Regional Variability in Growth and Leaf Functional Traits of Mitragyna speciosa in Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Soil Conditions
2.3. Plant Selection and Growth Measurement
2.4. Leaf Functional Trait Measurements
2.5. Data Analysis
3. Results
3.1. Soil Conditions
3.2. Kratom Growth
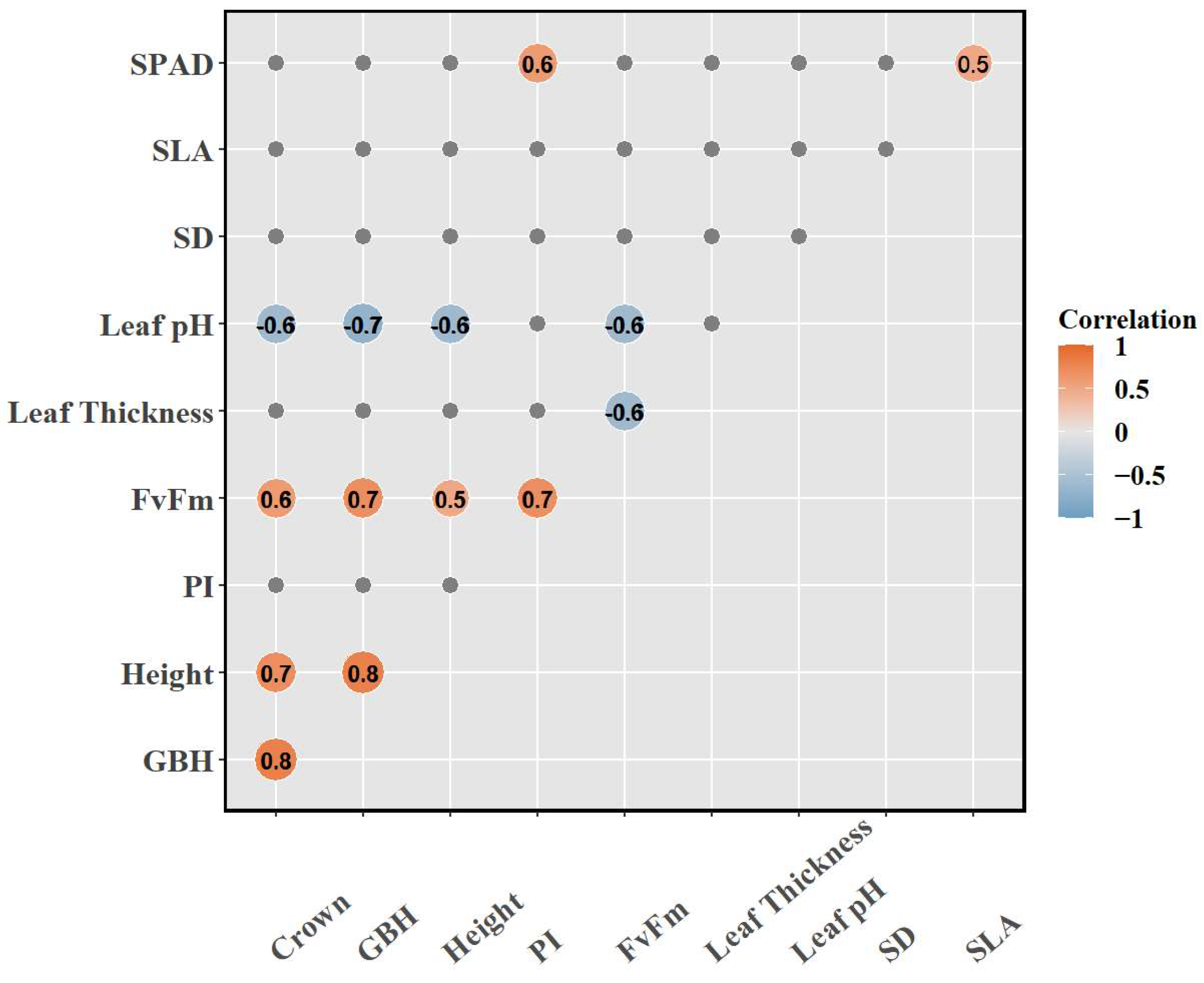
3.3. Leaf Functional Traits
3.4. Principal Component Analysis (PCA)
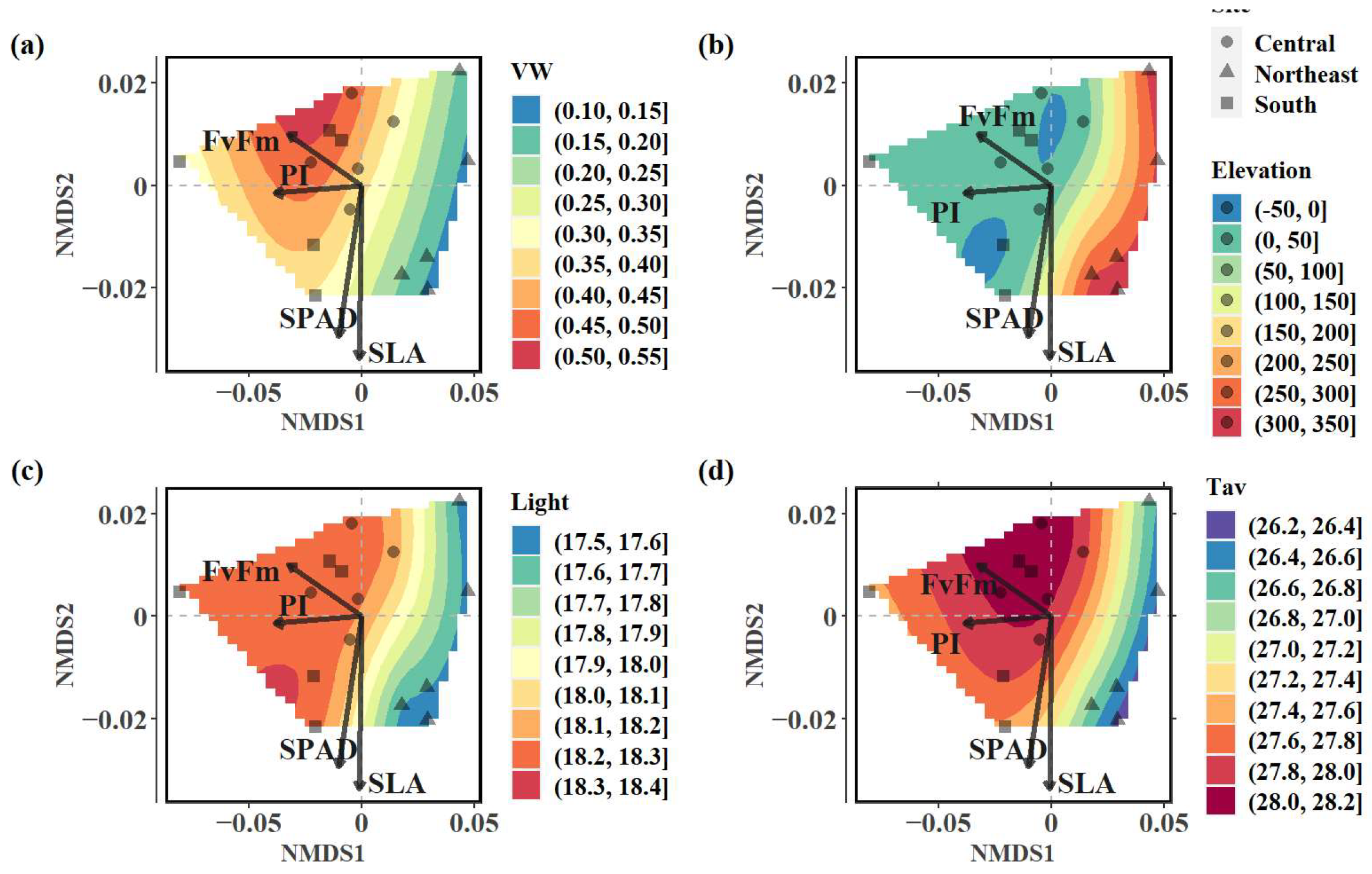
3.5. Non-Metric Multidimensional Scaling (NMDS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Firmansyah, A.; Sundalian, M.; Taufiq, M. Kratom (Mitragyna speciosa Korth) for a New Medicinal: A Review of Pharmacological and Compound Analysis. Biointerface Res. Appl. Chem. 2021, 11, 9704–9718. [Google Scholar]
- Singh, D.; Müller, C.P.; Vicknasingam, B.K. Kratom (Mitragyna speciosa) Dependence, Withdrawal Symptoms and Craving in Regular Users. Drug Alcohol Depend. 2014, 139, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Ngernsaengsaruay, C.; Leksungnoen, N.; Boonthasak, W.; Utharatsamee, S.; Racharak, P.; Leetanasakskul, K.; Pongamorn, P.; Saengbuapuean, A. Additional Knowledge on the Genus Mitragyna (Rubiaceae) in Thailand. Thai For. Bull. 2022, 50, 20–39. [Google Scholar] [CrossRef]
- Ghazalli, M.N.; Sah, M.S.M.; Mat, M.; Awang, K.; Jaafar, M.A.; Mirad, R.; Zaini, A.Z.; Nordin, A.R.M.; Rusli, N.M.; Mohamad, S.S.; et al. Leaf Anatomy and Micromorphology Characteristics of Ketum [Mitragyna speciosa (Korth.) Havil.] (Rubiaceae). Trop. Life Sci. Res. 2021, 32, 107. [Google Scholar] [CrossRef] [PubMed]
- Kruegel, A.C.; Grundmann, O. The Medicinal Chemistry and Neuropharmacology of Kratom: A Preliminary Discussion of a Promising Medicinal Plant and Analysis of Its Potential for Abuse. Neuropharmacology 2018, 134, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Halpenny, G.M. Mitragyna speciosa: Balancing Potential Medical Benefits and Abuse. ACS Med. Chem. Lett. 2017, 8, 897–899. [Google Scholar] [CrossRef]
- Grundmann, O. Patterns of Kratom Use and Health Impact in the US—Results from an Online Survey. Drug Alcohol Depend. 2017, 176, 63–70. [Google Scholar] [CrossRef]
- Singh, D.; Narayanan, S.; Vicknasingam, B.; Corazza, O.; Santacroce, R.; Roman-Urrestarazu, A. Changing Trends in the Use of Kratom (Mitragyna speciosa) in Southeast Asia. Hum. Psychopharmacol. Clin. Exp. 2017, 32, e2582. [Google Scholar] [CrossRef]
- Swogger, M.T.; Smith, K.E.; Garcia-Romeu, A.; Grundmann, O.; Veltri, C.A.; Henningfield, J.E.; Busch, L.Y. Understanding Kratom Use: A Guide for Healthcare Providers. Front. Pharmacol. 2022, 13, 801855. [Google Scholar] [CrossRef] [PubMed]
- Coe, M.A.; Pillitteri, J.L.; Sembower, M.A.; Gerlach, K.K.; Henningfield, J.E. Kratom as a Substitute for Opioids: Results from an Online Survey. Drug Alcohol Depend. 2019, 202, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Assanangkornchai, S.; Muekthong, A.; Sam-Angsri, N.; Pattanasattayawong, U. The Use of Mitragynine speciosa (“Krathom”), an Addictive Plant, in Thailand. Subst. Use Misuse 2007, 42, 2145–2157. [Google Scholar] [CrossRef]
- Chokprajakchat, S.; Techagaisiyavanit, W.; Iyavarakul, T.; Kuanliang, A. When Criminal Diversion Is a Temporary Solution: Rethinking Drug Rehabilitation Policy in Thailand. Curr. Issues Crim. Justice 2022, 34, 418–434. [Google Scholar] [CrossRef]
- Charoenratana, S.; Anukul, C.; Aramrattana, A. Attitudes towards Kratom Use, Decriminalization and the Development of a Community-Based Kratom Control Mechanism in Southern Thailand. Int. J. Drug Policy 2021, 95, 103197. [Google Scholar] [CrossRef] [PubMed]
- Phromchan, W.; Defri, I.; Saensano, C.; Chookaew, A.; Chiarawipa, R.; Sriwiriyajan, S. Morphological and Physiological Properties of Kratom (Mitragyna speciosa) Leaves: Macronutrients, Phytochemicals, Antioxidants, and Mitragynine Content. Plant Sci. Today 2024, 11, 762–770. [Google Scholar] [CrossRef]
- Leksungnoen, N.; Andriyas, T.; Ngernsaengsaruay, C.; Uthairatsamee, S.; Racharak, P.; Sonjaroon, W.; Kjelgren, R.; Pearson, B.J.; McCurdy, C.R.; Sharma, A. Variations in Mitragynine Content in the Naturally Growing Kratom (Mitragyna speciosa) Population of Thailand. Front. Plant Sci. 2022, 13, 1028547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sharma, A.; León, F.; Avery, B.; Kjelgren, R.; McCurdy, C.R.; Pearson, B.J. Plant Growth and Phytoactive Alkaloid Synthesis in Kratom [Mitragyna speciosa (Korth.)] in Response to Varying Radiance. PLoS ONE 2022, 17, e0259326. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. A Review of Light Interception in Plant Stands from Leaf to Canopy in Different Plant Functional Types and in Species with Varying Shade Tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Dörken, V.M.; Lepetit, B. Morpho-Anatomical and Physiological Differences between Sun and Shade Leaves in Abies Alba Mill.(Pinaceae, Coniferales): A Combined Approach. Plant Cell Environ. 2018, 41, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Givnish, T.J. Adaptation to Sun and Shade: A Whole-Plant Perspective. Funct. Plant Biol. 1988, 15, 63–92. [Google Scholar] [CrossRef]
- Boardman, N.K.T. Comparative Photosynthesis of Sun and Shade Plants. Annu. Rev. Plant Physiol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. Corrigendum to: New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef]
- Puglielli, G.; Varone, L.; Gratani, L.; Catoni, R. Specific Leaf Area Variations Drive Acclimation of Cistus Salvifolius in Different Light Environments. Photosynthetica 2017, 55, 31–40. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Raghubanshi, A.S. A Functional Trait Approach for Understanding Woody Species Assemblage in Tropical Deciduous Forest. MOJ Ecol. Environ. Sci. 2018, 3, 167–169. [Google Scholar]
- Torres-Leite, F.; Cavatte, P.C.; Garbin, M.L.; Hollunder, R.K.; Ferreira-Santos, K.; Capetine, T.B.; Soares, B.S.; Carrijo, T.T. Surviving in the Shadows: Light Responses of Co-Occurring Rubiaceae Species within a Tropical Forest Understory. Flora 2019, 261, 151487. [Google Scholar] [CrossRef]
- Xu, F.; Guo, W.; Xu, W.; Wei, Y.; Wang, R. Leaf Morphology Correlates with Water and Light Availability: What Consequences for Simple and Compound Leaves? Prog. Nat. Sci. 2009, 19, 1789–1798. [Google Scholar] [CrossRef]
- Schmitt, S.; Trueba, S.; Coste, S.; Ducouret, E.; Tysklind, N.; Heuertz, M.; Bonal, D.; Burban, B.; Herault, B.; Derroire, G. Seasonal Variation of Leaf Thickness: An Overlooked Component of Functional Trait Variability. Plant Biol. 2022, 24, 458–463. [Google Scholar] [CrossRef]
- Leenawarat, D.; Luang-on, J.; Buranapratheprat, A.; Ishizaka, J. Influences of Tropical Monsoon and El Niño Southern Oscillations on Surface Chlorophyll-a Variability in the Gulf of Thailand. Front. Clim. 2022, 4, 936011. [Google Scholar] [CrossRef]
- Asanok, L.; Kamyo, T.; Norsaengsri, M.; Salinla-um, P.; Rodrungruang, K.; Karnasuta, N.; Navakam, S.; Pattanakiat, S.; Marod, D.; Duengkae, P.; et al. Vegetation Community and Factors That Affect the Woody Species Composition of Riparian Forests Growing in an Urbanizing Landscape along the Chao Phraya River, Central Thailand. Urban For. Urban Green. 2017, 28, 138–149. [Google Scholar] [CrossRef]
- Kunstadter, P.R.; Chapman, E.C.; Sabhasri, S. Farmers in the Forest: Economic Development and Marginal Agriculture in Northern Thailand; University of Hawaii Press: Kolowalu St, HI, USA, 2019. [Google Scholar]
- Wiriyakitnateekul, W.; Kerdchana, C. Methods of Soil Analysis and Interpretation for Soil Survey and Classification: Physical Properties; Land Development Department, Bangkok: Bangkok, Thailand, 2016. [Google Scholar]
- Toková, L.; Igaz, D.; Aydin, E. Measurement of Volumetric Water Content by Gravimetric and Time Domain Reflectometry Methods at Field Experiment with Biochar and N Fertilizer. Acta Hortic. Regiotect. 2019, 22, 61–64. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Wang, C.; Liu, J.; Guo, Z.; Gao, L.; Ma, H.; Yuan, Z.; Yao, C.; Pan, X. Predicting the Soil Bulk Density Using a New Spectral PTF Based on Intact Samples. Geoderma 2024, 449, 117005. [Google Scholar] [CrossRef]
- Phogat, V.K.; Tomar, V.S.; Dahiya, R. Soil Physical Properties. Soil Sci. Introd. 2015, 1, 135–171. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 14. [Google Scholar]
- Walkley, A. A Critical Examination of a Rapid Method for Determining Organic Carbon in Soils—Effect of Variations in Digestion Conditions and of Inorganic Soil Constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Clemente, A.A.; Maciel, G.M.; Siquieroli, A.C.; Gallis, R.B.; Luz, J.M.; Sala, F.C.; Pereira, L.M.; Yada, R.Y. Nutritional Characterization Based on Vegetation Indices to Detect Anthocyanins, Carotenoids, and Chlorophylls in Mini-Lettuce. Agronomy 2023, 13, 1403. [Google Scholar] [CrossRef]
- Leksungnoen, N. Physiological Traits Contributing to Carbon Storage Variation in Monastery Bamboo and Pai Liang in Northeastern Thailand. Songklanakarin J. Sci. Technol. 2017, 39, 215–223. [Google Scholar] [CrossRef]
- Viljevac Vuletić, M.; Španić, V. Characterization of Photosynthetic Performance during Natural Leaf Senescence in Winter Wheat: Multivariate Analysis as a Tool for Phenotypic Characterization. Photosynthetica 2020, 58, 301–313. [Google Scholar] [CrossRef]
- Sonjaroon, W.; Kaveeta, L.; Chai-arree, W.; Klinsakorn, S.; Suksamrarn, A.; Jutamanee, K. Exogenous 7,8-Dihydro-8α-20-Hydroxyecdysone Application Improves Antioxidative Enzyme System, Photosynthesis, and Yield in Rice under High-Temperature Condition. Acta Physiol. Plant. 2016, 38, 202. [Google Scholar] [CrossRef]
- Arasa-Gisbert, R.; Arroyo-Rodríguez, V.; Meave, J.A.; Martínez-Ramos, M.; Lohbeck, M. Forest Loss and Treeless Matrices Cause the Functional Impoverishment of Sapling Communities in Old-Growth Forest Patches across Tropical Regions. J. Appl. Ecol. 2022, 59, 1897–1910. [Google Scholar] [CrossRef]
- Gao, J.; Wang, K.; Zhang, X. Patterns and Drivers of Community Specific Leaf Area in China. Glob. Ecol. Conserv. 2022, 33, e01971. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, Y.; Dong, F.; Chen, L.; Li, Z. Linkage between Leaf Anatomical Structure and Key Leaf Economic Traits across Co-Existing Species in Temperate Forests. Plant Soil 2024, 1–12. [Google Scholar] [CrossRef]
- Ye, Y.; Kitayama, K.; Onoda, Y. A Cost--Benefit Analysis of Leaf Carbon Economy with Consideration of Seasonal Changes in Leaf Traits for Sympatric Deciduous and Evergreen Congeners: Implications for Their Coexistence. New Phytol. 2022, 234, 1047–1058. [Google Scholar] [CrossRef]
- Lu, Z.; Ren, T.; Li, J.; Hu, W.; Zhang, J.; Yan, J.; Li, X.; Cong, R.; Guo, S.; Lu, J. Nutrition-Mediated Cell and Tissue-Level Anatomy Triggers the Covariation of Leaf Photosynthesis and Leaf Mass per Area. J. Exp. Bot. 2020, 71, 6524–6537. [Google Scholar] [CrossRef] [PubMed]
- Pauli, D.; White, J.W.; Andrade-Sanchez, P.; Conley, M.M.; Heun, J.; Thorp, K.R.; French, A.N.; Hunsaker, D.J.; Carmo-Silva, E.; Wang, G.; et al. Investigation of the Influence of Leaf Thickness on Canopy Reflectance and Physiological Traits in Upland and Pima Cotton Populations. Front. Plant Sci. 2017, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Tian, T.; Kou, M.; Liu, P.; Wang, L.; Hao, Z.; Yue, M. The Relationships between Photosynthesis and Stomatal Traits on the Loess Plateau. Glob. Ecol. Conserv. 2020, 23, e01146. [Google Scholar] [CrossRef]
- de Mendiburu, F.; de Mendiburu, M.F. Package ‘Agricolae’. R Packag. Version 2019, 1, 1143–1149. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Oksanen, J. Vegan: Community Ecology Package. 2010. Available online: http://vegan.r-forge.r-project.org (accessed on 15 September 2024).
- Team, R.C. R: A Language and Environment for Statistical Computing, R Foundation for Statistical. Computing 2023. [Google Scholar]
- Sengnon, N.; Vonghirundecha, P.; Chaichan, W.; Juengwatanatrakul, T.; Onthong, J.; Kitprasong, P.; Sriwiriyajan, S.; Chittrakarn, S.; Limsuwanchote, S.; Wungsintaweekul, J. Seasonal and Geographic Variation in Alkaloid Content of Kratom (Mitragyna speciosa (Korth.) Havil.) from Thailand. Plants 2023, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Veltri, C.; Grundmann, O. Current Perspectives on the Impact of Kratom Use. Subst. Abuse Rehabil. 2019, 10, 23–31. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slamka, P. Performance Index as a Sensitive Indicator of Water Stress in Triticum aestivum L. Plant Soil. Env. 2008, 54, 133–139. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon Yield of O2 Evolution and Chlorophyll Fluorescence Characteristics at 77 K among Vascular Plants of Diverse Origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and Metabolic Limitations to Photosynthesis under Drought and Salinity in C3 Plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Krause, A.G.H.; Weis, E. Chlorophyll Fluorescence and Photosynthesis: The Basics. Annu. Rev. Plant Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Klesse, S.; Wohlgemuth, T.; Meusburger, K.; Vitasse, Y.; von Arx, G.; Lévesque, M.; Neycken, A.; Braun, S.; Dubach, V.; Gessler, A.; et al. Long-Term Soil Water Limitation and Previous Tree Vigor Drive Local Variability of Drought-Induced Crown Dieback in Fagus Sylvatica. Sci. Total Environ. 2022, 851, 157926. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhao, X.; Qu, H.; Zuo, X.; Wang, S.; Huang, W.; Luo, Y.; Chen, M. Photosynthetic Performance and Growth Traits in Pennisetum centrasiaticum Exposed to Drought and Rewatering under Different Soil Nutrient Regimes. Acta Physiol. Plant. 2014, 36, 381–388. [Google Scholar] [CrossRef]
- Wall, A.; Heiskanen, J. Soil—Water Content and Air-Filled Porosity Affect Height Growth of Scots Pine in Afforested Arable Land in Finland. For. Ecol. Manage. 2009, 257, 1751–1756. [Google Scholar] [CrossRef]
- Osman, K.T.; Osman, K.T. Plant Nutrients and Soil Fertility Management. Soils Princ. Prop. Manag. 2013, 129–159. [Google Scholar]
- Zhang, Y.; Zhang, Q.; Wang, Y.; Lin, S.; Chen, M.; Cheng, P.; Du, M.; Jia, X.; Ye, J.; Wang, H. Study on the Effect of Magnesium on Leaf Metabolites, Growth and Quality of Tea Tree. Front. Plant Sci. 2023, 14, 1192151. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.S.; Proctor, J. Why Plants Grow Poorly on Very Acid Soils: Are Ecologists Missing the Obvious? J. Exp. Bot. 2001, 52, 791–799. [Google Scholar] [CrossRef]
- Sevillano, I.; Short, I.; Grant, J.; O’Reilly, C. Effects of Light Availability on Morphology, Growth and Biomass Allocation of Fagus sylvatica and Quercus robur Seedlings. For. Ecol. Manag. 2016, 374, 11–19. [Google Scholar] [CrossRef]
- Posada Hostettler, J.M.R.; Lechowicz, M.J.; Kitajima, K. Optimal Photosynthetic Use of Light by Tropical Tree Crowns Achieved by Adjustment of Individual Leaf Angles and Nitrogen Content. Ann. Botany 2009, 103, 795–805. [Google Scholar] [CrossRef]
- Brown, P.N.; Lund, J.A.; Murch, S.J. A Botanical, Phytochemical and Ethnomedicinal Review of the Genus Mitragyna Korth: Implications for Products Sold as Kratom. J. Ethnopharmacol. 2017, 202, 302–325. [Google Scholar] [CrossRef]
- Bloomfield, K.J.; Prentice, I.C.; Cernusak, L.A.; Eamus, D.; Medlyn, B.E.; Rumman, R.; Wright, I.J.; Boer, M.M.; Cale, P.; Cleverly, J.; et al. The Validity of Optimal Leaf Traits Modelled on Environmental Conditions. New Phytol. 2019, 221, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Hilal, B.; Khan, M.M.; Fariduddin, Q. Recent Advancements in Deciphering the Therapeutic Properties of Plant Secondary Metabolites: Phenolics, Terpenes, and Alkaloids. Plant Physiol. Biochem. 2024, 211, 108674. [Google Scholar] [CrossRef] [PubMed]



| Characteristics/Locations | South (S) (Control) | Central (C) | Northeast (NE) |
|---|---|---|---|
| 1. Temperature (°C) | 27.60 | 28.37 | 26.40 |
| 2. Light intensity (MJ m−2 day−1) | 18.26 | 18.20 | 17.57 |
| 3. Rainfall (mm) | 3450 | 1077 | 1507 |
| 4. Relative Humidity (RH) (%) | 79 | 75 | 72 |
| Characters/Locations | South (S) (Control) | Central (C) | Northeast (NE) | p-Value |
|---|---|---|---|---|
| Volumetric water content (VW) (unitless) | 0.38 ± 0.09 a | 0.45 ± 0.09 a | 0.15 ± 0.03 b | <0.001 *** |
| Bulk density (BD) (g cm−3) | 1.36 ± 0.14 | 1.24 ± 0.13 | 1.17 ± 0.02 | 0.110 NS |
| Porosity (%) | 48.66 ± 5.38 | 53.31 ± 4.83 | 55.89 ± 0.96 | 0.089 NS |
| Soil pH (unitless) | 5.31 ± 0.16 | 5.48 ± 0.88 | 5.37 ± 0.18 | 0.867 NS |
| Organic matter (OM) (%) | 1.41 ± 0.32 | 1.29 ± 0.72 | 0.91 ± 0.25 | 0.257 NS |
| Total carbon (C) (%) | 2.54 ± 0.33 | 2.60 ± 1.21 | 2.12 ± 0.22 | 0.218 NS |
| Total nitrogen (N) (%) | 0.21 ± 0.03 | 0.23 ± 0.09 | 0.20 ± 0.02 | 0.649 NS |
| Available phosphorus (P) (mg kg−1) | 22.76 ± 20.99 a | 44.04 ± 46.53 a | 1.85 ± 0.23 b | <0.001 *** |
| Exchangeable potassium (K) (mg kg−1) | 30.28 ± 6.23 b | 173.31 ± 115.72 a | 42.62 ± 23.59 b | <0.001 *** |
| Exchangeable calcium (Ca) (mg kg−1) | 252.36 ± 159.24 b | 1634.4 ± 713.36 a | 241.44 ± 48.66 b | <0.001 *** |
| Exchangeable magnesium (Mg) (mg kg−1) | 27.48 ± 15.86 c | 303.52 ± 60.84 a | 89.71 ± 19.74 b | <0.001 *** |
| Characters/Locations | South (S) (Control) | Central (C) | Northeast (NE) | p-Value |
|---|---|---|---|---|
| Specific leaf area (SLA) (cm2 g−1) | 161.83 ± 23.45 | 152.89 ± 14.12 | 165.32 ± 16.43 | 0.562 NS |
| Leaf thickness (mm) | 0.16 ± 0.01 | 0.16 ± 0.02 | 0.17 ± 0.01 | 0.056 NS |
| Chlorophyll content (SPAD) | 33.79 ± 3.53 | 31.66 ± 2.51 | 33.35 ± 3.48 | 0.557 NS |
| Leaf pH (Unitless) | 4.34 ± 0.25 b | 4.44 ± 0.14 ab | 4.72 ± 0.12 a | 0.014 * |
| Performance index (PI) (Unitless) | 3.26 ± 1.24 a | 2.36 ± 0.32 a | 1.55 ± 0.38 b | 0.001 ** |
| Quantum yield (Fv/Fm) (Unitless) | 0.80 ± 0.01 a | 0.80 ± 0.01 a | 0.72 ± 0.02 b | <0.001 *** |
| Stomatal density (SD) (stomata mm−2) | 352.40 ± 42.80 | 308.64 ± 19.54 | 334.80 ± 42.05 | 0.203 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chongdi, S.; Uthairatsamee, S.; Ngernsaengsaruay, C.; Andriyas, T.; Leksungnoen, N. Regional Variability in Growth and Leaf Functional Traits of Mitragyna speciosa in Thailand. Int. J. Plant Biol. 2025, 16, 24. https://doi.org/10.3390/ijpb16010024
Chongdi S, Uthairatsamee S, Ngernsaengsaruay C, Andriyas T, Leksungnoen N. Regional Variability in Growth and Leaf Functional Traits of Mitragyna speciosa in Thailand. International Journal of Plant Biology. 2025; 16(1):24. https://doi.org/10.3390/ijpb16010024
Chicago/Turabian StyleChongdi, Suthaporn, Suwimon Uthairatsamee, Chatchai Ngernsaengsaruay, Tushar Andriyas, and Nisa Leksungnoen. 2025. "Regional Variability in Growth and Leaf Functional Traits of Mitragyna speciosa in Thailand" International Journal of Plant Biology 16, no. 1: 24. https://doi.org/10.3390/ijpb16010024
APA StyleChongdi, S., Uthairatsamee, S., Ngernsaengsaruay, C., Andriyas, T., & Leksungnoen, N. (2025). Regional Variability in Growth and Leaf Functional Traits of Mitragyna speciosa in Thailand. International Journal of Plant Biology, 16(1), 24. https://doi.org/10.3390/ijpb16010024






