Alteration of Photosynthetic and Antioxidant Gene Expression in Sugarcane Infected by Multiple Mosaic Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Observation Area and Leaf Sample Collection
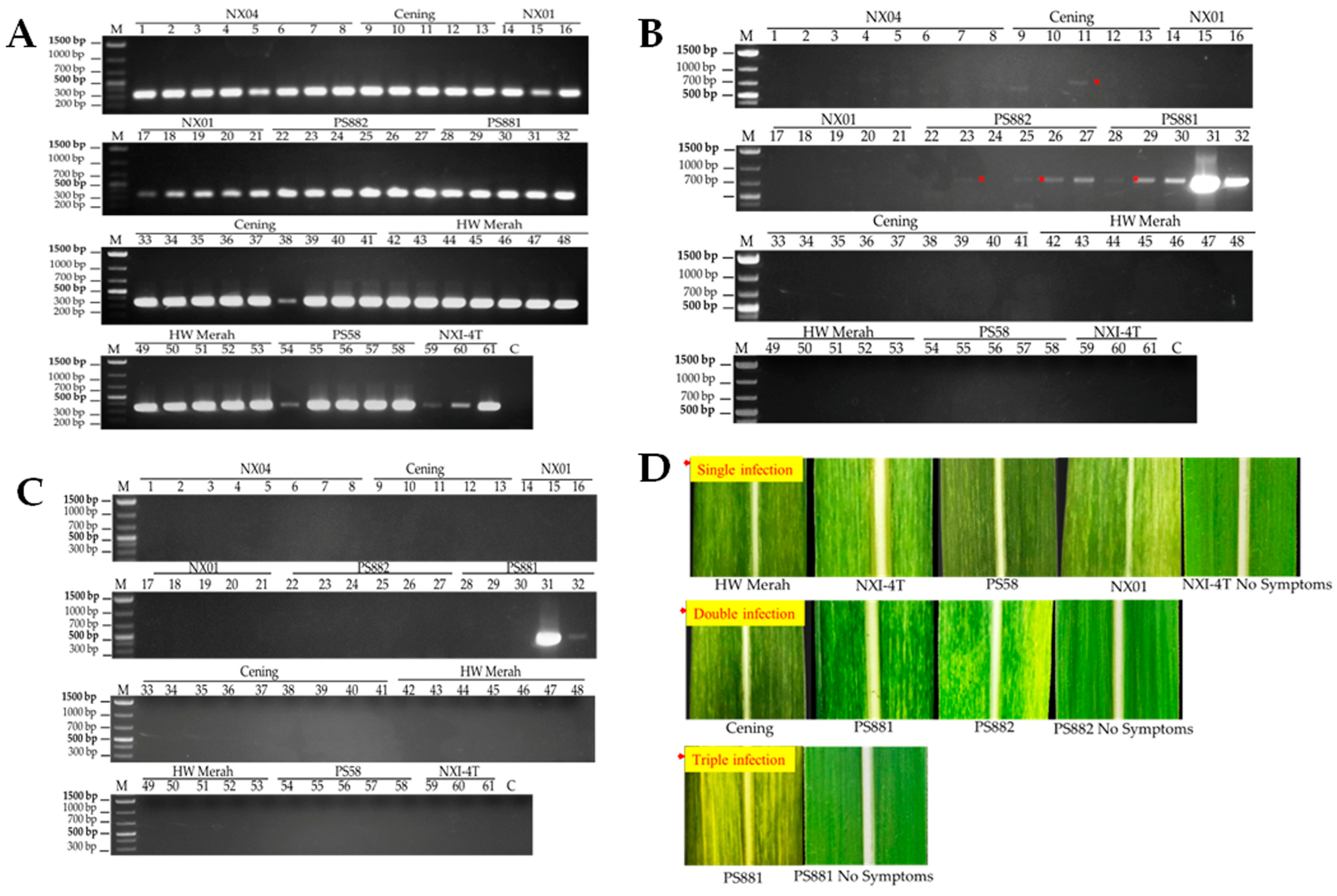
2.2. RNA Extraction and Mosaic Virus Determination
2.3. DNA Sequencing and Phylogenetic Analysis
2.4. Quantitative Real-Time PCR (RT-qPCR)
2.5. Protein Extraction and Immunoblotting
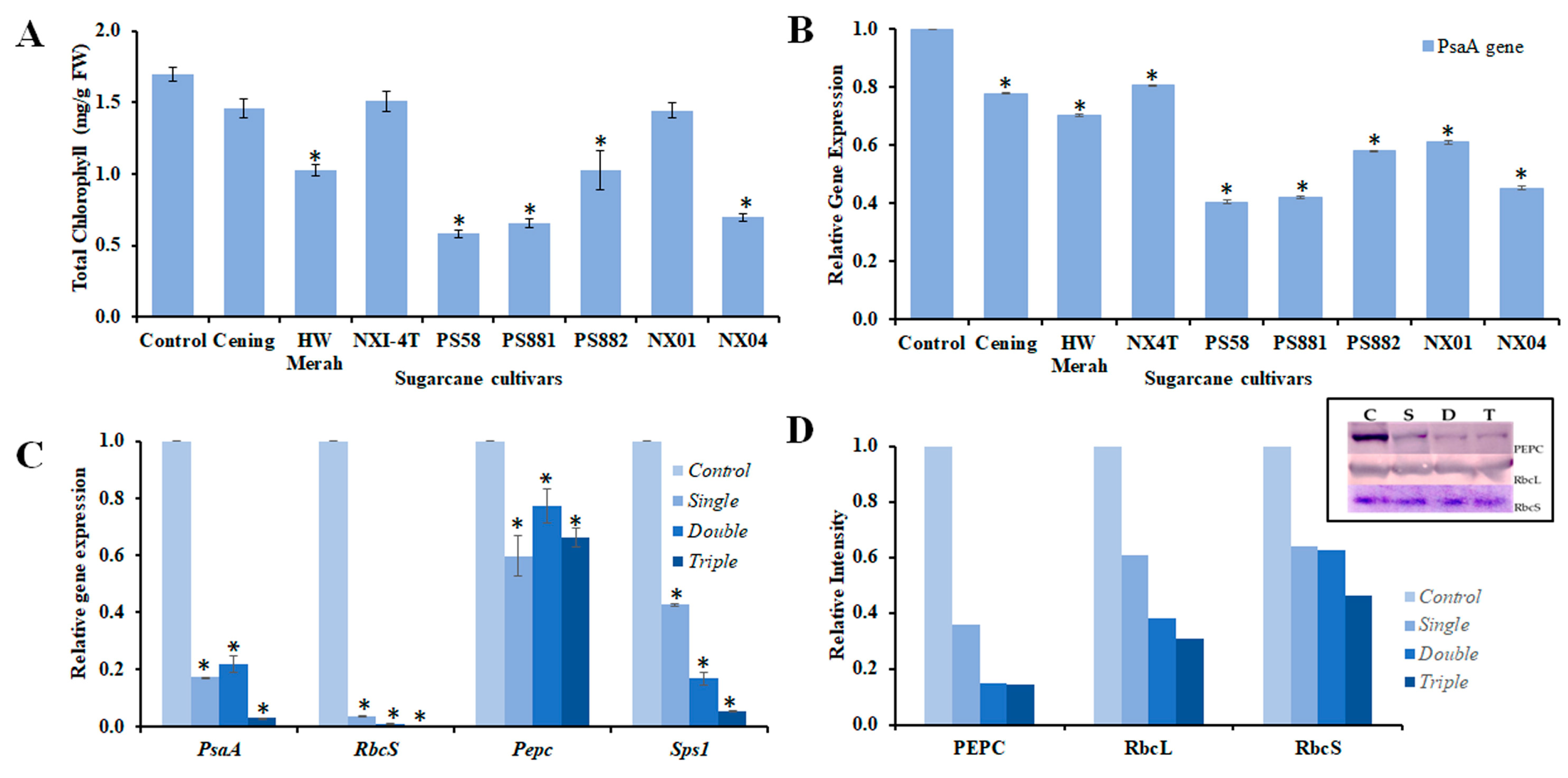
2.6. Analysis of the Total Chlorophyll of Leaves
- A645: absorbance at 645 nm;
- A663: absorbance at 663 nm;
- V: final volume of chlorophyll extract;
- W: fresh weight of leaf extract.
2.7. Statistical Analysis
3. Results
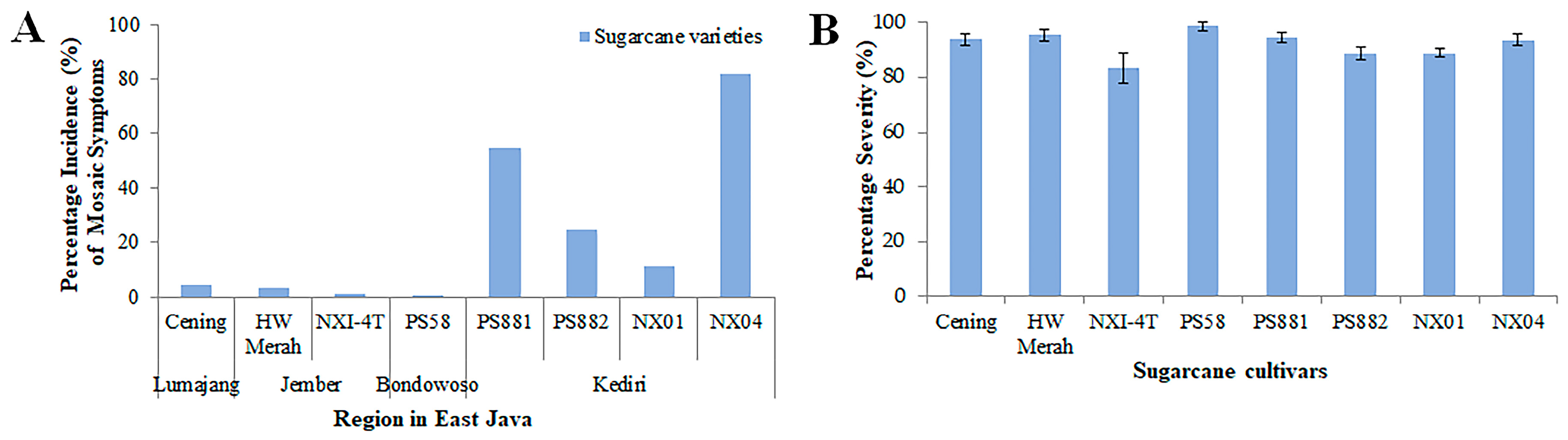
3.1. Mosaic Symptom, Incidence, and Severity
3.2. Phylogenetic Analysis of the Mosaic Virus Isolates
3.3. Expression of Apx and Cat Genes
3.4. Expression of Photosynthesis Related-Genes in Response to Mosaic Virus Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aono, A.H.; Pimenta, R.J.G.; Garcia, A.L.B.; Correr, F.H.; Hosaka, G.K.; Carrasco, M.M.; Cardoso-Silva, C.B.; Mancini, M.C.; Sforça, D.A.; Dos Santos, L.B.; et al. The Wild Sugarcane and Sorghum Kinomes: Insights Into Expansion, Diversification, and Expression Patterns. Front. Plant Sci. 2021, 12, 668623. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Hoang, Q.-T.; Nguyen, T.-T.; Pham, T.-A.; Cao, A.-D.; Pham, H.-D.; Le, V.-H.; Vu, T.-T.; Pham, N.-H.; Nguyen, T.-C.; et al. Research and Development Prospects for Sugarcane Industry in Vietnam. SugarTech 2022, 24, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Addy, H.; Nurmalasari; Wahyudi, A.; Sholeh, A.; Anugrah, C.; Iriyanto, F.; Darmanto, W.; Sugiharto, B. Detection and Response of Sugarcane against the Infection of Sugarcane Mosaic Virus (SCMV) in Indonesia. Agronomy 2017, 7, 50. [Google Scholar] [CrossRef]
- Putra, L.K.; Kristini, A.; Achadian, E.M.; Damayanti, T.A. Sugarcane streak mosaic virus in Indonesia: Distribution, Characterisation, Yield Losses and Management Approaches. SugarTech 2014, 16, 392–399. [Google Scholar] [CrossRef]
- Bertasello, L.E.T.; Da Silva, M.F.; Pinto, L.R.; Nóbile, P.M.; Carmo-Sousa, M.; Dos Anjos, I.A.; Perecin, D.; Spotti Lopes, J.R.; Gonçalves, M.C. Yellow Leaf Disease Resistance and Melanaphis sacchari Preference in Commercial Sugarcane Cultivars. Plants 2023, 12, 3079. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wang, Z.; Xu, F.; Pan, Y.-B.; Grisham, M.P.; Xu, L. Sugarcane Mosaic Disease: Characteristics, Identification and Control. Microorganisms 2021, 9, 1984. [Google Scholar] [CrossRef] [PubMed]
- Vamsi Krishna, G.; Manoj Kumar, V.; Kishore Varma, P.; Bhavani, B.; Vijaya Kumar, G. Identification of Resistance to Sugarcane mosaic virus, Sugarcane streak mosaic virus, and Sugarcane bacilliform virus in New Elite Sugarcane Accessions in India. Front. Microbiol. 2023, 14, 1276932. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ghanim, M.; Liu, Y. Editorial: Mixed Infections of Plant Viruses in Nature and the Impact on Agriculture. Front. Microbiol. 2022, 13, 922607. [Google Scholar] [CrossRef]
- He, E.-Q.; Bao, W.-Q.; Sun, S.-R.; Hu, C.-Y.; Chen, J.-S.; Bi, Z.-W.; Xie, Y.; Lu, J.-J.; Gao, S.-J. Incidence and Distribution of Four Viruses Causing Diverse Mosaic Diseases of Sugarcane in China. Agronomy 2022, 12, 302. [Google Scholar] [CrossRef]
- Filloux, D.; Fernandez, E.; Comstock, J.C.; Mollov, D.; Roumagnac, P.; Rott, P. Viral Metagenomic-Based Screening of Sugarcane from Florida Reveals Occurrence of Six Sugarcane-Infecting Viruses and High Prevalence of Sugarcane yellow leaf virus. Plant Dis. 2018, 102, 2317–2323. [Google Scholar] [CrossRef]
- Viswanathan, R.; Balamuralikrishnan, M.; Karuppaiah, R. Characterization and Genetic Diversity of Sugarcane streak mosaic virus Causing Mosaic in Sugarcane. Virus Genes 2008, 36, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Wei, Y.; Yuan, Y.; Khan, M.T.; Qin, L.; Powell, C.A.; Chen, B.; Zhang, M. Gene Expression Profiling of Reactive Oxygen Species (ROS) and Antioxidant Defense System following Sugarcane Mosaic Virus (SCMV) Infection. BMC Plant Biol. 2020, 20, 532. [Google Scholar] [CrossRef] [PubMed]
- Sawitri, W.D. Identification of Chinese Cabbage Sentrin as a Suppressor of Bax-Induced Cell Death in Yeast. J. Microbiol. Biotechnol. 2012, 22, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, Y.; Zhao, Y.; Zhang, J.; Luo, L.; Li, M.; Wang, J.; Yu, H.; Liu, G.; Yang, L.; et al. Deficient Plastidic Fatty Acid Synthesis Triggers Cell Death by Modulating Mitochondrial Reactive Oxygen Species. Cell Res. 2015, 25, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Huseynova, I.M.; Mirzayeva, S.M.; Sultanova, N.F.; Aliyeva, D.R.; Mustafayev, N.S.; Aliyev, J.A. Virus-Induced Changes in Photosynthetic Parameters and Peroxidase Isoenzyme Contents in Tomato (Solanum lycopersicum L.) Plants. Photosynthetica 2018, 56, 841–850. [Google Scholar] [CrossRef]
- Manimekalai, R.; Narayanan, J.; Ranjini, R.; Gokul, M.; Selvi, A.; Kumar, P.; Gomathi, R. Hydrogen Peroxide-Induced Oxidative Stress in Sugarcane and Response Expression Pattern of Stress-Responsive Genes through Quantitative RT-PCR. SugarTech 2018, 20, 681–691. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS Generated from Biotic Stress: Effects on Plants and Alleviation by Endophytic Microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Du, K.; Xie, J.; Sun, G.; Wang, P.; Chen, X.; Cao, Z.; Wang, B.; Chao, Q.; Li, X.; et al. Activated Malate Circulation Contributes to the Manifestation of Light-Dependent Mosaic Symptoms. Cell Rep. 2023, 42, 112333. [Google Scholar] [CrossRef]
- Ludwig, M. The Roles of Organic Acids in C4 Photosynthesis. Front. Plant Sci. 2016, 7, 647. [Google Scholar] [CrossRef]
- Chastain, C.J.; Chollet, R. Regulation of Pyruvate, Orthophosphate Dikinase by ADP-/Pi-Dependent Reversible Phosphorylation in C3 and C4 Plants. Plant Physiol. Biochem. 2003, 41, 523–532. [Google Scholar] [CrossRef]
- Huber, S.C.; Huber, J.L. Role and Regulation of Sucrose-Phosphate Synthase in Higher Plants. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1996, 47, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Yao, W.; Yu, K.; Qin, L.; Ruan, M.; Powell, C.A.; Chen, B.; Zhang, M. Photosynthetic Characterization and Expression Profiles of Sugarcane Infected by Sugarcane Mosaic Virus (SCMV). Photosynth. Res. 2021, 150, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Deng, Q.; Chen, J.; Shen, W. Physiological and Molecular Mechanisms Governing the Effect of Virus-Free Chewing Cane Seedlings on Yield and Quality. Sci. Rep. 2020, 10, 10306. [Google Scholar] [CrossRef] [PubMed]
- Zanini, A.A.; Di Feo, L.; Luna, D.F.; Paccioretti, P.; Collavino, A.; Rodriguez, M.S. Cassava common mosaic virus Infection Causes Alterations in Chloroplast Ultrastructure, function, and carbohydrate metabolism of cassava plants. Plant Pathol. 2021, 70, 195–205. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Shen, W.; Li, M.; Fu, Y.; Li, Z.; Li, J.; Liu, H.; Su, X.; Zhang, B.; et al. Integrated Transcriptome and microRNA Sequencing Analyses Reveal Gene Responses in Poplar Leaves Infected by the Novel Pathogen Bean Common Mosaic Virus (BCMV). Front. Plant Sci. 2023, 14, 1163232. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, I.M.; Peter Van Esse, H.; Ballester, A.-R.; Hogewoning, S.W.; Parra, N.O.; Paeleman, A.; Lievens, B.; Bovy, A.G.; Thomma, B.P.H.J. Differential Tomato Transcriptomic Responses Induced by Pepino Mosaic Virus Isolates with Differential Aggressiveness. Plant Physiol. 2011, 156, 301–318. [Google Scholar] [CrossRef]
- Pesti, R.; Kontra, L.; Paul, K.; Vass, I.; Csorba, T.; Havelda, Z.; Várallyay, É. Differential Gene Expression and Physiological Changes during Acute or Persistent Plant Virus Interactions May Contribute to Viral Symptom Differences. PLoS ONE 2019, 14, e0216618. [Google Scholar] [CrossRef] [PubMed]
- Tugume, A.K.; Mukasa, S.B.; Valkonen, J.P.T. Mixed Infections of Four Viruses, the Incidence and Phylogenetic Relationships of Sweet Potato Chlorotic Fleck Virus (Betaflexiviridae) Isolates in Wild Species and Sweetpotatoes in Uganda and Evidence of Distinct Isolates in East Africa. PLoS ONE 2016, 11, e0167769. [Google Scholar] [CrossRef]
- Syller, J. Facilitative and Antagonistic Interactions between Plant Viruses in Mixed Infections. Mol. Plant Pathol. 2012, 13, 204–216. [Google Scholar] [CrossRef]
- Moreno, A.B.; López-Moya, J.J. When Viruses Play Team Sports: Mixed Infections in Plants. Phytopathology 2020, 110, 29–48. [Google Scholar] [CrossRef]
- Redinbaugh, M.G.; Stewart, L.R. Maize Lethal Necrosis: An Emerging, Synergistic Viral Disease. Annu. Rev. Virol. 2018, 5, 301–322. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Wang, C.; Qian, Y.; Li, Z.; Hong, J.; Zhou, X. Further Characterization of Maize chlorotic mottle virus and Its Synergistic Interaction with Sugarcane mosaic virus in Maize. Sci. Rep. 2017, 7, 39960. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.H.; Barbedo, J.G.A.; Del Ponte, E.M.; Bohnenkamp, D.; Mahlein, A.-K. From Visual Estimates to Fully Automated Sensor-Based Measurements of Plant Disease Severity: Status and Challenges for Improving Accuracy. Phytopathol. Res. 2020, 2, 9. [Google Scholar] [CrossRef]
- Apriasti, R.; Widyaningrum, S.; Hidayati, W.N.; Sawitri, W.D.; Darsono, N.; Hase, T.; Sugiharto, B. Full Sequence of the Coat protein GENE is Required for the Induction of Pathogen-Derived Resistance against Sugarcane Mosaic Virus in Transgenic Sugarcane. Mol. Biol. Rep. 2018, 45, 2749–2758. [Google Scholar] [CrossRef]
- Liu, F.; Huang, N.; Wang, L.; Ling, H.; Sun, T.; Ahmad, W.; Muhammad, K.; Guo, J.; Xu, L.; Gao, S.; et al. A Novel L-ascorbate Peroxidase 6 Gene, ScAPX6, Plays an Important Role in the Regulation of Response to Biotic and Abiotic Stresses in Sugarcane. Front. Plant Sci. 2018, 8, 2262. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Anur, R.M.; Mufithah, N.; Sawitri, W.D.; Sakakibara, H.; Sugiharto, B. Overexpression of Sucrose Phosphate Synthase Enhanced Sucrose Content and Biomass Production in Transgenic Sugarcane. Plants 2020, 9, 200. [Google Scholar] [CrossRef]
- Pérez-Patricio, M.; Camas-Anzueto, J.; Sanchez-Alegría, A.; Aguilar-González, A.; Gutiérrez-Miceli, F.; Escobar-Gómez, E.; Voisin, Y.; Rios-Rojas, C.; Grajales-Coutiño, R. Optical Method for Estimating the Chlorophyll Contents in Plant Leaves. Sensors 2018, 18, 650. [Google Scholar] [CrossRef]
- Fiorentini, M.; Zenobi, S.; Giorgini, E.; Basili, D.; Conti, C.; Pro, C.; Monaci, E.; Orsini, R. Nitrogen and Chlorophyll Status Determination in Durum Wheat as Influenced by Fertilization and Soil Management: Preliminary Results. PLoS ONE 2019, 14, e0225126. [Google Scholar] [CrossRef]
- Bwalya, J.; Alazem, M.; Kim, K. Photosynthesis-Related Genes Induce Resistance against Soybean Mosaic Virus: Evidence for Involvement of the RNA Silencing Pathway. Mol. Plant Pathol. 2022, 23, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Studer, R.A.; Christin, P.-A.; Williams, M.A.; Orengo, C.A. Stability-Activity Tradeoffs Constrain the Adaptive Evolution of RubisCO. Proc. Natl. Acad. Sci. USA 2014, 111, 2223–2228. [Google Scholar] [CrossRef]
- Komínková, M.; Ben Mansour, K.; Komínek, P.; Brožová, J.; Střalková, R. Multiple Infections with Viruses of the Family Tymoviridae in Czech Grapevines. Viruses 2024, 16, 343. [Google Scholar] [CrossRef] [PubMed]
- Widyaningrum, S.; Pujiasih, D.R.; Sholeha, W.; Harmoko, R.; Sugiharto, B. Induction of Resistance to Sugarcane Mosaic Virus by RNA Interference Targeting Coat Protein Gene Silencing in Transgenic Sugarcane. Mol. Biol. Rep. 2021, 48, 3047–3054. [Google Scholar] [CrossRef] [PubMed]
- Hidayati, W.N.; Apriasti, R.; Addy, H.S.; Sugiharto, B. Distinguishing Resistances of Transgenic Sugarcane Generated from RNA Interference and Pathogen-Derived Resistance Approaches to Combating Sugarcane Mosaic Virus. Indones. J. Biotechnol. 2021, 26, 107. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Pai, H.; Hou, L.-Y.; Lee, S.-C.; Lin, T.-T.; Chang, C.-H.; Hsu, F.-C.; Hsu, Y.-H.; Lin, N.-S. Dual Resistance of Transgenic Plants against Cymbidium mosaic virus and Odontoglossum ringspot virus. Sci. Rep. 2019, 9, 10230. [Google Scholar] [CrossRef]
- Tatineni, S.; Sato, S.; Nersesian, N.; Alexander, J.; Quach, T.; Graybosch, R.A.; Clemente, T.E. Transgenic Wheat Harboring an RNAi Element Confers Dual Resistance Against Synergistically Interacting Wheat Streak Mosaic Virus and Triticum Mosaic Virus. MPMI 2020, 33, 108–122. [Google Scholar] [CrossRef]


| Primer | Sequence Information (5′-3′) | Amplicon Length (bp) | Annealing (°C) | Reference |
|---|---|---|---|---|
| CP-SCSMV-F | 5′-MTCTTCATCRGCCGCMTCRATAC-3′ | 335 bp | 53 | This study |
| CP-SCSMV-R | 5′-AGAACTGAACCCACTTGTACGCC-3′ | |||
| CP-SCMV-F | 5′-GACATATGGATGTAGATGCTGGTACGACA-3′ | 735 bp | 58 | [35] |
| CP-SCMV-R | 5′-ATGGATCCTAGTGGTGCTGCTGCACTCCC-3′ | |||
| CP-SrMV-F | 5′-GCAGATGCTGATGCGAAA-3′ | 480 bp | 52 | This study |
| CP-SrMV-R | 5′-ACGCTTCAGCTGCATCAC-3′ | |||
| PEPC-F | 5′-TGGGTGGTGACCGTGATGG-3′ | 129 bp | 60 | This study |
| PEPC-R | 5′-GCAGCGCCACATAGAGAGC-3′ | |||
| PsaA-F | 5′-AGGGGCTTATACCCTCAG-3′ | 121 bp | 52 | This study |
| PsaA-R | 5′-GGATTAGGTGCCTAACGGAC-3′ | |||
| RbcS-F | 5′-CACTAGCTTCGCCAAAGT-3′ | 205 bp | 55 | This study |
| RbcS-R | 5′-AAGCCTTCCTTGCTGAAC-3′ | |||
| SPS-F | 5′-GGGTCTCCATAGGACCATTA-3′ | 110 bp | 55 | This study |
| SPS-R | 5′-GGGGTGTTATTGTGTGAGTA-3′ | |||
| CatF | 5′-GCTCAGTTCGACAGGGAACG-3′ | 208 bp | 55 | [16] |
| CatR | 5′-CACGTGGATCCCTCAAGGTC-3′ | |||
| ApxF | 5′-GATTTGATTGCCGTGGCTGG-3′ | 134 bp | 55 | [36] |
| ApxR | 5′-TCTTCAGGAAGTTTGCCAGTTG-3′ | |||
| β-tubulinF | 5′-CATCTTCGTGTGGAATCCT-3′ | 129 bp | 55 | This study |
| β-tubulinR | 5′-AACTCCAGAACAGACCGTA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neliana, I.R.; Soleha, W.; Suherman; Darsono, N.; Harmoko, R.; Sawitri, W.D.; Sugiharto, B. Alteration of Photosynthetic and Antioxidant Gene Expression in Sugarcane Infected by Multiple Mosaic Viruses. Int. J. Plant Biol. 2024, 15, 757-768. https://doi.org/10.3390/ijpb15030055
Neliana IR, Soleha W, Suherman, Darsono N, Harmoko R, Sawitri WD, Sugiharto B. Alteration of Photosynthetic and Antioxidant Gene Expression in Sugarcane Infected by Multiple Mosaic Viruses. International Journal of Plant Biology. 2024; 15(3):757-768. https://doi.org/10.3390/ijpb15030055
Chicago/Turabian StyleNeliana, Intan Ria, Wardatus Soleha, Suherman, Nurmalasari Darsono, Rikno Harmoko, Widhi Dyah Sawitri, and Bambang Sugiharto. 2024. "Alteration of Photosynthetic and Antioxidant Gene Expression in Sugarcane Infected by Multiple Mosaic Viruses" International Journal of Plant Biology 15, no. 3: 757-768. https://doi.org/10.3390/ijpb15030055
APA StyleNeliana, I. R., Soleha, W., Suherman, Darsono, N., Harmoko, R., Sawitri, W. D., & Sugiharto, B. (2024). Alteration of Photosynthetic and Antioxidant Gene Expression in Sugarcane Infected by Multiple Mosaic Viruses. International Journal of Plant Biology, 15(3), 757-768. https://doi.org/10.3390/ijpb15030055







