The Optimization of In Vitro Culture Establishment and Shoot Proliferation of “GiSelA 17” (Prunus canescens × Prunus avium): A Novel Cherry Rootstock
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Planting Material
2.2. Sterilization of Planting Material
2.3. Culture Media Preparation
2.4. Culture Establishment and Conditions
2.5. Shoot Proliferation
2.6. Rooting
2.7. Hardening
2.8. Statistical Analysis
3. Results and Discussion
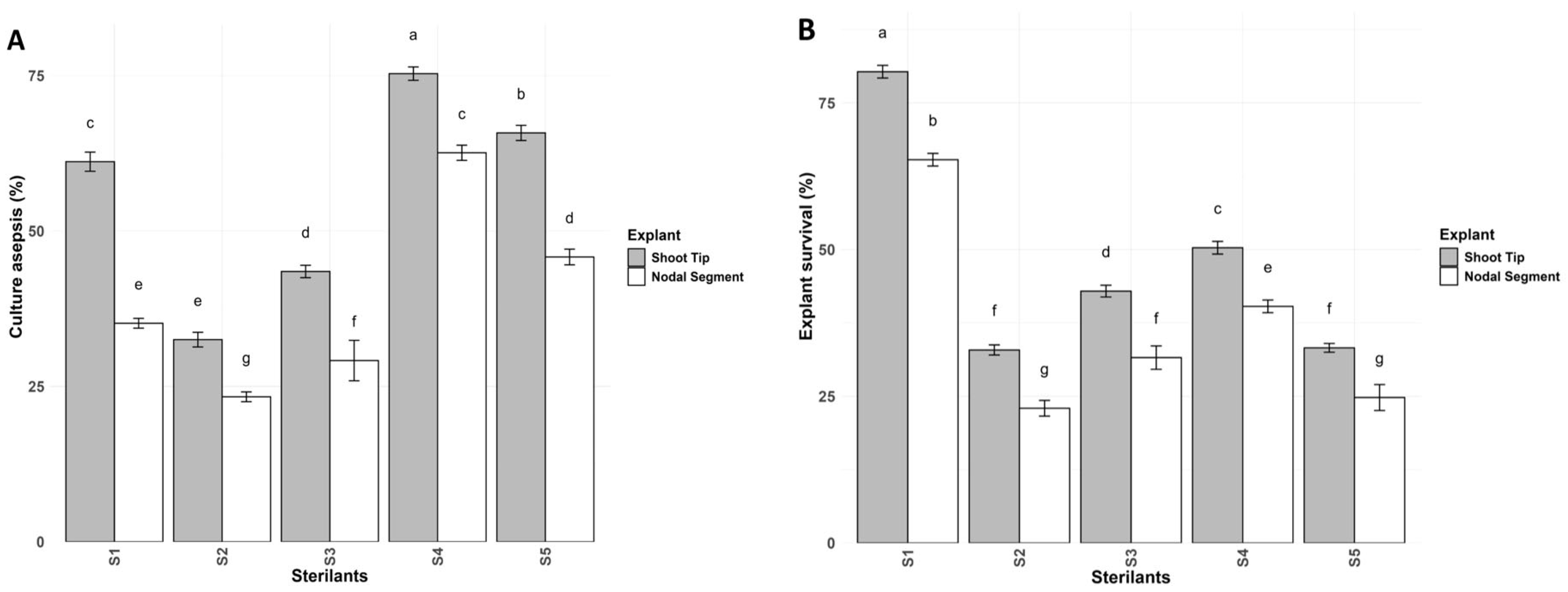
3.1. Sterilization Regime
Culture Asepsis and Explant Survival
3.2. Culture Establishment
Explant Survival, Establishment, and Necrosis
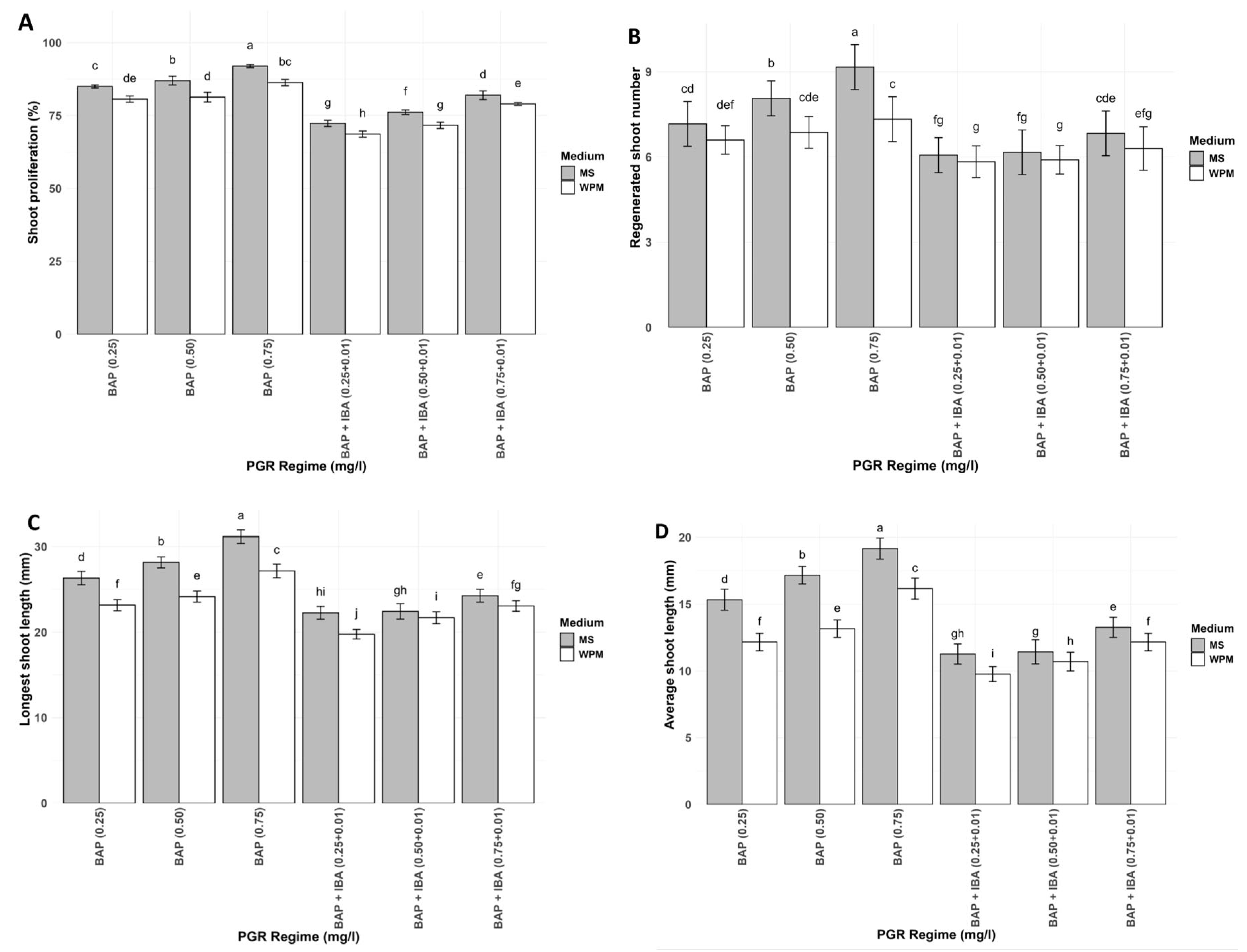
3.3. Shoot Regeneration
3.4. Rooting Process
3.5. Hardening Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GiSelA®17. Available online: https://battistinivivai.com/en/products/gisela-17-rootstock (accessed on 29 November 2024).
- Milkovich, M.; Mullinax, T.J. Good Fruit Grower: A Lot of Choices for Cherry Rootstocks. Available online: https://www.goodfruit.com/a-lot-of-choices-for-cherry-rootstocks/ (accessed on 28 November 2024).
- Wolfe, D.E.; Strang, J.; Becker, D.; Wright, S. Rootstocks for Kentucky Fruit Trees. University of Kentucky. College of Agriculture, Food and Environment. Cooperative Extension Service. Available online: http://www2.ca.uky.edu/agc/pubs/HO/HO82/HO82.pdf. (accessed on 2 December 2024).
- Lang, G.A. Critical concepts for sweet cherry training systems. Compact Fruit Tree 2001, 34, 70–75. [Google Scholar]
- Gregory, P.J.; Atkinson, C.J.; Bengough, A.G.; Else, M.A.; Fernandez-Fernandez, F.; Harrison, R.J.; Schmidt, S. Contributions of roots and rootstocks to sustainable, intensified crop production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Hrotko, K. Development in fruit trees production systems. AgroLife Sci. J. 2013, 2, 28–35. [Google Scholar]
- Ljubojevic, M. Horticulturalization of the 21st century cities. Sci. Hortic. 2021, 288, 110350. [Google Scholar] [CrossRef]
- Atkinson, C.; Else, M. Understanding how rootstocks dwarf fruit trees. Compact Fruit Tree 2001, 34, 46–49. [Google Scholar]
- Webster, A.D. Vigour mechanisms in dwarfing rootstocks for temperate fruit trees. Acta Hortic. 2002, 658, 29–41. [Google Scholar] [CrossRef]
- Hrotko, K. Progress in cherry rootstock research. Acta Hortic 2008, 795, 171–178. [Google Scholar] [CrossRef]
- Gjamovski, V.; Kiprijanovski, M. Influence of nine dwarfing apple rootstocks on vigour and productivity of apple cultivar ‘Granny Smith’. Sci. Hortic. 2011, 129, 742–746. [Google Scholar] [CrossRef]
- Marra, F.P.; Bianco, R.L.; La Mantia, M.; Caruso, T. Growth, yield and fruit quality of ‘Tropic Snow’ peach on size-controlling rootstocks under dry Mediterranean climates. Sci. Hortic. 2013, 160, 274–282. [Google Scholar] [CrossRef]
- Tworkoski, T.; Fazio, G. Hormone and growth interactions of scions and size-controlling rootstocks of young apple trees. Plant Growth Regul. 2016, 78, 105–119. [Google Scholar] [CrossRef]
- Yahmed, J.B.; Ghrab, M.; Mimoun, M.B. Eco-physiological evaluation of different scion-rootstock combinations of almond grown in Mediterranean conditions. Fruits 2016, 71, 185–193. [Google Scholar] [CrossRef]
- Bujdoso, G.; Magyar, L.; Hrotko, K. Long term evaluation of growth and cropping of sweet cherry (Prunus avium L.) varieties on different rootstocks under Hungarian soil and climatic conditions. Sci. Hortic. 2019, 256, 108613. [Google Scholar] [CrossRef]
- Webster, T. Dwarfing rootstocks: Past, present and future. Compact Fruit Tree 2002, 35, 67–72. [Google Scholar]
- Santos, A.; Santos-Ribeiro, R.; Cavalheiro, J.; Cordeiro, V.; Lousada, J.L. Initial growth and fruiting of ‘Summit’ sweet cherry (Prunus avium) on five rootstocks. N. Z. J. Crop Hortic. Sci. 2006, 34, 269–277. [Google Scholar] [CrossRef]
- Wocior, S. The effect of rootstock on the growth and yielding of ‘Regina’ cherry trees. Folia Hortic. 2008, 20, 15–22. [Google Scholar] [CrossRef]
- Lopez-Ortega, G.; Garcia-Montiel, F.; Bayo-Canha, A.; Frutos-Ruiz, C.; Frutos-Tomas, D. Rootstock effects on the growth, yield and fruit quality of sweet cherry cv. ‘Newstar’ in the growing conditions of the region of Murcia. Sci. Hortic. 2016, 198, 326–335. [Google Scholar] [CrossRef]
- Dziedzic, E.; Błaszczyk, J.; Kaczmarczyk, E. Postharvest properties of sweet cherry fruit depending on rootstock and storage conditions. Folia Hortic. 2017, 29, 113–121. [Google Scholar] [CrossRef]
- Pal, M.D.; Mitre, I.; Asanica, A.C.; Sestraș, A.F.; Peticila, A.G.; Mitre, V. The influence of rootstock on the growth and fructification of cherry cultivars in a highdensity cultivation system. Not. Bot. Horti Agrobot. 2017, 45, 451–457. [Google Scholar] [CrossRef]
- Balducci, F.; Capriotti, L.; Mazzoni, L.; Medori, I.; Albanesi, A.; Giovanni, B.; Giampieri, F.; Mezzetti, B.; Capocasa, F. The rootstock effects on vigor, production and fruit quality in sweet cherry (Prunus avium L.). J. Berry Res. 2019, 9, 249–265. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Lugli, S.; Tugnoli, A.; Boini, A.; Perulli, G.D.; Bresilla, K.; Venturi, M.; Grappadelli, L.C. Sweet cherry water relations and fruit production efficiency are affected by rootstock vigor. J. Plant Physiol. 2019, 237, 43–50. [Google Scholar] [CrossRef]
- Callesen, O. Recent developments in cherry rootstock research. Acta Hortic. 1998, 468, 219–228. [Google Scholar] [CrossRef]
- Wertheim, S.J. Rootstock Guide: Apple, Pear, Cherry, European Plum; Fruit Research Station: Wilhelminadorp, The Netherlands, 1998. [Google Scholar]
- Milosevic, T.; Milosevic, N.; Mladenovic, J. Combining fruit quality and main antioxidant attributes in the sour cherry: The role of new clonal rootstock. Sci. Hortic. 2020, 265, 109236. [Google Scholar] [CrossRef]
- Solonkin, A.; Nikolskaya, O.; Seminchenko, E. The effect of low-growing rootstocks on the adaptability and productivity of sour cherry varieties (Prunus cerasus L.) in Arid Conditions. Horticulturae 2022, 8, 400. [Google Scholar] [CrossRef]
- Cline, J.A. Planting density and size-controlling rootstocks influence the performance of Montmorency tart cherry (Prunus cerasus L.). Can. J. Plant Sci. 2019, 100, 16–28. [Google Scholar] [CrossRef]
- Barac, G.; Ognjanov, V.; Vidakovic, D.O.; Doric, D.; Ljubojevic, M.; Dulic, J.; Miodragovic, M.; Gasic, K. Genetic diversity and population structure of European ground cherry (Prunus fruticose Pall.) using SSR markers. Sci. Hortic. 2017, 224, 374–383. [Google Scholar] [CrossRef]
- Ljubojevic, M.; Sebolt, A.; Ognjanov, V.; Iezzoni, A. Heritability of anatomical characteristics in cherry interspecific hybrids. J. Plant Growth Regul. 2022, 41, 965–982. [Google Scholar] [CrossRef]
- Hrotko, K. Potentials in Prunus mahaleb L. for cherry rootstock breeding. Sci. Hortic. 2016, 205, 70–78. [Google Scholar] [CrossRef]
- Bosnjak, A.M.; Keresa, S.; Jercic, I.H.; Baric, M. The effect of cytokinin type and explant orientation on axillary shoot proliferation and in vitro rooting of ‘GiSelA 5’ cherry rootstock. J. Food Agric. Environ. 2012, 10, 616–620. [Google Scholar]
- Bhojwani, S.S.; Dantu, P.K. Micropropagation. In Plant Tissue Culture: An Introductory Text; Bhojwani, S.S., Dantu, P.K., Eds.; Springer: New Delhi, India, 2013. [Google Scholar]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent applications of plant cell culture technology in cosmetics and foods. Eng. Life Sci. 2020, 21, 68–76. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant Propagation by Tissue Culture; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 1. [Google Scholar]
- Cardoso, J.C.; Lee Tseng Sheng, G.; Teixeira da Silva, J.A. Micropropagation in the Twenty-First Century. In Plant Cell Culture Protocols; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Boonsnongcheep, P.; Benyakan, P. Factors affecting micropropagation of Cannabis sativa L.: A review. Pharm. Sci. Asia. 2020, 47, 21–29. [Google Scholar] [CrossRef]
- Adhikary, D.; Kulkarni, M.; El-Mezawy, A.; Mobini, S.; Elhiti, M.; Gjuric, R.; Ray, A.; Polowick, P.; Slaski, J.J.; Jones, M.; et al. Medical cannabis and industrial hemp tissue culture: Present status and future potential. Front. Plant Sci. 2021, 12, 627240. [Google Scholar] [CrossRef]
- Borsai, O.; Clapa, D.; Magdea, A.; Harta, M.; Andrecan, A.; Mitre, V. Effects of different culture media and plant growth regulators on micropropagation of ‘GiSelA 5’ cherry rootstock. Sci. Papers Ser. B Hortic. 2020, 64, 33–40. [Google Scholar]
- Marin, J.A.; Garcia, E.; Lorente, P.; Andreu, P.; Arbeloa, A. Assessing effect of rootstock micropropagation on field performance of grafted peach varieties by fitting mixed-effects models: A longitudinal study. Plants 2023, 12, 674. [Google Scholar] [CrossRef]
- Lacuzzi, N.; Salamone, F.; Farruggia, D.; Tortorici, N.; Vultaggio, L.; Tuttolomondo, T. Development of a new micropropagation protocol and transfer of in vitro plants to in vivo conditions for cascade hop. Plants 2023, 12, 2877. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yu, Q.; Li, D.; Xu, K.; Di, Z.; Zhang, Y.; Yu, Y.; Zheng, J.; Zhang, Y. In vitro propagation, shoot regeneration, callus induction, and suspension from lamina explants of Sorbus caloneura. For. Res. 2023, 3, 7. [Google Scholar] [CrossRef]
- Nacheva, L.; Dimitrova, N.; Koleva-Valkova, L.; Stefanova, M.; Ganeva, T.; Nesheva, M.; Tarakanov, I.; Vassilev, A. In vitro multiplication and rooting of plum rootstock ‘Saint Julien’ (Prunus domestica subsp. insititia) under fluorescent light and different LED spectra. Plants 2023, 12, 2125. [Google Scholar]
- Tariverdi, Z.; Nughabi, K.A.; Piri, S. Propagation of rootstocks of ‘GiSelA 5’ based on tissue culture method. Biosci. Biotech. Res. Asia 2017, 14, 1395–1399. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, V.; Thakur, M. In vitro rooting and hardening of clonal cherry rootstock “GiSelA 5” (Prunus cerasus × Prunus canescens). Int. J. Agr. Sci. 2020, 90, 1032–1035. [Google Scholar] [CrossRef]
- Sharma, K.; Thakur, M.; Sharma, V. Cost effective in vitro propagation of GiSelA 5 cherry rootstock. Indian J. Hortic. 2020, 77, 597–602. [Google Scholar] [CrossRef]
- Jafarlou, A.M.; Pirivatlo, S.P.; Salehi, B.; Mogbli, A.H.H. Establishing an efficient in vitro propagation system for sweet cherry rootstocks “GiSelA 12” and “Maxma14” and assessment of genetic homogeneity by ISSR markers. J. Crop Sci. Biotechnol. 2021, 24, 449–460. [Google Scholar] [CrossRef]
- Nicolae, I.; Venat, O.; Peticila, A.; Ștefanut, M.M.; Hoza, D. Use of different hormones on in vitro propagation of “GiSela 5” cherry rootstock. Sci. Papers Ser. B Hortic. 2022, 66, 407–412. [Google Scholar]
- Sarropoulou, V.; Sperdouli, I.; Adamakis, I.D.; Grigoriadou, K. The use of different LEDs wavelength and light intensities for in vitro proliferation of cherry rootstock: Influence on photosynthesis and photomorphogenesis. Plant Cell Tiss. Organ. Cult. 2023, 152, 317–330. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Propag Soc. 1980, 30, 421–427. [Google Scholar]
- Zhang, A.; Wang, H.; Shao, Q.; Xu, M.; Zhang, W.; Li, M. Large-scale in vitro propagation of Anoectochilus roxburghii for commercial application: Pharmaceutically important and ornamental plant. Ind. Crop. Prod. 2015, 70, 158–162. [Google Scholar] [CrossRef]
- Mamatha, D.S.; Nishani, S.; Mahantesha, B.N.; Hegde, L.; Shet, R.; Prasad, D.S. Tissue culture studies on garsden Rauvolfia (Rauvolfia tetraphylla L.). J. Pharm. Innov. 2021, 10, 1205–1210. [Google Scholar]
- Patil, M.; Bharathi, T.U.; Usharani, T.R.; Kumar, R.; Kulkarni, B.S. Standardization of sterilization protocol for explants and its suitability for direct organogenesis in tuberose cv. Arka Vaibhav. J. Hortic. Sci. 2023, 18, 173–180. [Google Scholar] [CrossRef]
- Supreetha, B.G.; Prakasha, D.P.; Kulapati Hipparagi, P.G. Optimizing sterilization protocols for in vitro culture establishment of finger lime (Citrus australasica F. Muell): A comprehensive investigation. J. Pharm. Innov. 2023, 12, 355–361. [Google Scholar]
- Shaheen, S.; Ankanna, S.; Pattanaik, S.; Savithramma, N. Standardization of explant sterilization for in vitro propagation of Pterocarpus Santalinus L.F.-An endemic, endangered multipurpose medicinal tree taxon. Int. J. Creat. Res. Thoughts 2023, 11, 307–317. [Google Scholar]
- Chokheli, V.; Bakulin, S.; Ermolaeva, O.; Rajput, V.; Azarov, A.S.; Kumari, A.; Stepanenko, V.; Bushkova, A.; Dmitriev, P.; Martinez-Montero, M.E.; et al. An Efficient Method for Micropropagation of Red-List Herbaceous Plant Species (Hedysarum cretaceum). OBM Genet. 2023, 7, 1–14. [Google Scholar] [CrossRef]
- Sereda, M.; Petrenko, V.; Kapralova, O.; Chokheli, V.; Varduni, T.; Dmitriev, P.; Minkina, T.; Sushkova, S.; Barbashev, A.; Dudnikova, T.; et al. Establishment of an In Vitro Micropropagation Protocol for Hibiscus moscheutos L. ‘Berry Awesome’. Horticulturae 2023, 10, 21. [Google Scholar] [CrossRef]
- Ansar, S.; Iqbal, M.J.T.R. Effect of dietary antioxidant on mercuric chloride induced lung toxicity and oxidative stress. Toxin Rev. 2015, 34, 168–172. [Google Scholar] [CrossRef]
- Palei, S.; Das, A.K.; Dash, D.K.; Rout, G.R. Effect of surface sterilant for reducing microbial contamination of field grown strawberry explants intended for in vitro culture. Int. Chem. Stud. 2017, 5, 1476–1479. [Google Scholar]
- Kuppusamy, S.; Ramanathan, S.; Sengodagounder, S.; Senniappan, C.; Shanmuganathan, R.; Brindhadevi, K.; Kaliannan, T. Optimizing the sterilization methods for initiation of the five different clones of the Eucalyptus hybrid species. Biocatal. Agric. Biotechnol. 2019, 22, 101361. [Google Scholar] [CrossRef]
- Kumar, M.; Prasad, Y.; Yadav, A.; Kumar, A. Effects of two different surface sterilization (sodium hypochlorite and mercuric chloride) agents under in-vitro leaf explant in Gerbera (Gerbera jamesonii Bolus). J. Pharm. Innov. 2021, 10, 1346–1349. [Google Scholar]
- Pal, D.; Kumar, M.; Kumar, A.; Sengar, R.S.; Yadav, M.K.; Pal, A.; Singh, K.P.; Pandey, V. Standardization of Surface Sterilization for in vitro Cloning of Pomegranate (Punica granatum L.) cv. Bhagwa. Biol. Forum. 2022, 14, 653–656. [Google Scholar]
- Pandey, V.; Kumar, A.; Kumar, V.; Prakash, S.; Gangwar, L.K.; Sengar, R.S.; Kumar, M.; Pal, A.; Pal, D.; Kumar, A. Standardize the aseptic environment protocol for strawberry in vitro cloning (Fragaria × ananassa Duch.). J. Pharm. Innov. 2023, 12, 1443–1449. [Google Scholar]
- Bhyravi, B.M.; Athani, S.I.; Prakasha, D.P.; Anil, I.S.; MS, K.; Nagesh Naik, D.A.; Puspha, T.N.; Yallesh Kumar, H.S. Effect of different sterilization agents on in vitro culture establishment of guava cv. Arka Kiran. J. Pharm. Innov. 2023, 12, 1761–1766. [Google Scholar]
- Munir, M.; Iqbal, S.; Baloch, J.U.D.; Khakwani, A.A. In vitro explant sterilization and bud initiation studies of four strawberry cultivars. J. Appl. Hortic. 2015, 17, 192–198. [Google Scholar] [CrossRef]
- Ferdous, M.H.; Billah, A.M.; Mehraj, H.; Taufique, T.; Uddin, A.F.M.J. BAP and IBA pulsing for in vitro multiplication of banana cultivars through shoot-tip culture. J. Biosci. Agric. Res. 2015, 3, 87–95. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, V.; Sharma, D.P.; Kumari, G.; Vivek, M. In Vitro Propagation of Virus Indexed Gisela-5 (Prunus cerasus × Prunus canescens)-Clonal Cherry Rootstock. Int. J. Crop Sci. Tech. 2016, 2, 87–99. [Google Scholar]
- Amiri, S.; Mohammadi, R. Establishment of an efficient in vitro propagation protocol for Sumac (Rhus coriaria L.) and confirmation of the genetic homogeneity. Sci. Rep. 2021, 11, 173. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, V.; Luharch, R. Propagation of plum (Prunus salicina L.) cultivar Frontier in vitro through control of shoot tip necrosis (STN) and validation of genetic integrity using ISSR markers. Plant Physiol. Rep. 2021, 26, 238–246. [Google Scholar] [CrossRef]
- Bahgat, H.; Hassan, S.A.M.; Salaheldin, S.; Abou Ellail, M. Tissue culture protocol establishment of Artemisia annua L. plant and artemisinin production. SVU-Int. J. Agric. Sci. 2021, 3, 73–83. [Google Scholar] [CrossRef]
- Ai, X.; Wen, Y.; Wang, B. Assessment of genetic stability on in vitro propagation of Ardisia crenata var. bicolorusing ISSR markers. Int. J. Plant Biol. 2023, 14, 218–227. [Google Scholar] [CrossRef]
- Goyal, S.; Chatterjee, V.; Kulkarni, V.M.; Bhat, V. Plant regeneration through somatic embryogenesis in cell suspensions of Cenchrus ciliaris L. Plant Methods. 2023, 19, 110. [Google Scholar] [CrossRef]
- Welehaweria, M.; Sbhatu, D.B. In vitro micropropagation of Aloe elegans Tod. using offshoot cuttings. BMC Res. Notes 2023, 16, 215. [Google Scholar] [CrossRef]
- Kodad, S.; Melhaoui, R.; Hano, C.; Addi, M.; Sahib, N.; Elamrani, A.; Abid, M.; Mihamou, A. Effect of culture media and plant growth regulators on shoot proliferation and rooting of internode explants from Moroccan native Almond (Prunus dulcis Mill.) genotypes. Int. J. Agron. 2021, 2021, 9931574. [Google Scholar] [CrossRef]
- Shi, J.L.; Dong, Z.D.; Song, C.H.; Xie, B.Y.; Zheng, X.B.; Song, S.W.; Jiao, J.; Wang, M.; Bai, T.H. Establishment of an efficient micropropagation system in enhancing rooting efficiency via stem cuttings of apple rootstock M9T337. Hort. Sci. 2021, 48, 63–72. [Google Scholar] [CrossRef]
- Sivakumar, P.; Visalakshi, M. In vitro micropropagation of banana cv. Poovan (AAB). J. Appl. Hortic. 2021, 23, 37–41. [Google Scholar] [CrossRef]
- Hajare, S.T.; Chauhan, N.M.; Kassa, G. Effect of growth regulators on in vitro micropropagation of potato (Solanum tuberosum L.) Gudiene and Belete varieties from Ethiopia. Sci. World J. 2021, 2021, 5928769. [Google Scholar] [CrossRef] [PubMed]
- Chirumamilla, P.; Gopu, C.; Jogam, P.; Taduri, S. Highly efficient rapid micropropagation and assessment of genetic fidelity of regenerants by ISSR and SCoT markers of Solanum khasianum Clarke. Plant Cell Tissue Organ. Cult. 2021, 144, 397–407. [Google Scholar] [CrossRef]
- Sushmarani, Y.S.; Venkatesha Murthy, P.; Deeksha Raj, N. Influence of BAP with TDZ growth regulators on in vitro regeneration in chrysanthemum (Dendranthema grandiflora T.) cv. Marigold. J. Pharmcog. Phytochem. 2021, 10, 1171–1176. [Google Scholar]
- Kim, T.D.; Kim, N.H.; Park, E.J.; Lee, N.N. High-frequency regeneration of plants in vitro from seedling-derived apical bud explants of Tilia mandshurica Rupr. & Maxim. J. Plant Biotechnol. 2021, 48, 54–61. [Google Scholar]
- Cabral-Miramontes, J.P.; Chavez-Simental, J.A.; Pulido-Díaz, C.; González-Portillo, M.; Goche-Télles, J.R.; Barragán-Hernández, V.M. In vitro propagation of apple tree from mature zygotic embryos. Rev. Mexicanacienc. Agríc. 2022, 13, 603–616. [Google Scholar]
- Iqbal, Z.; Javad, S.; Naz, S.; Shah, A.A.; Shah, A.N.; Paray, B.A.; Gulnaz, N.; Abdelsalam, N.R. Elicitation of the in vitro cultures of selected varieties of Vigna radiata L. with zinc oxide and copper oxide nanoparticles for enhanced phytochemicals production. Front. Plant Sci. 2022, 13, 908532. [Google Scholar] [CrossRef]
- Jafari, M.; Daneshvar, M.H. Prediction and optimization of indirect shoot regeneration of Passiflora caerulea using machine learning and optimization algorithms. BMC Biotechnol. 2023, 23, 27. [Google Scholar] [CrossRef]
- Klanrit, P.; Kitwetcharoen, H.; Thanonkeo, P.; Thanonkeo, S. In vitro propagation of Philodendron erubescens ‘Pink Princess’ and Ex Vitro Acclimatization of the Plantlets. Horticulturae 2023, 9, 688. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant growth regulation in cell and tissue culture in vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef]





| S. No. | Bio-Inoculants | Details |
|---|---|---|
| 1 | Prerak | Prerak is a product containing a consortium of bacteria containing Azospirillium, Azotobacter, Phosphate Solubilizing Bacteria, and Potassium Mobilizing Bacteria that fixes Nitrogen (N), solubilizes Phosphorus (P) and zinc (Zn) and helps in the mobilization of Potassium (K). It is a product of Grow Indigo company. |
| 2 | PSB | Phosphate Solubilizing Bacteria is a product which solubilizes Phosphorus (P) for improving the growth of plantlets. It is a product of Grow Indigo company. |
| 3 | Rallis Gold | Rallis Gold is a bio-inoculant of Rallis India Ltd. containing a mycorrhizal-based product which helps in the uptake of Phosphorus (P) and increases the root surface area of plantlets. It is a product of Rallis India Pvt. Ltd. company. |
| Treatments | Survival (%) | Average Root Length (mm) |
|---|---|---|
| Ralligold (1.0 mg/10.0 mL distilled water) | 70.33 +/− 0.36 | 113.01 +/− 1.23 |
| Phosphate Solubilizing Bacteria (1.0 mL/10.0 mL distilled water) + Trichoderma viride (1.0 mg/10.0 mL distilled water) | 78.33 +/− 0.47 | 122.94 +/− 1.74 |
| Prerak (1.0 mL/10.0 mL distilled water) | 54.66 +/− 0.29 | 87.75 +/− 1.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzoor, I.; Bhat, K.M.; Mir, M.A.; Nair, N.M.; Pandit, A.H.; Kulsum, U.; Quadri, S.; Deshmukh, S.; Pasternak, T. The Optimization of In Vitro Culture Establishment and Shoot Proliferation of “GiSelA 17” (Prunus canescens × Prunus avium): A Novel Cherry Rootstock. Int. J. Plant Biol. 2025, 16, 33. https://doi.org/10.3390/ijpb16010033
Manzoor I, Bhat KM, Mir MA, Nair NM, Pandit AH, Kulsum U, Quadri S, Deshmukh S, Pasternak T. The Optimization of In Vitro Culture Establishment and Shoot Proliferation of “GiSelA 17” (Prunus canescens × Prunus avium): A Novel Cherry Rootstock. International Journal of Plant Biology. 2025; 16(1):33. https://doi.org/10.3390/ijpb16010033
Chicago/Turabian StyleManzoor, Ikra, Khalid Mushtaq Bhat, Mohammad Amin Mir, Narendran M. Nair, Aashiq Hussain Pandit, Ume Kulsum, Shoeb Quadri, Smithal Deshmukh, and Taras Pasternak. 2025. "The Optimization of In Vitro Culture Establishment and Shoot Proliferation of “GiSelA 17” (Prunus canescens × Prunus avium): A Novel Cherry Rootstock" International Journal of Plant Biology 16, no. 1: 33. https://doi.org/10.3390/ijpb16010033
APA StyleManzoor, I., Bhat, K. M., Mir, M. A., Nair, N. M., Pandit, A. H., Kulsum, U., Quadri, S., Deshmukh, S., & Pasternak, T. (2025). The Optimization of In Vitro Culture Establishment and Shoot Proliferation of “GiSelA 17” (Prunus canescens × Prunus avium): A Novel Cherry Rootstock. International Journal of Plant Biology, 16(1), 33. https://doi.org/10.3390/ijpb16010033






