjee, A.; Roychoudhury, A. Abiotic Stress, Generation of Reactive Oxygen Species, and Their Consequences: An Overview. In Reactive Oxygen Species in Plants: Boon or Bane—Revisiting the Role of ROS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 33–50. ISBN 9781119324928. [Google Scholar]
- Bensoussan, N.; Estrella Santamaria, M.; Zhurov, V.; Diaz, I.; Grbić, M.; Grbić, V. Plant-Herbivore Interaction: Dissection of the Cellular Pattern of Tetranychus urticae Feeding on the Host Plant. Front. Plant Sci. 2016, 7, 212761. [Google Scholar] [CrossRef]
- Park, Y.L.; Lee, J.H. Leaf Cell and Tissue Damage of Cucumber Caused by Twospotted Spider Mite (Acari: Tetranychidae). J. Econ. Entomol. 2002, 95, 952–957. [Google Scholar] [CrossRef]
- Livinali, E.; Sperotto, R.A.; Ferla, N.J.; de Souza, C.F.V. Physicochemical and Nutritional Alterations Induced by Two-Spotted Spider Mite Infestation on Strawberry Plants. Electron. J. Biotechnol. 2014, 17, 193–198. [Google Scholar] [CrossRef]
- Modarres Najafabadi, S.S.; Bagheri, A.; Seyahooei, M.A. Cucumber Cultivar Responses to Two Tetranychid Mites, Two-Spotted Spider Mite and Strawberry Spider Mite in Greenhouses. Syst. Appl. Acarol. 2019, 24, 1383–1393. [Google Scholar] [CrossRef]
- Ghongade, D.S.; Sood, A.K. Economic Injury Level for Tetranychus Urticae Koch on Parthenocarpic Cucumber under Protected Environment in North-Western Indian Himalayas. Phytoparasitica 2021, 49, 893–905. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Rodríguez-Serrano, M.; Corpas, F.J.; Gómez, M.; Del Río, L.A.; Sandalio, L.M. Cadmium-Induced Subcellular Accumulation of O2− and H2O2 in Pea Leaves. Plant Cell Environ. 2004, 27, 1122–1134. [Google Scholar] [CrossRef]
- Zhang, C.; Turgeon, R. Mechanisms of Phloem Loading. Curr. Opin. Plant Biol. 2018, 43, 71–75. [Google Scholar] [CrossRef]
- Keller, I.; Rodrigues, C.M.; Neuhaus, H.E.; Pommerrenig, B. Improved Resource Allocation and Stabilization of Yield under Abiotic Stress. J. Plant Physiol. 2021, 257, 153336. [Google Scholar] [CrossRef]
- Lal, M.K.; Sharma, N.; Adavi, S.B.; Sharma, E.; Altaf, M.A.; Tiwari, R.K.; Kumar, R.; Kumar, A.; Dey, A.; Paul, V.; et al. From Source to Sink: Mechanistic Insight of Photoassimilates Synthesis and Partitioning under High Temperature and Elevated [CO2]. Plant Mol. Biol. 2022, 110, 305–324. [Google Scholar] [CrossRef]
- Çetin, H.; Arslan, D.; Musa Özcan, M. Influence of Eriophyid Mites (Aculus olearius Castagnoli and Aceria oleae (Nalepa) (Acarina: Eriophyidae)) on Some Physical and Chemical Characteristics of Ayvalık Variety Olive Fruit. J. Sci. Food Agric. 2011, 91, 498–504. [Google Scholar] [CrossRef]
- Kemerich, G.T.; Johann, L.; Silva, D.E.; Ferla, N.J.; Volken de Souza, C.F. Effect of Mite Biological Control on the Physicochemical Properties and Bioactive Compounds Profile in Grapes of Merlot Variety. Phytoparasitica 2022, 50, 501–511. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Ferla, N.J.; Botton, M. European Red Spider Mite Panonychus ulmi (Koch) (Tetranychidae) Occurrence of Vineyards in Rio Grande Do Sul, Brazil. Ciência Rural. 2008, 38, 1758–1761. [Google Scholar] [CrossRef]
- Johann, L.; Klock, C.L.; Ferla, N.J.; Botton, M. Acarofauna (Acari) Associada à Videira (Vitis vinifera L.) No Estado Do Rio Grande Do Sul. Biociências 2009, 17, 1–19. [Google Scholar]
- Monetti, L.; Fernandez, N. Seasonal Populations Dynamics of the European Red Mite (Panonychus ulmi) and Its Predator Neoseiulus californicus in a Sprayed Apple Orchard in Argentina (Acari: Tetranychidae, Phytoseiidae). Acarologia 1995, 36, 325–331. [Google Scholar]
- De Resende, J.T.V.; Filho, R.B.D.L.; Ribeiro, L.K.; Corrêa, J.V.W.; Maciel, C.D.d.G.; Youssef, K. Strawberry Genotypes with Resistance to Tetranychus urticae Mediated by Leaf Trichomes. Ciência Agrotecnologia 2020, 44, e006920. [Google Scholar] [CrossRef]
- Oliveira, H.; Fadini, M.A.M.; Venzon, M.; Rezende, D.; Rezende, F.; Pallini, A. Evaluation of the Predatory Mite Phytoseiulus macropilis (Acari: Phytoseiidae) as a Biological Control Agent of the Two-Spotted Spider Mite on Strawberry Plants under Greenhouse Conditions. Exp. Appl. Acarol. 2009, 47, 275–283. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and Raffinose Constitute a Novel Function to Protect Plants from Oxidative Damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Schneider, T.; Keller, F. Raffinose in Chloroplasts Is Synthesized in the Cytosol and Transported across the Chloroplast Envelope. Plant Cell Physiol. 2009, 50, 2174–2182. [Google Scholar] [CrossRef]
- Peshev, D.; Vergauwen, R.; Moglia, A.; Hideg, É.; Van Den Ende, W. Towards Understanding Vacuolar Antioxidant Mechanisms: A Role for Fructans? J. Exp. Bot. 2013, 64, 1025–1038. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Yan, C.; Yang, N.; Wang, X.; Wang, Y. VqBGH40a Isolated from Chinese Wild Vitis Quinquangularis Degrades Trans-Piceid and Enhances Trans-Resveratrol. Plant Sci. 2021, 310, 110989. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Properties of Resveratrol: A Structure–Activity Insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Basu, S.; Varsani, S.; Louis, J. Altering Plant Defenses: Herbivore-Associated Molecular Patterns and Effector Arsenal of Chewing Herbivores. Mol. Plant-Microbe Interact. 2018, 31, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Fidler, J.; Graska, J.; Gietler, M.; Nykiel, M.; Prabucka, B.; Rybarczyk-Płońska, A.; Muszyńska, E.; Morkunas, I.; Labudda, M. PYR/PYL/RCAR Receptors Play a Vital Role in the Abscisic-Acid-Dependent Responses of Plants to External or Internal Stimuli. Cells 2022, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
- Nykiel, M.; Gietler, M.; Fidler, J.; Prabucka, B.; Rybarczyk-Płońska, A.; Graska, J.; Boguszewska-Mańkowska, D.; Muszyńska, E.; Morkunas, I.; Labudda, M. Signal Transduction in Cereal Plants Struggling with Environmental Stresses: From Perception to Response. Plants 2022, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Graska, J.; Fidler, J.; Gietler, M.; Prabucka, B.; Nykiel, M.; Labudda, M. Nitric Oxide in Plant Functioning: Metabolism, Signaling, and Responses to Infestation with Ecdysozoa Parasites. Biology 2023, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Bouwmeester, H.J.; Dicke, M.; Kappers, I.F. Transcriptional and Metabolite Analysis Reveal a Shift in Direct and Indirect Defences in Response to Spider-Mite Infestation in Cucumber (Cucumis sativus). Plant Mol. Biol. 2020, 103, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Labudda, M.; Różańska, E.; Czarnocka, W.; Sobczak, M.; Dzik, J.M. Systemic Changes in Photosynthesis and Reactive Oxygen Species Homeostasis in Shoots of Arabidopsis thaliana Infected with the Beet Cyst Nematode Heterodera schachtii. Mol. Plant Pathol. 2018, 19, 1690–1704. [Google Scholar] [CrossRef] [PubMed]
- Labudda, M.; Różańska, E.; Gietler, M.; Fidler, J.; Muszyńska, E.; Prabucka, B.; Morkunas, I. Cyst Nematode Infection Elicits Alteration in the Level of Reactive Nitrogen Species, Protein S-Nitrosylation and Nitration, and Nitrosoglutathione Reductase in Arabidopsis thaliana Roots. Antioxidants 2020, 9, 795. [Google Scholar] [CrossRef]
- Gietler, M.; Fidler, J.; Labudda, M.; Nykiel, M. Abscisic Acid—Enemy or Savior in the Response of Cereals to Abiotic and Biotic Stresses? Int. J. Mol. Sci. 2020, 21, 4607. [Google Scholar] [CrossRef]
- Woźniak, A.; Kęsy, J.; Glazińska, P.; Glinkowski, W.; Narożna, D.; Bocianowski, J.; Rucińska-Sobkowiak, R.; Mai, V.C.; Krzesiński, W.; Samardakiewicz, S.; et al. The Influence of Lead and Acyrthosiphon Pisum (Harris) on Generation of Pisum sativum Defense Signaling Molecules and Expression of Genes Involved in Their Biosynthesis. Int. J. Mol. Sci. 2023, 24, 10671. [Google Scholar] [CrossRef]
- Pingault, L.; Luong, T.K.N.; Louis, J.; Hein, G. Wheat Transcriptomic Responses to Extended Feeding by Wheat Curl Mites. Sci. Rep. 2022, 12, 12535. [Google Scholar] [CrossRef]
- Kiani, M.; Bryan, B.; Rush, C.; Szczepaniec, A. Transcriptional Responses of Resistant and Susceptible Wheat Exposed to Wheat Curl Mite. Int. J. Mol. Sci. 2021, 22, 2703. [Google Scholar] [CrossRef] [PubMed]
- Hoseinzadeh, A.H.; Soorni, A.; Shoorooei, M.; Mahani, M.T.; Amiri, R.M.; Allahyari, H.; Mohammadi, R. Comparative Transcriptome Provides Molecular Insight into Defense-Associated Mechanisms against Spider Mite in Resistant and Susceptible Common Bean Cultivars. PLoS ONE 2020, 15, e0228680. [Google Scholar] [CrossRef] [PubMed]
- Zumajo-Cardona, C.; Aguirre, M.; Castillo-Bravo, R.; Mizzotti, C.; Di Marzo, M.; Banfi, C.; Mendes, M.A.; Spillane, C.; Colombo, L.; Ezquer, I. Maternal Control of Triploid Seed Development by the TRANSPARENT TESTA 8 (TT8) Transcription Factor in Arabidopsis thaliana. Sci. Rep. 2023, 13, 1316. [Google Scholar] [CrossRef] [PubMed]
- Nesi, N.; Debeaujon, I.; Jond, C.; Pelletier, G.; Caboche, M.; Lepiniec, L. The TT8 Gene Encodes a Basic Helix-Loop-Helix Domain Protein Required for Expression of DFR and BAN Genes in Arabidopsis Siliques. Plant Cell 2000, 12, 1863–1878. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Grain, D.; Bobet, S.; Le Gourrierec, J.; Thévenin, J.; Kelemen, Z.; Lepiniec, L.; Dubos, C. Complexity and Robustness of the Flavonoid Transcriptional Regulatory Network Revealed by Comprehensive Analyses of MYB–BHLH–WDR Complexes and Their Targets in Arabidopsis Seed. New Phytol. 2014, 202, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The Flavonoid Biosynthetic Pathway in Arabidopsis: Structural and Genetic Diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Garcia, A.; Arnaiz, A.; Rosa-Diaz, I.; Romero-Hernandez, G.; Diaz, I.; Martinez, M. Comparative Transcriptomics Reveals Hidden Issues in the Plant Response to Arthropod Herbivores. J. Integr. Plant Biol. 2021, 63, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Martinez, D.; Martinez, M.; Diaz, I.; Estrella Santamaria, M. Spider Mite Egg Extract Modifies Arabidopsis Response to Future Infestations. Sci. Rep. 2021, 11, 17692. [Google Scholar] [CrossRef] [PubMed]
- Arena, G.D.; Ramos-González, P.L.; Rogerio, L.A.; Ribeiro-Alves, M.; Casteel, C.L.; Freitas-Astúa, J.; Machado, M.A. Making a Better Home: Modulation of Plant Defensive Response by Brevipalpus Mites. Front. Plant Sci. 2018, 9, 1147. [Google Scholar] [CrossRef]
- Chen, L.; Cui, Y.; Yao, Y.; An, L.; Bai, Y.; Li, X.; Yao, X.; Wu, K. Genome-Wide Identification of WD40 Transcription Factors and Their Regulation of the MYB-BHLH-WD40 (MBW) Complex Related to Anthocyanin Synthesis in Qingke (Hordeum vulgare L. Var. nudum Hook. f.). BMC Genom. 2023, 24, 166. [Google Scholar] [CrossRef]
- Viola, I.L.; Alem, A.L.; Jure, R.M.; Gonzalez, D.H. Physiological Roles and Mechanisms of Action of Class I TCP Transcription Factors. Int. J. Mol. Sci. 2023, 24, 5437. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Molina, A. Ethylene Response Factor 1 Mediates Arabidopsis Resistance to the Soilborne Fungus Fusarium oxysporum. Mol. Plant-Microbe Interact. 2007, 17, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed]
| Mite Family | Species | Plant | Cultivar | Time of Infestation | MDA (%) | References |
|---|---|---|---|---|---|---|
| Eriophyidae | Aceria tosichella | Hordeum vulgare | Airway | 18 * | ↑27 | [34] |
| Colomerus vitis | Vitis vinifera | Ghalati (Su) | 7 * | ↑1 | [32] | |
| 14 * | ↑83 | |||||
| 28 * | ↑94 | |||||
| Rishbaba (Su) | 7 * | ↓2 | ||||
| 14 * | ↑119 | |||||
| 28 * | ↑191 | |||||
| Neyshaboori (Su) | 7 * | ↑19 | ||||
| 14 * | ↑130 | |||||
| 28 * | ↑191 | |||||
| Muscat (Su) | 7 * | ↑17 | ||||
| 14 * | ↑203 | |||||
| 28* | ↑361 | |||||
| White Thompson (Re) | 7 * | ↑24 | ||||
| 14 * | ↑135 | |||||
| 28 * | ↑360 | |||||
| Sahebi (Re) | 7 * | ↑23 | ||||
| 14 * | ↑172 | |||||
| 28 * | ↑202 | |||||
| Koladari (Re) | 7 * | ↑19 | ||||
| 14 * | ↑145 | |||||
| 28 * | ↑294 | |||||
| Atabaki (Re) | 7 * | ↑28 | ||||
| 14 * | ↑159 | |||||
| 28 * | ↑397 | |||||
| Tetranychidae | Tetranychus macfarlanei | Plumbago zeylanica | − | 0 * | ↑2 | [39] |
| 15 * | ↑83 | |||||
| 30 * | ↑100 | |||||
| 60 * | ↑190 | |||||
| 90 * | ↑225 | |||||
| 120 * | ↑109 | |||||
| Tetranychus urticae | Phaseolus vulgaris | Bronco | 55 * | ↑75 | [31] | |
| Medicago truncatula | Ecotype + | 1 * | ↑33 | [41] | ||
| Ocimum basilicum | − | 1 * | ↑267 | [42] | ||
| 7 * | ↑292 | |||||
| 14 * | ↑339 | |||||
| Melissa officinalis | − | 1 * | ↑16 | |||
| 7 * | ↑23 | |||||
| 14 * | ↑78 | |||||
| Tetranychidae | Tetranychus urticae | Salvia officinalis | − | 1 * | ↑42 | [42] |
| 7 * | ↑55 | |||||
| 14 * | ↑71 | |||||
| Glycine max | Williams (Re) | 30 * | ↑14 | [73] | ||
| 33 * | ↑24 | |||||
| 37 * | ↑51 | |||||
| 40 * | ↑83 | |||||
| 44 * | ↑46 | |||||
| 47 * | ↑59 | |||||
| Bonus (Su) | 30 * | ↑28 | ||||
| 33 * | ↑16 | |||||
| 37 * | ↑71 | |||||
| 40 * | ↑58 | |||||
| 44 * | ↑40 | |||||
| 47 * | ↑35 | |||||
| Ocimum basilicum | Sweet basil (Su) | 1 * | ↑263 | [33] | ||
| 7 * | ↑300 | |||||
| 14 * | ↑250 | |||||
| Purpurascens (Su) | 1 * | ↑1400 | ||||
| 7 * | ↑2720 | |||||
| 14 * | ↑2460 | |||||
| Fino Verde (Su) | 1 * | ↑663 | ||||
| 7 * | ↑1175 | |||||
| 14 * | ↑2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wurlitzer, W.B.; Labudda, M.; Silveira, J.A.G.; Matthes, R.D.; Schneider, J.R.; Ferla, N.J. From Signaling to Stress: How Does Plant Redox Homeostasis Behave under Phytophagous Mite Infestation? Int. J. Plant Biol. 2024, 15, 561-585. https://doi.org/10.3390/ijpb15030043
Wurlitzer WB, Labudda M, Silveira JAG, Matthes RD, Schneider JR, Ferla NJ. From Signaling to Stress: How Does Plant Redox Homeostasis Behave under Phytophagous Mite Infestation? International Journal of Plant Biology. 2024; 15(3):561-585. https://doi.org/10.3390/ijpb15030043
Chicago/Turabian StyleWurlitzer, Wesley Borges, Mateusz Labudda, Joaquim Albenisio G. Silveira, Ronice Drebel Matthes, Julia Renata Schneider, and Noeli Juarez Ferla. 2024. "From Signaling to Stress: How Does Plant Redox Homeostasis Behave under Phytophagous Mite Infestation?" International Journal of Plant Biology 15, no. 3: 561-585. https://doi.org/10.3390/ijpb15030043
APA StyleWurlitzer, W. B., Labudda, M., Silveira, J. A. G., Matthes, R. D., Schneider, J. R., & Ferla, N. J. (2024). From Signaling to Stress: How Does Plant Redox Homeostasis Behave under Phytophagous Mite Infestation? International Journal of Plant Biology, 15(3), 561-585. https://doi.org/10.3390/ijpb15030043







