Abstract
Coffee cultivation facilitates foreign trade, which is important to the Mexican economy, particularly to the coffee growers of Jilotepec, Veracruz. However, in this region, the soil in which the coffee plants are grown is acidic and has low nutrient availability, making plants susceptible to pests and diseases. In this context, the use of mycorrhizal fungi has gained importance, due to the benefits that they provide in terms of the transport of nutrients and the development of plants, contributing to a reduction in the use of chemical fertilizers. This work aimed to determine the dominant Arbuscular mycorrhizal fungi (AMF) in the soil of coffee farms and evaluate the potential of sorghum as a trap plant for these organisms. As a result, ten morphotypes of AMF were detected in the coffee soil, with Glomus and Acaulospora being the dominant genera. It was found that their presence was related to the pH, clay, organic matter, and total carbon of the soil from the farms. The abundance of spores increased significantly (p < 0.05) between the initial count in the soil and the final count after propagation in the sorghum trap plants. The characteristic structures of mycorrhizal colonization and a high percentage of mycorrhizal colonization of the roots of the trap plants (Sorghum vulgare) were observed at 120 days after sowing. It is concluded that Glomus sp1, Glomus sp2, Glomus sp3, Glomus sp4, Rhizophagus clarus, and Acaulospora scrobiculata are the dominant morphotypes in the considered coffee plantation soils and that sorghum has high potential for favoring the propagation of native AMF through increasing their abundance and favoring high mycorrhizal colonization.
1. Introduction
Coffee is cultivated in 14 states of Mexico; however, most of the production in the country (90%) is concentrated in Chiapas, Veracruz, Puebla, and Oaxaca. In 2021, Mexico ranked 12th in the world for coffee production. The countries that stand out in the production of the Arabica type are Brazil, Colombia, Ethiopia, and Honduras, which together account for 71.9% of the global production; meanwhile, in the case of the Robusta type, Vietnam, Brazil, and Indonesia account for 77.6% of the global production. The winners of the global coffee trade at present are the large corporations, which is incongruous considering that the cultivation and production of coffee is carried out by small producers [1]. Fairtrade International [2] indicated that around 12.5 million coffee farms, of which 95% are less than five hectares in size, are owned by small farmers.
Coffee plantations are cultivated at elevations ranging from 300 to almost 2000 m above sea level and in areas with diverse climates, soils, and vegetation; however, coffee plantations develop better between 600 and 1200 m above sea level and are found mainly in hilly and mountainous areas on the slopes facing the Gulf of Mexico and the Pacific. The coffee-growing areas coincide with very rich and diverse regions, and coffee cultivation areas play a very important role in the dynamics of basins and in the conservation of soils, as they help to prevent the loss of soil on slopes [3].
Despite the coffee production areas in the state of Veracruz being extensive, they face various problems; for example, there are issues related to the soil and its composition, as the soil is typically acidic and deficient in nutrients. The soils where coffee is produced in Mexico are of volcanic origin and have a pH of 4.5–5.2 and a low availability of essential macronutrients [4], which results in a low production of the coffee crop. To address these issues, producers have resorted to the application of fertilizers; however, the excessive use of chemical fertilizers creates other problems, such as eutrophication, soil degradation and, subsequently, the loss of soil biodiversity [5].
In the soil, interactions occur between microorganisms, including mycorrhizae, which represent symbiotic associations established between fungi and plant roots [6]. AMF have been shown to have potential as a sustainable alternative that allows for the optimization of resources, as they offer multiple benefits such as greater tolerance to abiotic stressors [7], bioremediation after the accumulation of heavy metals [8], bioprotection [9], improvement of the structure and aggregation of the soil [10], and promotion of the nutrition of plants [11].
AMF have a beneficial effect on soil structure, because the mycelium forms stable aggregates in the soil through glomalin, a glycoprotein produced by AMF hyphae acting as a binder to unite the soil microaggregates [12,13]. These aggregates improve water infiltration, reduce surface runoff, control soil erosion, reduce nutrient and organic matter losses, increase gas exchange, improve water and mineral retention, especially potassium, and thus increase crop productivity [14].
Sustainability in coffee production has become a requirement for the largest consumers, such as Europeans, which makes it a requirement for market access. To meet this demand, different alternative systems have been developed, which are called sustainable, organic, and special by some, as they have the intention of conserving biodiversity. Among the approaches for producing, marketing, and consuming coffee, organic coffee production is considered a holistic production management system that promotes and improves the health of agroecosystems, particularly the health, biological cycles, and activity of the soil. These achievements are reached through practices that avoid the use of chemical products as well as of genetically modified organisms, sewage, sweeteners, and synthetic preservatives [15]. The application of AMF as bioinoculants in coffee plantations can minimize the use of chemical fertilizers in agricultural practices and guarantee agricultural sustainability through improving symbiotic associations with plants. However, given their biotrophic nature, these microorganisms cannot be propagated in artificial culture media in the laboratory. This has complicated the development of large-scale production methods, thus limiting their commercial exploitation. Hence, the use of host trap plants with favorable sporulation and mycorrhizal colonization qualities is highly relevant for the propagation of native AMF [16]. Another important factor is the use of native or local AMF strains, which provide better results without the introduction of allochthonous organisms that compete with or lead to an imbalance of native organisms and are local resources typical of coffee-growing areas.
The objective of this study was to determine the dominant native AMF in the soil of coffee farms and evaluate the potential of sorghum as a trap plant for their propagation, with the purpose of promoting the adoption of this technique among coffee producers in the region. As a hypothesis, we expected a high dominance of species of the genera Glomus and Acaulospora, as they are generalists, and that S. vulgare would be efficient in promoting the multiplication of AMF morphospecies native to coffee plantations through favoring sporulation and mycorrhizal colonization.
2. Materials and Methods
Study sites. The study sites were established in in the center of the state of Veracruz, Mexico. Five shade-grown coffee farms that carry out conventional or traditional management of Coffea arabica var. Costa Rica were selected (Figure 1). The characteristics of each of the sites are detailed in Table 1.

Figure 1.
Coffee plantations in the center of the state of Veracruz: (a) San Isidro; (b) Los bambus; (c) La barranca; (d) Tuzamapan; and (e) San Marcos.

Table 1.
Geographic location, elevation, mean annual precipitation, mean annual temperature, and management type of the study sites.
The five farms in this study are in the center of the state of Veracruz, Mexico, in the Coatepec–Jilotepec coffee-growing area, located at an altitude between 1125 and 1636 masl. The average annual temperature in the area fluctuates between 19.4 and 27 °C. Coffee producers in this region are distributed in the Coatepec, Cosautlán, Emiliano Zapata, Jilotepec, Ixhuacán de los Reyes, Teocelo, and Xico municipalities. Most of the crops are grown under shade, which provides structural complexity and high diversification in the tree and understory strata.
The plants that predominate in coffee plantations include Inga vera (chalahuite), Inga jinicuil (jinicuil), Trema micrantha (ixpepe), Grevillea robusta (grevilia), Persea schiedeana (chinine), Fuchsia arborescens (aretillo), Leucaena leucocephala (huaje), and Macadamia sp. (macadamia). The coffee grown in the study area belongs to the C. arabica species, mainly the Costa Rica variety. From 2012 to 2017, rust (Hemileia vastatrix) devastated almost all the coffee plantations in this area and, so, improved varieties resistant to H. vastatrix are now planted. The farms in this region are mostly managed conventionally (with agrochemicals), but a minority carry out organic practices. At least 50% of the producers apply chemical fertilizers (1–2 fertilizers/year), while the other 50% apply organic fertilizers. Most of them carry out weed control through a mechanical method; however, some farms apply chemical control using glyphosate [17]. Producers tend to apply NPK fertilizers, and some of the commercial products that they use most are YaraMila®, ACTIVA NPK17, and SUMAGRO PSD
Physicochemical analysis of the soils. The physicochemical analyses of the soil samples from the coffee farms were carried out in accordance with NOM 021-RECNAT-2000 [18]. Organic matter (OM) and organic carbon (CO) were quantified using the modified Walkley–Black method [19], the pH was measured using the electrometric method, the cation exchange capacity (CEC) was determined with 1 N ammonium acetate (pH 7.0), total nitrogen (N) was determined using a micro-Kjeldahl apparatus [20], the available phosphorus (P) was measured using the Bray–Kurtz method [21], and the retained P was quantified using the Blakemore method [22]. The analyses were carried out at the Soil, Plant, and Water Analysis Laboratory of the Institute of Ecology, AC (Table 2).

Table 2.
Physicochemical characteristics of the coffee plantations evaluated: pH, available phosphorus (P), retained P, organic matter, organic carbon, cation exchange capacity (CEC), field capacity (FC), bulk density, clay, silt, sand, texture, total C, total N, and soil type.
Sampling. Soil sampling was carried out on five coffee farms. On each farm, five sampling points were established, each separated by a distance of 50 m to ensure that they were independent. At each point, a coffee plant was considered the center, from which two axes measuring 1 m were defined: one north–south and another east–west. At the end of each axis, a 250 g soil sample was taken at a depth of 0–15 cm. The samples were dried at room temperature, after which a physicochemical analysis was performed. Another soil sample was used. The isolation and counting of the AMF spores were carried out at the beginning and the end (at 120 days) of the experiment with the trap plants. The AMF spores were separated by wet sieving and decantation [23]. A total of 50 g of soil was placed in a flask with 250 mL of water, and the sample was vigorously shaken for 10 min. Subsequently, the flask was left to rest for 10–15 min. The supernatant was passed through a series of Tyler sieves with 750, 250, 150, and 50 µm apertures. The supernatant from the last sieve was placed in a 50 mL falcon tube, which was subsequently centrifuged at 2000 rpm for 5 min (Thermo Ice Centra CL2 Centrifuge). Once the sample was centrifuged, it was decanted, and a 70% sucrose solution was added. After vigorous shaking, the sample was centrifuged again at 2500 rpm for 1 min. The supernatant was passed through a 50 µm sieve, washed under running water, and placed in plastic bottles.
The samples were subsequently placed in Petri dishes and observed under a stereoscopic microscope (Carl Zeiss). With the help of a micropipette (0.5–10 µL Science Med), the spores were mounted in permanent preparations in polyvinyl alcohol with or without Melzer solution for observation under a compound microscope (Carl Zeiss). The identification and classification of the morphotypes was based on INVAM [24] and Redecker et al. [25], according to morphological characteristics such as size, color, wall characteristics, and the presence of supporting hyphae. The abundance of morphotypes was quantified with a colony counter (Luzeren).
Propagation test in Sorghum trap plants. Soil from the coffee farms was used for the AMF propagation test in the trap plant S. vulgare var. su miel II, and five pots (5 kg each) were prepared with soil from each of the farms, along with sterile sand and perlite in the same proportion (1:1:1), and kept in a greenhouse for 120 days (5 seeds per pot). Irrigation was carried out every third day with a modified Hewitt nutrient solution without phosphorus.
At the end of the experiment, irrigation was suspended, the spore morphotypes were isolated and counted, and the percentage of mycorrhization was quantified. The abundance of spores in the trap plant substrate was compared with the number of spores counted in the initial soil obtained from the coffee plantation.
Thinning and staining of the roots of the trap plants (S. vulgare). After 120 days, the roots of the trap plants were extracted, washed under water, cut into small pieces, and placed in test tubes. The roots were stained following the thinning and staining technique [26]. For this purpose, 10% KOH was added until the roots were completely covered, and then the roots were placed in a water bath for 15 min. The roots were then washed with running water to remove KOH. Next, 10% HCL was added, and the mixture was left for 3 min. The mixture was washed again with running water until the remaining HCL was removed. Then, 0.05% trypan blue was added, and the roots were placed in a water bath for 10 min. Finally, the stained roots were placed in 5% lactic acid for observation.
Measuring the percentage of mycorrhization. The percentage of mycorrhization was quantified according to the modified magnified intersections method [27]. The magnified intersections method can offer the best quantitative description of colonization that can be made, given the inherent difficulties associated with interpreting fungal material observed in roots, according to McGonigle et al. [28]. The roots were mounted in KOH (10%) on microscope slides and covered with 40 × 22 mm coverslips. The roots were aligned parallel to the longitudinal axis of the slides and observed at 40× magnification. The position on the surface of the root at which the center of the eyepiece crosshairs entered its side was taken as the point of intersection.
To measure the colonized root percentage, we used the following criteria: whenever a hypha, vesicle, or arbuscule was present in a longitudinal portion of the root, and the axis of the reticule that crossed that root touched any of these structures, colonization was considered to have occurred (+); otherwise, it was considered lacking (−). The counts were expressed as percentages, calculated using the following formula [27,28]:
Five replicates were made per treatment (pot), and, for each replicate, 20 root intersections were counted; in total, 100 intersections were observed per treatment.
Statistical analysis. Spore abundance is expressed as the total number of spores found in 100 g of soil. The species abundance distributions were plotted (Whittaker plots) to elucidate the AMF dominance patterns in each of the samples. The distributions were obtained by plotting the AMF abundance (from the most to least the abundant species).
To identify differences in spore abundance between the initial count in the coffee-growing soil and the final count after propagation in the sorghum trap plants, we conducted one-way analysis of variance after determining whether the data met the assumption of a normal distribution and homogeneity of variance (using the Kolmogorov–Smirnov and the Bartlett tests, respectively).
The percentages of root colonization were compared between the samples using one-way ANOVA (with 5 replicates). When the effects of factors were significant in the ANOVA, Tukey’s HSD post-hoc test was run to test for pair-wise mean differences at p ≤ 0.05. These analyses were performed using the Statistica 12.0 software. A Pearson correlation analysis was performed with a significance level of p ≤ 0.05 between the abundance of spores of the morphotypes detected in the initial samples and the physicochemical characteristics of the soil from the farms.
3. Results
Morphotypes and Abundance of the AMF Spores
A total of 10 spore morphotypes were distinguished in the initial and final samples on the substrate. In most of the farms, four morphospecies of the genus Glomus were detected, along with two Rhizophagus, three Aculospora, and one Gigaspora, except in the bamboo farm, where only two morphospecies of Acaulopsora were detected.
The abundance of spores increased significantly (p < 0.05) between the initial count in the coffee-growing soil and the final count after propagation in sorghum trap plants for 120 days. This was observed for all the sites analyzed. For the San Isidro farm soil, the number of spores increased from 136 to 918; for Los bambus farm, the number of spores increased from 89 to 867; for La barranca farm soil, the number of spores increased from 130 to 801; for the Tuzamapan farm soil, the number of spores increased from 195 to 990; and, for the San Marcos farm soil, the number of spores increased from 209 to 809. The greatest increase was detected for Los bambus farm soil (9.7 times), while the smallest increase was detected for the San Marcos farm soil (3.87 times); see Table 3.

Table 3.
Morphotypes and spore abundance of AMF in 50 g of coffee plantation soil at the beginning of the experiment (initial) and after 120 days (final) on trap plants (S. vulgare) in the greenhouse.
The results demonstrate the presence of morphotypes of the Glomus and Acaulospora genera in all the coffee plantations, as well as a large increase in these genera through propagation in S. vulgare trap plants. It is important to highlight that the abundance of morphotypes belonging to the Glomus genus (Glomus sp3 and Glomus sp1) increased more than that of the Acaulospora genus. Within the Acaulospora genus, the number of A. scrobiculata spores increased on the trap plants. The Gigaspora genus was represented by a single morphotype (Gigaspora sp1), and the number of spores of this morphotype did not increase in the S. vulgare trap plants; see Table 3.
In the initial sampling, the Glomus sp3 morphotype dominated in the San Isidro and La barranca farm soils. For the Los bambus farm, the Glomus sp3, Glomus sp4, and A. scrobiculata morphotypes were dominant. The Glomus sp1 morphotype dominated in the Tuzamapan and San Marcos farms soil. In the correlation analyses, positive relationships of more than 80% were observed between Glomus sp2, Glomus sp3, Acaulospora sp1, and Gigaspora with soil pH (4.09 to 5.34); Glomus sp2, Glomus sp4, Acaulospora sp1, and Gigaspora with clay content (29.8 to 49.8); Rizhophagus intraradices and A. scrobiculata with retained P content (81.63 to 89.8%); and Acaulospora sp1 with organic matter content (ranging from 3.93 to 12.46%) and total carbon (2.28 to 7.23); see Table 3 and Table 4.

Table 4.
Pearson correlation analysis between the abundance of spores of the morphotypes detected in the initial sampling and the physicochemical characteristics of the soil of the farms.
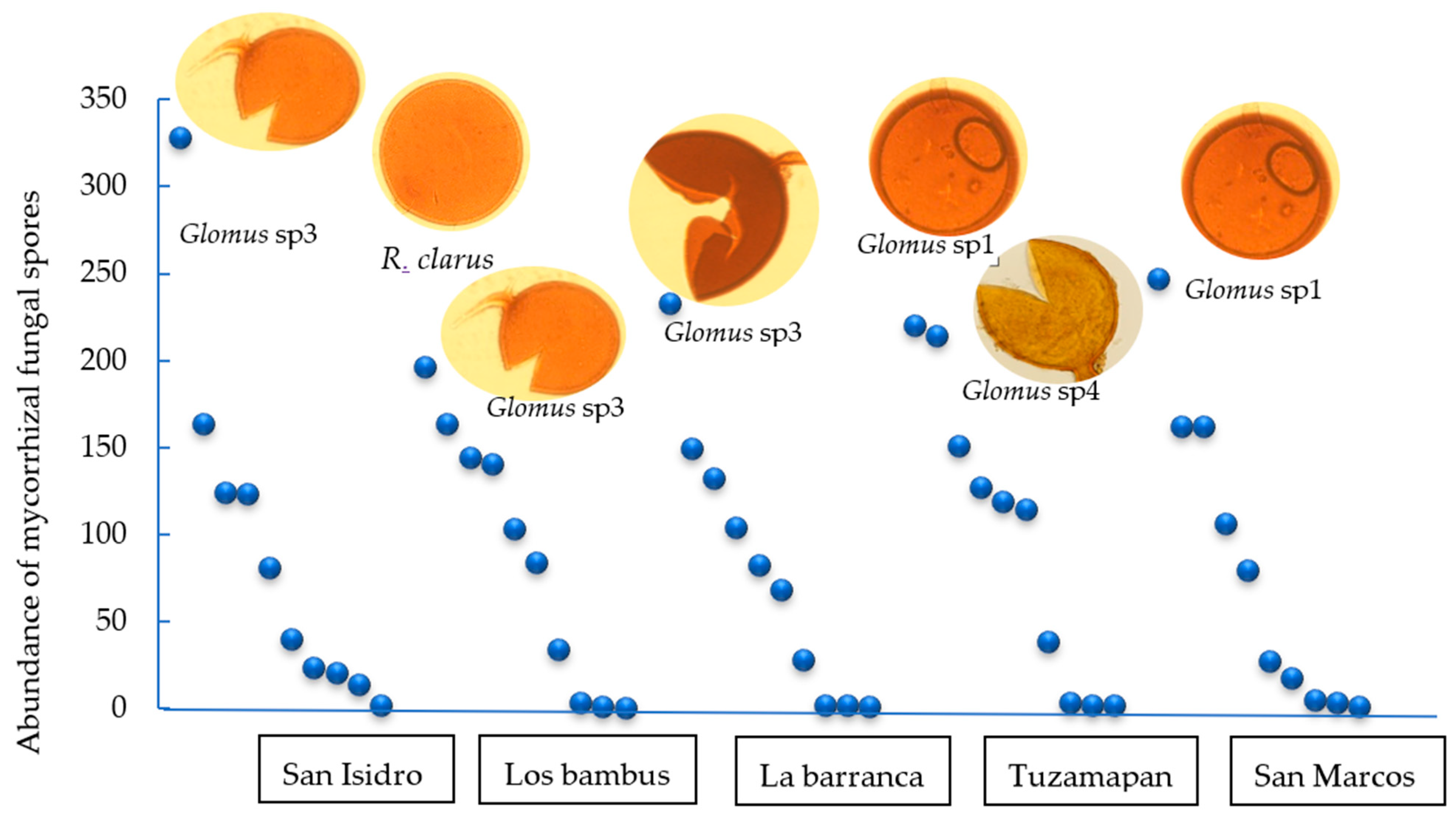
On the other hand, assessments after propagation in the trap plants showed that Glomus sp3 dominated in the San Isidro and La barranca soils, and Glomus sp1 dominated in the Tuzamapan and San Marcos soils. In the Los bambus farm soil, the Glomus sp3 and R. clarus morphotypes were dominant (Figure 2).
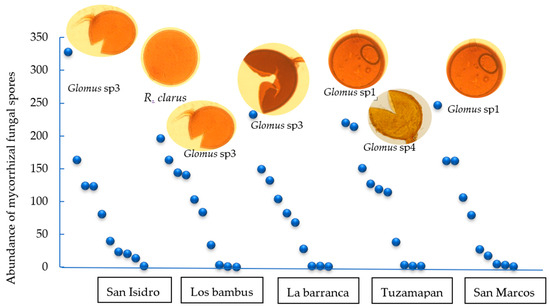
Figure 2.
Whittaker plots (rank/abundance plots) illustrating the dominant morphotypes of AMF in trap plants (S. vulgare) inoculated with coffee plantation soil after 120 days in the greenhouse. The morphospecies are plotted in sequence from highest to lowest abundance along the X-axis. The Y-axis represents the total spore abundance of each morphospecies.
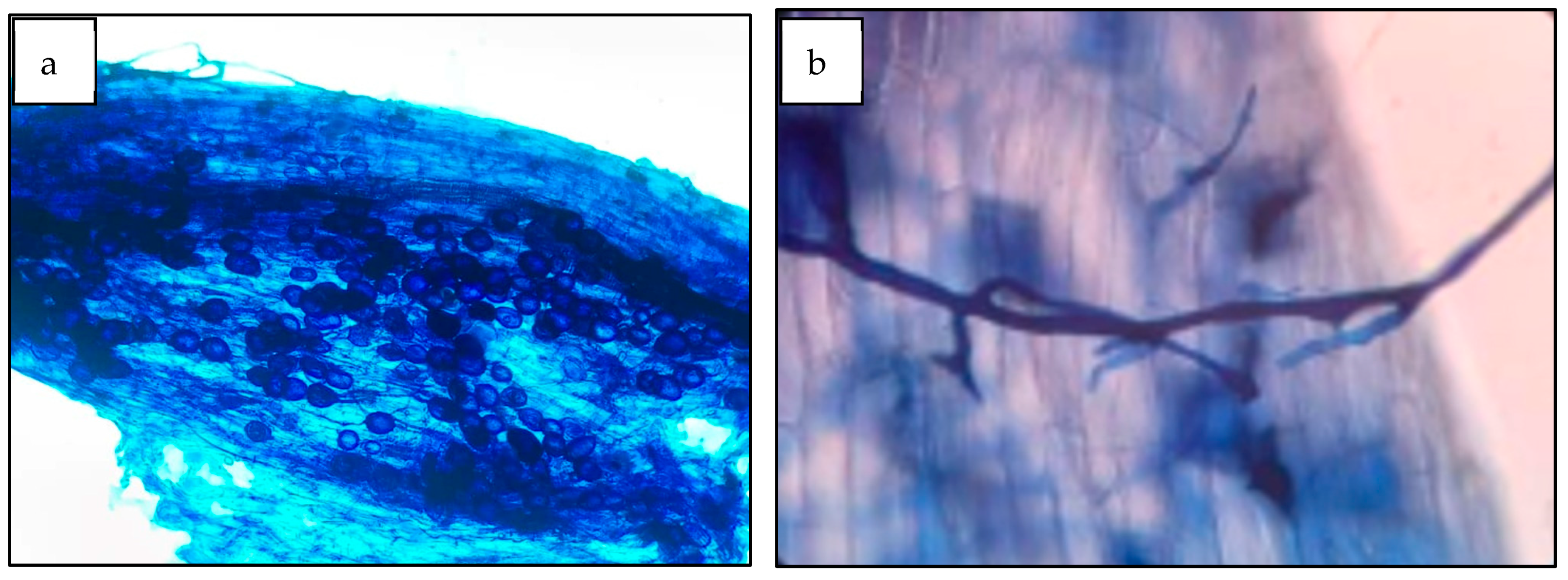
Mycorrhizal colonization of the trap plants (S. vulgare). Characteristic AMF structures (Figure 3), such as hyphae, vesicles, arbuscules, and spores, were observed on the roots of the trap plants (S. vulgare) at 120 days after sowing. The mycorrhizal colonization percentages did not significantly differ between the trap plants inoculated with soil from different coffee farms (p > 0.05) and ranged from 71 to 80%. Furthermore, the differences between the values were not significant, and the highest colonization rate was observed at the San Isidro farm (80%).

Figure 3.
Roots of Sorghum trap plant (S. vulgare) with mycorrhizal colonization: (a) spores inside the roots; (b) hyphae penetrating the roots.
4. Discussion
In recent decades, the use of AMF has received a lot of attention due to their significant potential to improve plant yields and, therefore, agricultural production. The great benefits provided by AMF in terms of nutrient absorption and tolerance to water stress are well known; however, few studies have specifically analyzed the effects of these microorganisms in coffee plantations run by indigenous communities, even though coffee is an important agricultural product at both the global and the national levels. Although there is a lot of information on the use of mycorrhizae as a biofertilizer and a vast list of products on the market, there are no conclusive results, as the commercial products typically have low inoculum concentrations, which reduces their effectiveness in the field; furthermore, the manufacturers do not generally reveal their composition. In this context, producing native AMF inocula provides several benefits that facilitate the production of viable and low-cost inocula. However, few studies have propagated and inoculated autochthonous AMF from coffee plantations; most studies have instead used allochthonous bioinoculants or lack information on their origins [29].
Knowing the origin of an AMF for its use as a biofertilizer is interesting, as the soil and climatic conditions in which the strains are isolated are crucial to predict the response of the treated plants. Native AMF have greater possibilities to adapt to regional climatic conditions than non-native AMF. Therefore, it is of biotechnological interest to determine AMF strains native to agricultural soils or natural ecosystems that are suitable in terms of different factors, particularly those which can be used for the formulation of biofertilizers for coffee-growing areas in Mexico [30]. To successfully propagate native AMF species, it is of utmost relevance to consider both the host plants and the dominant AMF species, as these have greater possibilities of competing efficiently and ensuring the success of inoculation in the field. However, constant monitoring is recommended, as Bertolini [31] reported that the high reproductive capacity of generalist species—although favoring their establishment—could lead to the displacement of other, more efficient native species.
In this work, we showed that the dominant morphotypes of coffee plantations in the central region of Veracruz are Glomus sp1 and Glomus sp3, which seem to manifest as generalists, as they were abundant and frequent in all the evaluated farms. On the other hand, the Gigaspora genus was detected with low frequency and abundance. This result could be due to the low number of spores detected at the beginning of the experiment or to poor compatibility with the trap plant. The genus Glomus has been reported to have a wide distribution [32], which is why it is considered a generalist genus [33]. In this work, Glomus was the predominant genus; its dominance in the coffee soils analyzed may be due to the fact that it has a highly infective extraradical mycelium, while other genera such as Gigaspora frequently develop from spores [34].
In other studies of coffee plantations in Mexico, the Glomus and Acaulospora genera have generally been highlighted as dominant in coffee plantations in Veracruz [35,36] and Chiapas [31,37,38]. Studies of coffee plantations in Costa Rica indicated that the Gigasporaceae and Acaulosporaceae families are dominant [39]. In Ecuadorian coffee plantations [40], Funneliformis mosseae, Gigaspora gigantea, and Scutellospora spp. were the dominant species. After a molecular and phylogenetic analysis in Saudi Arabia, Mahdi et al. [41] reported Glomus as the dominant genus, followed by Claroideoglomus, Acaulospora, and Gigaspora. Bertolini et al. [37] found that C. arabica plants have higher AMF diversity, compared with Robusta (C. canephora) plantations, and the authors emphasized the presence of species exclusive to C. arabica plantations. Hence, it is important to continue studying the functional compatibility of native AMF species with different coffee tree species and/or varieties, to better utilize their benefits as biofertilizers.
AMF are found in all types of soils and can colonize any plant that establishes symbiosis with them; however, the physicochemical conditions of the soil may generate a certain type of specificity with respect to the host plant, depending on its responses to certain AMF species. Other relevant parameters that affect the abundance of AMF spores are the physicochemical characteristics of the soil (e.g., texture, organic matter content, pH, and N and P availability). These characteristics determine the distribution of microorganisms [42]. In this study, the abundance of morphospecies in the Acaulospora, Glomus, and Gigaspora genera was related to the soil pH and clay content, possibly as a slight increase in soil pH generates lower aluminum saturation and better cation exchange capacity in clays, reducing stress in fungal populations; this produces higher population densities and favors sporulation [43]. Nutrient deficiency and metal toxicity (Mn, Fe, and Al) are characteristics of acidic andosol soils—such as coffee soils—in this region, with iron (Fe3+) toxicity being the main factor that limits plant growth in this type of soil. It was reported that the optimal pH for developing AMF is between 6 and 7; however, it was also pointed out that the Acaulospora and Glomus genera comprise many species that can adapt to degraded, poorly fertile soils with low pH values [44]. The acidity level is an important factor that controls P availability, which, in turn, determines an endophyte’s efficiency, the germination percentage, and the development of AMF spores [45]. Therefore, to select species that are efficient in a wide pH range, it is very important to account for the effects of pH on the efficiency of the association and multiplication mechanisms in AMF species selection studies. Unlike other studies carried out on coffee plantations in Mexico [31,35,36,37], species of the Scutellospora and Entrophospora genera were not observed in the farms we analyzed. This result may have been influenced by the state of disturbance at the coffee plantations generated by the management practices developed there (e.g., weed control, soil conditions, and chemical fertilization), in agreement with the conclusion of Arias et al. [35] regarding other coffee farms in Veracruz, where these factors interfered in the abundance and composition of AMF in coffee plantations.
In the propagation of AMF, it is very important to estimate their abundance, as this parameter allows for the estimation of the possibility of mycorrhization that will be reflected in the growth and development of the plants; however, to evaluate the success of mycorrhization, the percentage of colonization must be measured.
Considering the results of this study, we recommend using the trap plant S. vulgare to propagate AMF spores that are native to coffee plantations. Initially, there were 89–209 spores in 50 g of soil; meanwhile, after 120 days on the trap plant, sporulation increased to 801–990 spores in 50 g of soil. Likewise, this species was found to be ideal for promoting high mycorrhization (71–80%). Kormanic and McGraw [46] defined five degrees of mycorrhizal colonization: null (0%), low (1–25%), moderate (26–50%), high (51–75%), and very high (76–100%). According to these categories, the extent of mycorrhizal colonization in this study was very high.
The reported values are superior to those of Yusnizar et al. [47] in Indonesia, who used maize as a trap plant (111 spores/50 gr. soil), and those of Robles-Gonzalez et al. [38] in Chiapas, who used coffee, corn, bean, and grass (139 spores/50 gr. substrate). Other studies detected relatively low mycorrhization (5.8–36.9%); however, this colonization was reported for coffee plants while using a substrate with spores propagated in corn [48,49]. Fernández-Martín [29] obtained colonization percentages of 45–60% in coffee plants using sorghum as a trap plant; however, they used an allochthonous AMF strain (G. clarum). The INVAM [24] has routinely used the sorghum plant for its germplasm bank, which is considered an excellent host for a wide range of AMF under greenhouse conditions; however, it has not previously been used to propagate native AMF from coffee plantations. The obtained results demonstrated the presence of morphotypes of the Glomus and Acaulospora genera in all the coffee plantations and a large increase in these genera through propagation in S. vulgare trap plants. Although there are different AMF propagation methods—including monosporic cultivation [50], the use of solid substrates [51] and aeroponic crops [52], and hydroponics [53]—storing propagated AMF spores requires technical skills and preliminary knowledge that farmers do not have. Therefore, using a spore propagation technique with trap plants to produce native inocula is ideal for managing coffee plantations in the study region.
Mycorrhizal inoculation is necessary among coffee producers, as the coffee plant is stressed in the transplant stage; therefore, it must have a biological system that allows it to tolerate environmental changes [54]. Using mycorrhizae for coffee cultivation allows for a reduction in fertilizer use, which positively affects the coffee grower’s profits; it also generates positive ecological and environmental impacts as it sustains the agroecosystem through providing environmental services such as carbon capture, soil protection, aquifer recharge, and improved air quality, among others [55]. Using AMF in coffee plantations can help to establish organic farms and allow producers to access the certified coffee market, which has better prices.
This study is part of a broader strategy that involves promoting the use of native biotechnological resources among coffee growers in this region, which is a relevant socio-environmental aspect of our research. Since 2020, various participatory workshops have been held to raise awareness of bioinoculants and to encourage the adoption of simple techniques, such as using trap plants to multiply local AMF germplasms. The propagation of native AMF in coffee plantations using trap plants offers an alternative for sustainable coffee production, as producers can first use this inoculum as a substrate for coffee seedlings in the greenhouse phase and then use it to subsequently renovate their farms.
5. Conclusions
A total of 10 AMF morphotypes were detected in the soil of the considered coffee farms, with Glomus sp1, Glomus sp2, Glomus sp3, Glomus sp4, R. clarus, and A. scrobiculata being the dominant morphotypes. The sorghum trap plants were found to have high potential for favoring the propagation of native AMF through increasing their abundance and promoting a high mycorrhizal colonization.
Author Contributions
Conceptualization, R.M.A.M.; methodology, R.M.A.M., Y.d.l.C.E., L.C.R.M., and Y.d.C.P.-R.; validation, R.M.A.M. and Y.d.l.C.E.; writing—original draft, R.M.A.M., Y.d.l.C.E. and Y.d.C.P.-R.; writing—review and editing, R.M.A.M. and Y.d.C.P.-R.; supervision, R.M.A.M. and Y.d.l.C.E.; project administration, L.C.R.M.; funding acquisition, L.C.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by COVEICYDET for the CP1111 2113/2023 project: “Impulso a la cafeticultura sustentable mediante innovaciones tecnológicas para el uso del agua y suelo en Jilotepec, Veracruz”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors thank Elizabeth Abigail López Martínez for her help in processing the samples. The authors thank the owners of the farms for allowing access to take the soil samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- SIAP (Servicio de Información Agroalimentaria y Pesquera). Panorama Agroalimentario. Available online: https://drive.google.com/file/d/1FWHntHMgjw_uOse_MsOF9jZQDAm_FOD9/view (accessed on 10 December 2023).
- Fairetrade International. Available online: https://www.fairtrade.net/ (accessed on 1 April 2023).
- Moguel, P.; Toledo, V.M. Biodiversity conservation in traditional coffee systems of Mexico. Biol. Conserv. 1999, 13, 11–21. [Google Scholar] [CrossRef]
- Geissert, D.; Ibañez, A. Calidad y ambiente fisicoquímico de los suelos. In Agroecosistemas Cafetaleros del Estado de Veracruz Biodiversidad, Manejo y Conservación; Manson, R.H., Hernández, V., Gallina, O.S., Mehltreter, K., Eds.; Instituto de Ecología: Mexico City, Mexico, 2008; pp. 15–44. [Google Scholar]
- Rodríguez-Eugenio, N.; McLaughlin, M.; Pennock, D. La Contaminación del Suelo: Una Realidad Oculta; FAO: Italia, Roma, 2019; p. 144. [Google Scholar]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Micología 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Pérez-Moncada, U.A.; Gómez, M.R.; Serralde-Ordoñez, D.P.; Peñaranda-Rolón, A.M.; Wilches-Ortiz, W.A.; Ramírez, L.; Rengifo-Estrada, G.A. Hongos formadores de micorrizas arbusculares (HFMA) como estrategia para reducir la absorción de cadmio en plantas de cacao (Theobroma cacao). Terra Latinoam. 2019, 37, 121–130. [Google Scholar] [CrossRef]
- Mohamed, I.; Eid, K.E.; Abbas, M.H.H.; Salem, A.A.; Ahmed, N.; Ali, M.; Shah, G.M.; Fang, C. Use of plant growth promoting Rhizobacteria (PGPR) and mycorrhizae to improve the growth and nutrient utilization of common bean in a soil infected with white rot fungi. Ecotoxicol. Environ. Saf. 2019, 171, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.V.; Pedroso, D.D.F.; Curi, N.; Carneiro, M.A.C. Do different arbuscular mycorrhizal fungi affect the formation and stability of soil aggregates? Cienc. Agrotecnologia 2019, 43, e003519. [Google Scholar] [CrossRef]
- Muñoz, M.G. Análisis de Expresión de Genes de Respuesta al Estrés Hídrico en Plantas de Sorghum bicolor (L.) Moench en Presencia y Ausencia de Asociaciones con Hongos Micorrízicos. Doctoral Dissertation, Universidad Autónoma de Aguascalientes, Aguascalientes, Mexico, 2018. [Google Scholar]
- Singh, A.K.; Chen, C.; Wu, J.; Yang, B.; Zakari, S.; Jiang, X.J.; Singh, N.; Liu, W. The role of glomalin in mitigation of multiple soil degradation problems. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1–35. [Google Scholar] [CrossRef]
- Lehmann, E.F.; Leifheit, A.; Rillig, M.C. Mycorrhizas and soil aggregation. In Mycorrhizal Mediation of Soil: Fertility, Structure and Carbon Storage; Johnson, N.C., Gehring, C., Jansa, J., Eds.; Elsevier: New York, NY, USA, 2017; pp. 241–262. [Google Scholar]
- Parihar, M.; Rakshit, A.; Meena, V.S.; Gupta, V.K.; Rana, K.; Choudhary, M.; Jatav, H.S. The potential of arbuscular mycorrhizal fungi in C cycling: A review. Arch. Microbiol. 2019, 202, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cruz, M.A.; Schwentesius, R.R.; Meraz, A.M.d.R.; Lobato, G.A.; Gómez, T. Agricultura, Apicultura y Ganadería Orgánicas de México-2005-Situación–Retos–Tendencias, 1st ed.; PIAI-CIESTAAM: Texcoco, Mexico, 2005; pp. 1–65. [Google Scholar]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Maeder, P.; Wiemken, A.; Boller, T. Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agric. Ecosyst. Environ. 2009, 134, 257–268. [Google Scholar] [CrossRef]
- Quiroz, A.C. Estrategias de Gestión Para la Producción Sustentable de Café Diferenciado en Jilotepec, Veracruz. Master’s Dissertation, Universidad Veracruzana, Veracruz, Mexico, 2023. [Google Scholar]
- NORMA Oficial Mexicana NOM-021-SSA1-2021, Salud Ambiental. Criterio Para Evaluar la Calidad del Aire Ambiente, con Respecto al Monóxido de Carbono (CO). Valores Normados para la Concentración de Monóxido de Carbono (CO) en el Aire ambiente, Como Medida de Protección a la Salud de la Población. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5634084&fecha=29/10/2021#gsc.tab=0 (accessed on 26 April 2023).
- Bahadori, M.; Tofighi, H. A modified Walkley-Black method based on spectrophotometric procedure. Commun. Soil Sci. Plant Anal. 2016, 47, 213–220. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Bray, R.; Kurtz, L. Determination of total, organic and available forms of phosphorus in soil. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Metson, A.J.; Blakemore, L.C.; Rhoades, D.A. Methods for the Determination of Soil Organic Carbon: A Review, and Application to New Zealand Soils. N. Z. J. Sci. 1979, 22, 205–228. [Google Scholar]
- Gerdemann, J.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Invam (International Culture Collection of Vesicular Arbuscular Mycorrhizal Fungi). Available online: http://invam.caf.wvu.edu/ (accessed on 10 January 2024).
- Redecker, D.; Schüßler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trejo, D.; Zulueta, R.; Lara, L. Manual de Prácticas Para el Estudio de la Simbiosis Micorrizógena Arbuscular, 1st ed.; Universidad Veracruzana: Veracruz, Mexico, 2008; pp. 1–137. [Google Scholar]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martín, F.; Rivera-Espinosa, R.A.; Hernández-Jiménez, A.; Herrera-Peraza, R.A.; Fernández-Suárez, K. Inoculación de hongos micorrízicos arbusculares y diferentes relaciones suelo: Humus de lombriz sobre el crecimiento del cafeto (Coffea arabica L.) cv. Catuaí bajo la etapa de vivero. Rev. Chapingo Ser. Hortic. 2005, 11, 175–184. [Google Scholar] [CrossRef]
- Quiñones-Aguilar, E.E.; Hernández-Cuevas, L.V.; López Pérez, L.; Rincón-Enríquez, G. Efectividad de hongos micorrízicos arbusculares nativos de rizósfera de Agave como promotores de crecimiento de papaya. Terra Latinoam. 2019, 37, 163–174. [Google Scholar] [CrossRef]
- Bertolini, V.; Montaño, N.M.; Chimal, E.; Varela, L.; Gómez, J.; Martínez, J.M. Abundancia y riqueza de hongos micorrizógenos arbusculares en cafetales de Soconusco, Chiapas, México. Rev. Biol. Trop. 2018, 66, 91–105. [Google Scholar] [CrossRef][Green Version]
- Öpik, M.; Moora, M.; Liira, J.; Zobel, M. Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J. Ecol. 2006, 94, 778–790. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Mäder, P.; Boller, T.; Wiemken, A. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 2003, 69, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.M.; Reader, R.J. Host plant benefit from association with arbuscular mycorrhizal fungi: Variation due to differences in size of mycelium. Biol. Fertil. Soils 2002, 36, 357–366. [Google Scholar] [CrossRef]
- Arias, R.M.; Heredia, G.; Sosa, V.; Fuentes-Ramírez, L.E. Diversity and abundance of arbuscular mycorrhizal fungi spores under different coffee production systems and in a tropical montane cloud forest patch in Veracruz, Mexico. Agrofor. Syst. 2012, 85, 179–193. [Google Scholar] [CrossRef]
- Posada, R.H.; Sánchez, M.; Heredia, G.; Sieverding, E. Effects of soil physical and chemical parameters, and farm management practices on arbuscular mycorrhizal fungi communities and diversities in coffee plantations in Colombia and Mexico. Agrofor. Syst. 2018, 92, 555–574. [Google Scholar] [CrossRef]
- Bertolini, V.; Montaño, N.M.; Salazar, B.L.; Chimal, E.; Varela, L. Diversidad de hongos micorrizógenos arbusculares en plantaciones de café (Coffea arabica) del volcán Tacaná, Chiapas, México. Act. Bot. Mex. 2020, 127, 1–16. [Google Scholar] [CrossRef]
- Robles-González, K.K.; Álvarez-Solís, J.D.; Bertolini, V.; Pérez-Luna, Y.C. Diversidad y propagación de hongos micorrízicos arbusculares nativos de un cafetal orgánico en Chiapas, México. Rev. Fitotec. Mex. 2023, 46, 147. [Google Scholar] [CrossRef]
- Prates Júnior, P.; Moreira, B.C.; da Silva, M.d.C.S.; Veloso, T.G.R.; Stürmer, S.L.; Fernandes, R.B.A.; Mendonça, E.D.S.; Kasuya, M.C.M. Agroecological coffee management increases arbuscular mycorrhizal fungi diversity. PLoS ONE 2019, 14, e0209093. [Google Scholar] [CrossRef] [PubMed]
- Urgiles-Gómez, N.; Avila-Salem, M.E.; Loján, P.; Encalada, M.; Hurtado, L.; Araujo, S.; Cornejo, P. Plant growth-promoting microorganisms in coffee production: From isolation to field application. Agronomy 2021, 11, 1531. [Google Scholar] [CrossRef]
- Mahdi, S.S.; Hassan, G.; Samoon, S.; Rather, H.; Dar, S.A.; Zehra, B. Bio-fertilizers in organic agriculture. J. Phytol. 2010, 2, 42–54. [Google Scholar]
- de Souza, T.A.F.; Freitas, H. Arbuscular mycorrhizal fungal community assembly in the Brazilian tropical seasonal dry forest. Ecol. Process. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Peña-Venegas, C.P.; Cardona, G.I.; Arguelles, J.H.; Arcos, A.L. Micorrizas arbusculares del sur de la amazonia colombiana y su relación con algunos factores fisicoquímicos y biológicos del suelo. Acta Amaz. 2007, 37, 327–336. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Anderson, K.; Morton, J.B. Arbuscular mycorrhizal communities in tropical forests are affected by host tree species and environment. Oecologia 2023, 135, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Bano, A.; Malik, R.N. Role of arbuscular mycorrhizal fungi in phytoremediation of heavy metals and effects on growth and biochemical activities of wheat (Triticum aestivum L.) plants in Zn contaminated soils. AJB 2016, 15, 872–883. [Google Scholar] [CrossRef]
- Kormanik, P.P.; McGraw, A.C. Quantification of vesicular-arbuscular mycorrhizae in plant roots. In Methods and Principles of Mycorrhizal Research; Schenck, N., Ed.; American Phytopathological Society: St. Paul, MN, USA, 1982; pp. 37–45. [Google Scholar]
- Yusnizar, Y.; Syafruddin, S.; Hifnalisa, H.; Karim, A.; Fikrinda, F.; Latifurrahmi, L. Propagation of arbuscular mycorrhizal fungi (AMF) spores from arabica coffee (Coffea arabica L.) plantations in Bener Meriah Regency. AGROTEK 2024, 8, 55–61. [Google Scholar] [CrossRef]
- Vallejos-Torres, G.; Saboya, A.; Arévalo, L. Efecto Bioprotector de Micorrizas Arbusculares en la Reducción de Roya (Hemileia vastatrix) en la Región San Martín. Rev. Agrotec. Amaz. 2021, 1, 34–44. [Google Scholar] [CrossRef]
- Del Aguila, K.M.; Vallejos-Torres, G.; Arévalo, L.A.; Becerra, A.G. Inoculación de consorcios micorrícicos arbusculares en Coffea arabica, variedad Caturra en la región San Martín. Inf. Tecnol. 2018, 29, 137–146. [Google Scholar] [CrossRef]
- Selvakumar, G.; Krishnamoorthy, R.; Kim, K.; Sa, T. Propagation technique of arbuscular mycorrhizal fungi isolated from coastal reclamation land. Eur. J. Soil. Biol. 2016, 74, 39–44. [Google Scholar] [CrossRef]
- Douds, D.D., Jr.; Nagahashi, G.; Pfeffer, P.E.; Kayser, W.M.; Reider, C. On-farm production and utilization of arbuscular mycorrhizal fungus inoculum. Can. J. Plant Sci. 2005, 85, 15–21. [Google Scholar] [CrossRef]
- Mohammad, A.; Khan, A.G.; Kuek, C. Improved aeroponic culture of inocula of arbuscular mycorrhizal fungi. Mycorrhiza 2000, 9, 337–339. [Google Scholar] [CrossRef]
- Tajini, F.; Suriyakup, P.; Vailhe, H.; Jansa, J.; Drevon, J.J. Assess suitability of hydroaeroponic culture to establish tripartite symbiosis between different AMF species, beans, and rhizobia. BMC Plant Biol. 2009, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Acosta, E.; Trejo-Aguilar, D.; Ferrera-Cerrato, R.; Rivera-Fernández, A.; González-Chávez, M.C. Arbuscular mycorrhizal fungi in coffee growth (Coffea arabica L.) varieties Garnica, Catimor, Caturra and Catuaí. Agroproductividad 2018, 11, 61–67. [Google Scholar]
- Ruelas-Monjardín, L.C.; Nava-Tablada, M.E.; Cervantes, J.; Barradas, V.L. Importancia ambiental de los agroecosistemas cafetaleros bajo sombra en la zona central montañosa del estado de Veracruz, México. Madera Bosques 2014, 20, 27–40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).