Abstract
The β-thalassemias are a group of monogenic hereditary hematological disorders caused by deletions and/or mutations of the β-globin gene, leading to low or absent production of adult hemoglobin (HbA). For β-thalassemia, sirolimus has been under clinical consideration in two trials (NCT03877809 and NCT04247750). A reduced immune response to anti-SARS-CoV-2 vaccination has been reported in organ recipient patients treated with the immunosuppressant sirolimus. Therefore, there was some concern regarding the fact that monotherapy with sirolimus would reduce the antibody response after SARS-CoV-2 vaccination. In the representative clinical case reported in this study, sirolimus treatment induced the expected increase of fetal hemoglobin (HbF) but did not prevent the production of anti-SARS-CoV-2 IgG after vaccination with mRNA-1273 (Moderna). In our opinion, this case report should stimulate further studies on β-thalassemia patients under sirolimus monotherapy in order to confirm the safety (or even the positive effects) of sirolimus with respect to the humoral response to anti-SARS-CoV-2 vaccination. In addition, considering the extensive use of sirolimus for the treatment of other human pathologies (for instance, in organ transplantation, systemic lupus erythematosus, autoimmune cytopenia, and lymphangioleiomyomatosis), this case report study might be of general interest, as large numbers of patients are currently under sirolimus treatment.
1. Introduction
The β-thalassemias are a heterogenous group of monogenic hereditary hematological disorders caused by deletions and/or mutations of the β-globin gene [1,2,3]. Since these alterations are associated with a total (β0) or a partial (β+) suppression of the expression of this gene, causing the absence or reduction of adult hemoglobin (HbA) production, the reactivation of the silent γ-globin genes can ameliorate the clinical parameters of β-thalassemia patients in association with a “de novo” production of fetal hemoglobin (HbF) [4,5,6,7,8].
The mTOR (mammalian target of rapamycin) inhibitor sirolimus [9,10,11] has been proposed as a potent HbF inducer in vitro [12,13] in experimental animals [14,15,16] and when administered to patients carrying sickle-cell disease (SCD) [17,18] and β-thalassemia [19] traits. On the other hand, mTOR inhibitors retain immunomodulating properties [20]. Therefore, especially considering the coronavirus disease 2019 (COVID-19) pandemic [21,22], the use of mTOR inhibitors should be carefully monitored with respect to potential effects on humoral (i.e., production of neutralizing antibodies) [23] and cellular (i.e., memory T-cell) [24,25] responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigens. This is of great relevance for the following considerations: (a) sirolimus is the prototype of a large class of mTOR inhibitor analogues; (b) it was approved by the FDA (U.S. Food and Drug Administration) as an immunosuppressant in combination with cyclosporine and corticosteroids for prophylaxis of organ rejection; and (c) sirolimus is extensively employed in long-term monotherapy for several diseases, including the treatment of solid organ transplantation [26,27,28], systemic lupus erythematosus [29], autoimmune cytopenia [30], and lymphangioleiomyomatosis [31]. An extended list of diseases that can be treated with sirolimus can be found in Zuccato et al. and Gamberini et al. [19,32]. Moreover, sirolimus and mTOR inhibitors have been evaluated for the possible promotion of health span in adults [33].
Concerning this issue, reduced immune response to the SARS-CoV-2 vaccine has been observed in organ recipients receiving sirolimus-based immunosuppressant therapy [34]. Accordingly, the production of anti-SARS-CoV-2 antibodies in patients receiving sirolimus has been monitored in recent studies performed on liver and kidney transplant recipients [35,36].
Sirolimus is at present employed in two clinical trials conducted on β-thalassemia patients (NCT03877809 and NCT04247750) [32]. These two trials are based on the use of low dosages of sirolimus with the main objective of verifying its efficacy as an in vivo HbF inducer, aiming to reduce the number of transfusions needed with overall good tolerability [19,32]. In this context, for determining the possible effects of sirolimus on anti-SARS-CoV-2 vaccination, β-thalassemia patients participating in the clinical trial NCT04247750 are very informative.
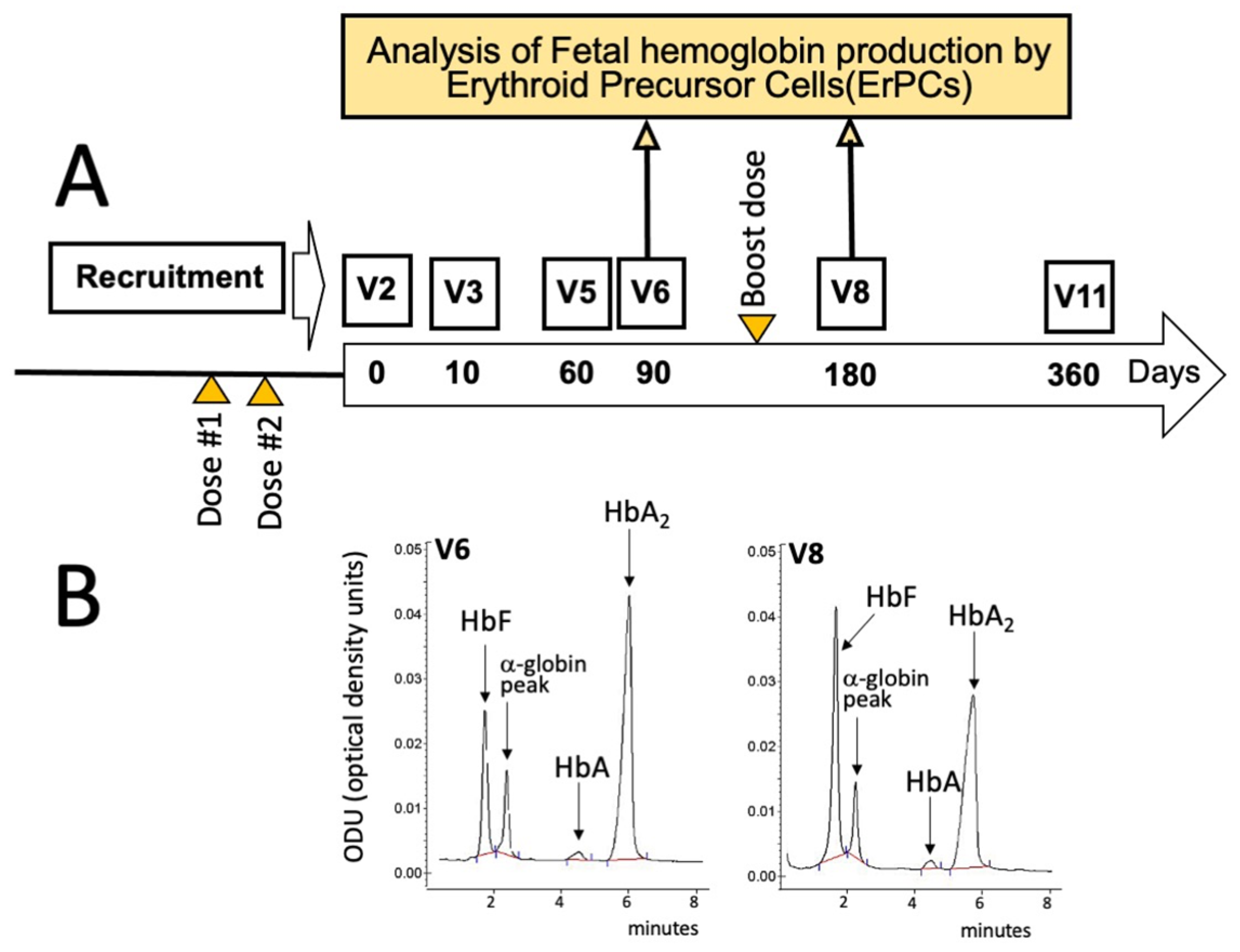
According to the protocol summarized in Figure 1A and Figure 2, all the enrolled β-thalassemia patients received two doses of either the BNT162b2 (Pfizer–BioNTech) or the mRNA-1273 (Moderna) vaccines before starting a daily intake of 2 mg of sirolimus as described by Gamberini et al. [32] and by Zuccato et al. [19]. In addition, all the patients received a booster dose about 6 months later (Figure 1A), between V6 and V8.
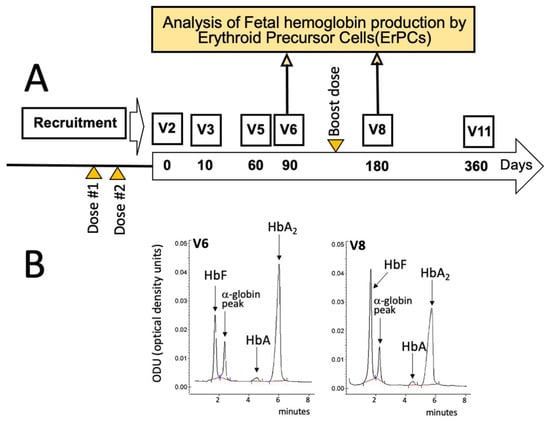
Figure 1.
Scheme depicting some key activities within the T04247750 clinical trial (A), along with the timing of SARS-CoV-2 vaccination (orange triangles) and blood sampling (yellow arrows) considered in this report. The large longitudinal arrow in panel A identifies the sirolimus treatment that usually started 20 days after the administration of the second dose of the mRNA-1273 (Moderna) vaccine. (B) HPLC analysis of the hemoglobins of EPO-cultured ErPCs isolated from the sirolimus-treated patient at V6 (left side of panel (B)) and V8 (right side of panel (B)) and cultured as described by Zuccato et al. [19] and by Gamberini et al. [32].
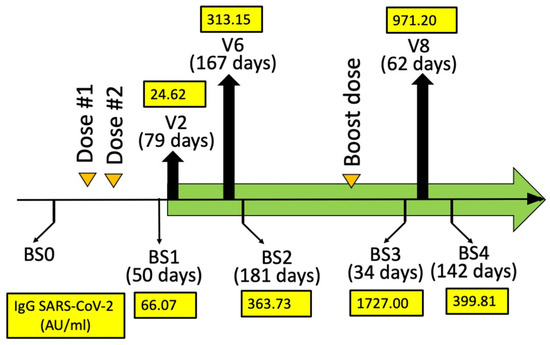
Figure 2.
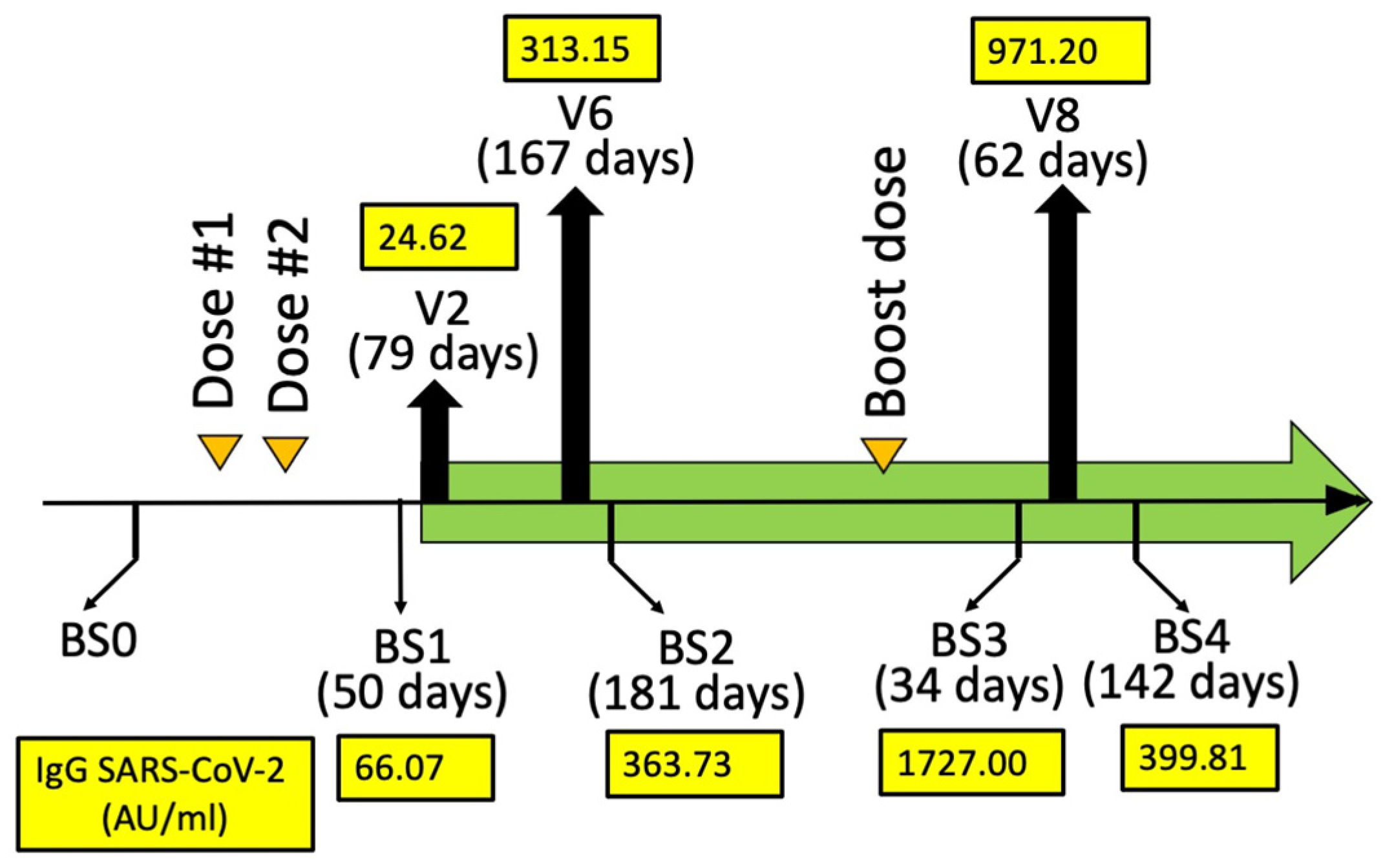
Humoral immune response (AU/mL) quantifying the anti-SARS-CoV-2 IgG at BS1, BS2, BS3, and BS4. The days from mRNA-1273 (Moderna) dose #2 (BS1 and BS2) and booster dose #3 (BS3 and BS4) are indicated in parenthesis. A blood sampling was programmed (BS0) just before the vaccination in order to exclude SARS-CoV-2 infection. Two blood samplings (BS1 and BS2) were programmed after 30–50 (BS1) and 150–200 (BS2) days from BNT162b2 dose #2. Two blood samplings (BS3 and BS4) were programmed after 30–50 (BS3) and 110–160 (BS4) days from BNT162b2 booster dose #3.
For the analysis of the effects of sirolimus on parameters of erythropoiesis, visits were programmed after 10 (V3), 90 (V6), 180 (V8), and 360 (V11) days after starting sirolimus intake (Figure 1A). In this report, the HbF production by erythroid precursor cells (ErPCs) isolated from the patient at V6 and V8 and differentiated by culturing with erythropoietin (EPO) was considered. The HbF production by these cells was determined in order to confirm the response of the patient to sirolimus treatment. For determination of anti-SARS-CoV-2 antibodies, blood sampling was performed before the administration of the first dose (BS0), after 30–50 (BS1) and 150–200 (BS2) days from the second dose, then after 30–50 (BS3) and 110–160 days (BS4) from the booster dose (Figure 2). These two blood samplings (early and late) have been programmed, considering several studies indicating that immunity gradually waned after having received the second dose of vaccine [37,38,39].
2. Case Presentation
The studied case was a male transfusion-dependent TM (Thalassemia Major) patient, 41 years old, homozygous for the β039-Thalassemia mutation.
He started the first blood transfusion when he was 2 years old, was splenectomized in 1996, and participated in the NCT04247750 clinical trial. More clinical information is reported in Table 1.

Table 1.
Clinical parameters and therapies.
According to the general scheme of the study depicted in Figure 1A and Figure 2, he started the intake of sirolimus after about 20 days from the second dose of mRNA-1273 (Moderna) anti-SARS-CoV-2 vaccine. The response of this patient to sirolimus treatment is documented by the HPLC (High-Performance Liquid Chromatography) analysis of the erythropoietin (EPO)-differentiated ErPCs, in agreement with the protocols and the methods described in Gamberini et al. [32] and Zuccato et al. [19].
Figure 1B shows that a sirolimus-mediated increase of HbF was appreciable when EPO-induced ErPCs from the patient treated for 90 days (V6) with sirolimus were compared with ErPCs after 180 days of sirolimus treatment (V8). The HbF content increased from 21.99% (V6) to 40.52% (V8) (1.84-fold). These preliminary analyses support the concept that the patient was responding to sirolimus treatment.
The response to vaccination was determined by quantifying anti-RBD (Spike) antibodies (Abbott Laboratories, Wiesbaden, Germany). The values of the anti-SARS-CoV-2 IgG (immunoglobulin G) (AU/mL) were determined in BS1, BS2, BS3, and BS4 and reported in Figure 2. The highest BS1/BS2 SARS-CoV-2 IgG value of the propositus of this case report study was 362.73. This value is coherent with the data obtained when BS3 and BS4 were considered. In fact, the BS3 SARS-CoV-2 IgG value of the propositus was 1727.00 (Figure 2), which is a value much higher than those found in BS1/BS2. Interestingly, the BS4 value was also high, suggesting that the production of anti-SARS-CoV-2 IgG was durable after more than three months from booster dose #3 of the mRNA-1273 (Moderna) vaccine.
Taken together, these data demonstrated that in this patient, sirolimus allowed the production of SARS-CoV-2 IgGs after vaccination with mRNA-1273 (Moderna) [37].
3. Discussion
A reduced immune response to anti-SARS-CoV-2 vaccination has been observed in organ recipients receiving immunosuppressant sirolimus-based treatment [33]. Therefore, there was some concern regarding the fact that monotherapy with sirolimus would reduce the antibody response after SARS-CoV-2 vaccination. On the other hand, recent studies have concluded that negative effects of sirolimus are not present in sirolimus-treated patients [34,35]. For instance, Cheng et al. reported that the neutralizing antibody responses to a SARS-CoV-2 vaccine were unchanged in sirolimus-treated lymphangioleiomyomatosis patients, suggesting that monotherapy with sirolimus or other mTOR inhibitors does not prevent antibody responses to SARS-CoV-2 vaccines [40]. In other studies, a positive, beneficial effect of sirolimus and mTOR inhibitors on anti-SARS-CoV-2 antibody production was reported [24,41,42]. For instance, an immunosuppressive regimen based on low doses of the mTOR inhibitor everolimus was found by de Boer et al. to be associated with a higher humoral response rate after COVID-19 vaccination in elderly kidney transplant recipients [42].
This issue is of relevance for patients with hematological diseases, including β-thalassemia and sickle-cell disease (SCD). In this respect, sirolimus has been proposed as a possible therapeutic strategy for in vivo enhancement of HbF production in β-thalassemia and SCD [8] and is under clinical consideration for β-thalassemia in two trials (NCT03877809 and NCT04247750) [19,33]. However, the immune response of SARS-CoV-2 vaccines was unknown in β-thalassemia patients on monotherapy with sirolimus and other mTOR inhibitors. In this respect, β-thalassemia patients participating in the clinical trial NCT04247750 might be very informative. According to the protocol shown in Figure 1A and Figure 2, all the enrolled β-thalassemia patients received two doses of either the BNT162b2 (Pfizer–BioNTech) or the mRNA-1273 (Moderna) vaccines before starting with the daily intake of 2 mg of sirolimus as described by Gamberini et al. [32] and by Zuccato et al. [19]. The anti-SARS-CoV-2 IgG levels (Figure 2) were found to be comparable to those reported elsewhere of β-thalassemia patients (not treated with sirolimus) [43,44,45].
Our data are also of interest when considered together with our previously published observation on the effects of sirolimus on memory T-cells of sirolimus treated β-thalassemia patients [46]. In this study, Zurlo et al. found that sirolimus treatment has a positive impact on the biological activity and number of memory CD4+ and CD8+ T cells releasing IFN-γ following stimulation with antigenic stimuli present in immunological memory [46]. As a final comment, we would like to underline that our study might be considered of interest to β-thalassemia and SCD patients living in counties where these diseases are widespread and there is a need to control the COVID-19 pandemic with extensive anti-SARS-CoV-2 vaccination programs.
4. Conclusions
The results shown in Figure 2 demonstrate that sirolimus treatment does not prevent the production of anti-SARS-CoV-2 IgG after vaccination with mRNA-1273 (Moderna). In our opinion, this case report study should stimulate further studies on β-thalassemia patients under sirolimus monotherapy. The aim of these further investigations should verify and confirm the safety (or even the positive effects) of sirolimus with respect to the responses to anti-SARS-CoV-2 vaccination. In addition, considering the extensive use of sirolimus for the treatment of other human pathologies (for instance, in organ transplantation, systemic lupus erythematosus, autoimmune cytopenia, and lymphangioleiomyomatosis) [26,27,28,29,30,31], this case report study might be of general interest, as large numbers of patients are currently under sirolimus treatment or will be treated in future clinical trials with sirolimus.
Author Contributions
Conceptualization, M.R.G. and R.G.; methodology, M.Z., L.C.C. and C.Z.; validation, M.R.G., M.Z. and R.G.; formal analysis, M.R.G., A.F. and R.G.; investigation, M.R.G. and M.Z.; resources, M.R.G.; data curation, M.R.G. and A.F.; writing—original draft preparation, M.R.G. and R.G.; writing—review and editing, R.G. and A.F.; supervision, A.F. and R.G.; project administration, M.R.G. and R.G.; funding acquisition, R.G. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by Wellcome Trust (United Kingdom, Innovator Award 208872/Z/17/Z), by the UE FP7 THALAMOSS Project (THALAssaemia MOdular Stratification System for personalized therapy of β-thalassemia, grant No-306201-FP7-Health-2012-INNOVATION-1), by AIFA (Agenzia Italiana del Farmaco, Italy, AIFA-2016-02364887), and by the MUR-FISR COVID-miRNAPNA Project (FISR2020IP_04128). This study was supported by Pfizer Grant WI227770 (SIRFORTHAL—Sirolimus for beta-thalassemia patients: from pre-clinical research to a multicenter clinical trial).
Institutional Review Board Statement
The analysis of the effects of sirolimus on immunophenotype and immune response is included in the protocol approved by the Ethical Committee in charge of human studies at Arcispedale S. Anna, Ferrara (release of the approval, 17 April 2019).
Informed Consent Statement
The patients provided written informed consent for the publication of the information included in the present article.
Data Availability Statement
All of the data produced in the present work are contained in the manuscript. Other information related to the present study is available upon reasonable request to the corresponding authors.
Acknowledgments
The authors wish to thank all the patients involved in this study and the Veneta Association for the Fight against Thalassemia (AVLT-Elio Zago) for their support. M.Z., L.C.C. and C.Z. were supported by “Associazione Tutti per Chiara”).
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Weatherall, D.J. Phenotype-genotype relationships in monogenic disease: Lessons from the thalassaemias. Nat. Rev. Genet. 2001, 2, 245–255. [Google Scholar] [CrossRef]
- Galanello, R.; Origa, R. Β-thalassemia. Orphanet J. Rare Dis. 2010, 5, 11. [Google Scholar] [CrossRef]
- Origa, R. β-Thalassemia. Genet. Med. 2017, 19, 609–619. [Google Scholar] [CrossRef]
- Sripichai, O.; Fucharoen, S. Fetal hemoglobin regulation in β-thalassemia: Heterogeneity, modifiers and therapeutic approaches. Expert. Rev. Hematol. 2016, 9, 1129–1137. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.; Yu, L.; Cai, R.; Ma, X.; Zheng, C.; Zhou, Y.; Liu, Q.; Wei, X.; Lin, L.; et al. KLF1 mutations are relatively more common in a thalassemia endemic region and ameliorate the severity of β-thalassemia. Blood 2014, 124, 803–811. [Google Scholar] [CrossRef]
- Musallam, K.M.; Sankaran, V.G.; Cappellini, M.D.; Duca, L.; Nathan, D.G.; Taher, A.T. Fetal hemoglobin levels and morbidity in untransfused patients with β-thalassemia intermedia. Blood 2012, 119, 364–367. [Google Scholar] [CrossRef]
- Nuinoon, M.; Makarasara, W.; Mushiroda, T.; Setianingsih, I.; Wahidiyat, P.A.; Sripichai, O.; Kumasaka, N.; Takahashi, A.; Svasti, S.; Munkongdee, T.; et al. A genome-wide association identified the common genetic variants influence disease severity in β0-thalassemia/hemoglobin E. Hum. Genet. 2010, 127, 303–314. [Google Scholar] [CrossRef]
- Prosdocimi, M.; Zuccato, C.; Cosenza, L.C.; Borgatti, M.; Lampronti, I.; Finotti, A.; Gambari, R. A Rational Approach to Drug Repositioning in β-thalassemia: Induction of Fetal Hemoglobin by Established Drugs. Wellcome Open Res. 2022, 7, 150. [Google Scholar] [CrossRef]
- Sehgal, S.N. Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant. Proc. 2003, 35 (Suppl. 3), 7S–14S. [Google Scholar] [CrossRef]
- Kahan, B.D. Sirolimus: A new agent for clinical renal transplantation. Transplant. Proc. 1997, 29, 48–50. [Google Scholar] [CrossRef]
- Mao, B.; Zhang, Q.; Ma, L.; Zhao, D.S.; Zhao, P.; Yan, P. Overview of Research into mTOR Inhibitors. Molecules 2022, 27, 5295. [Google Scholar] [CrossRef]
- Fibach, E.; Bianchi, N.; Borgatti, M.; Zuccato, C.; Finotti, A.; Lampronti, I.; Prus, E.; Mischiati, C.; Gambari, R. Effects of rapamycin on accumulation of alpha-, β- and gamma-globin mRNAs in erythroid precursor cells from β-thalassaemia patients. Eur. J. Haematol. 2006, 77, 437–441. [Google Scholar] [CrossRef]
- Pecoraro, A.; Troia, A.; Calzolari, R.; Scazzone, C.; Rigano, P.; Martorana, A.; Sacco, M.; Maggio, A.; Marzo, R.D. Efficacy of Rapamycin as Inducer of Hb F in Primary Erythroid Cultures from Sickle Cell Disease and β-Thalassemia Patients. Hemoglobin 2015, 39, 225–229. [Google Scholar] [CrossRef]
- Khaibullina, A.; Almeida, L.E.; Wang, L.; Kamimura, S.; Wong, E.C.; Nouraie, M.; Maric, I.; Albani, S.; Finkel, J.; Quezado, Z.M.N. Rapamycin increases fetal hemoglobin and ameliorates the nociception phenotype in sickle cell mice. Blood Cells Mol. Dis. 2015, 55, 363–372. [Google Scholar] [CrossRef]
- Wang, J.; Tran, J.; Wang, H.; Guo, C.; Harro, D.; Campbell, A.D.; Eitzman, D.T. mTOR Inhibition improves anaemia and reduces organ damage in a murine model of sickle cell disease. Br. J. Haematol. 2016, 174, 461–469. [Google Scholar] [CrossRef]
- Lechauve, C.; Keith, J.; Khandros, E.; Fowler, S.; Mayberry, K.; Freiwan, A.; Thom, C.S.; Delbini, P.; Romero, E.B.; Zhang, J.; et al. The autophagy-activating kinase ULK1 mediates clearance of free α-globin in β-thalassemia. Sci. Transl. Med. 2019, 11, eaav4881. [Google Scholar] [CrossRef]
- Gaudre, N.; Cougoul, P.; Bartolucci, P.; Dörr, G.; Bura-Riviere, A.; Kamar, N.; Del Bello, A. Improved Fetal Hemoglobin With mTOR Inhibitor-Based Immunosuppression in a Kidney Transplant Recipient with Sickle Cell Disease. Am. J. Transplant. 2017, 17, 2212–2214. [Google Scholar] [CrossRef]
- Al-Khatti, A.A.; Alkhunaizi, A.M. Additive effect of sirolimus and hydroxycarbamide on fetal haemoglobin level in kidney transplant patients with sickle cell disease. Br. J. Haematol. 2019, 185, 959–961. [Google Scholar] [CrossRef]
- Zuccato, C.; Cosenza, L.C.; Zurlo, M.; Gasparello, J.; Papi, C.; D’Aversa, E.; Breveglieri, G.; Lampronti, I.; Finotti, A.; Borgatti, M.; et al. Expression of γ-globin genes in β-thalassemia patients treated with sirolimus: Results from a pilot clinical trial (Sirthalaclin). Ther. Adv. Hematol. 2022, 13, 20406207221100648. [Google Scholar] [CrossRef]
- Sorrenti, V.; Benedetti, F.; Buriani, A.; Fortinguerra, S.; Caudullo, G.; Davinelli, S.; Zella, D.; Scapagnini, G. Immunomodulatory and Antiaging Mechanisms of Resveratrol, Rapamycin, and Metformin: Focus on mTOR and AMPK Signaling Networks. Pharmaceuticals 2022, 15, 912. [Google Scholar] [CrossRef]
- Jia, S.; Li, Y.; Fang, T. System dynamics analysis of COVID-19 prevention and control strategies. Environ. Sci. Pollut. Res. Int. 2022, 29, 3944–3957. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Wlodkowic, D. The 2020 race towards SARS-CoV-2 specific vaccines. Theranostics 2021, 11, 1690–1702. [Google Scholar] [CrossRef]
- Netti, G.S.; Infante, B.; Troise, D.; Mercuri, S.; Panico, M.; Spadaccino, F.; Catalano, V.; Gigante, M.; Simone, S.; Pontrelli, P.; et al. mTOR inhibitors improve both humoral and cellular response to SARS-CoV-2 messenger RNA BNT16b2 vaccine in kidney transplant recipients. Am. J. Transplant. 2022, 22, 1475–1482. [Google Scholar] [CrossRef]
- Tunbridge, M.; Perkins, G.B.; Singer, J.; Salehi, T.; Ying, T.; Grubor-Bauk, B.; Barry, S.; Sim, B.; Hissaria, P.; Chadban, S.J.; et al. Rapamycin and inulin for booster vaccine response stimulation (RIVASTIM)-rapamycin: Study protocol for a randomised, controlled trial of immunosuppression modification with rapamycin to improve SARS-CoV-2 vaccine response in kidney transplant recipients. Trials 2022, 23, 780. [Google Scholar] [CrossRef]
- Zurlo, M.; Nicoli, F.; Borgatti, M.; Finotti, A.; Gambari, R. Possible effects of sirolimus treatment on the long-term efficacy of COVID-19 vaccination in patients with β-thalassemia: A theoretical perspective. Int. J. Mol. Med. 2022, 49, 33. [Google Scholar] [CrossRef]
- Vasquez, E.M. Sirolimus: A new agent for prevention of renal allograft rejection. Am. J. Health Syst. Pharm. 2000, 57, 437–448. [Google Scholar] [CrossRef]
- Schaffer, S.A.; Ross, H.J. Everolimus: Efficacy and safety in cardiac transplantation. Expert. Opin. Drug Saf. 2010, 9, 843–854. [Google Scholar] [CrossRef]
- Tang, C.Y.; Shen, A.; Wei, X.F.; Li, Q.D.; Liu, R.; Deng, H.J.; Wu, Y.Z.; Wu, Z.J. Everolimus in de novo liver transplant recipients: A systematic review. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 461–469. [Google Scholar] [CrossRef]
- Ji, L.; Xie, W.; Zhang, Z. Efficacy and safety of sirolimus in patients with systemic lupus erythematosus: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2020, 50, 1073–1080. [Google Scholar] [CrossRef]
- Li, H.; Ji, J.; Du, Y.; Huang, Y.; Gu, H.; Chen, M.; Wu, R.; Han, B. Sirolimus is effective for primary relapsed/refractory autoimmune cytopenia: A multicenter study. Exp. Hematol. 2020, 89, 87–95. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, M.; Xiang, B.; Chen, S.; Ji, Y. The efficacy and safety of pharmacological treatments for lymphangioleiomyomatosis. Respir. Res. 2020, 21, 55. [Google Scholar] [CrossRef]
- Gamberini, M.R.; Prosdocimi, M.; Gambari, R. Sirolimus for Treatment of β-Thalassemia: From Pre-Clinical Studies to the Design of Clinical Trials. Health Educ. 2021, 4, 425–435. [Google Scholar]
- Kaeberlein, T.L.; Green, A.S.; Haddad, G.; Hudson, J.; Isman, A.; Nyquist, A.; Rosen, B.S.; Suh, Y.; Zalzala, S.; Zhang, X.; et al. Evaluation of off-label rapamycin use to promote healthspan in 333 adults. Geroscience 2023, 1–12. [Google Scholar] [CrossRef]
- Mazzola, A.; Todesco, E.; Drouin, S.; Hazan, F.; Marot, S.; Thabut, D.; Varnous, S.; Soulié, C.; Barrou, B.; Marcelin, A.G.; et al. Poor Antibody Response After Two Doses of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine in Transplant Recipients. Clin. Infect. Dis. 2022, 74, 1093–1096. [Google Scholar] [CrossRef]
- Lee, W.C.; Hung, H.C.; Lee, J.C.; Huang, C.G.; Huang, P.W.; Gu, P.W.; Wang, Y.C.; Cheng, C.H.; Wu, T.H.; Lee, C.F.; et al. Adjustment of Immunosuppressants to Facilitate Anti-COVID-19 Antibody Production after mRNA Vaccination in Liver Transplant Recipients. Viruses 2023, 15, 678. [Google Scholar] [CrossRef]
- Rahbar, M.; Kazemi, R.; Salehi, H.; Ghasemi, P.; Naghizageh, M.; Dehghani, S.; Gholamnejad, M.; Pishkuhi, M.A.; Aghamir, S.M.K. Evaluation of SARS-CoV-2 Serum Level in Patients Vaccinated with Sinopharm/BBIBP-CorV With Kidney Transplantation. Transplant. Proc. 2022, 54, 2663–2667. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity After the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Khatib, H.A.A.; Coyle, P.; Ayoub, H.H.; Kanaani, Z.A.; et al. Waning of BNT162b2 Vaccine Protection Against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Dorabawila, V.; Easton, D.; Bauer, U.E.; Kumar, J.; Hoen, R.; Hoefer, D.; Wu, M.; Lutterloh, E.; Conroy, M.B.; et al. COVID-19 Vaccine Effectiveness in New York State. N. Engl. J. Med. 2021, 386, 116–127. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Y.; Hu, D.; Zhou, W.; Liu, C.; Tian, X.; Zhang, H.; Xu, Y.-C.; Xu, K.-F. Humoral response to inactivated SARS-CoV-2 vaccines in patients on sirolimus alone. Sci. China Life Sci. 2022, 65, 2118–2120. [Google Scholar] [CrossRef]
- Banjongjit, A.; Phirom, S.; Phannajit, J.; Jantarabenjakul, W.; Paitoonpong, L.; Kittanamongkolchai, W.; Wattanatorn, S.; Prasithsirikul, W.; Eiam-Ong, S.; Avihingsanon, Y.; et al. Benefits of Switching Mycophenolic Acid to Sirolimus on Serological Response after a SARS-CoV-2 Booster Dose among Kidney Transplant Recipients: A Pilot Study. Vaccines 2022, 10, 1685. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.E.; Berger, S.P.; van Leer-Buter, C.C.; Kroesen, B.J.; van Baarle, D.; Sanders, J.F.; OPTIMIZE Study Group. Enhanced Humoral Immune Response After COVID-19 Vaccination in Elderly Kidney Transplant Recipients on Everolimus Versus Mycophenolate Mofetil-containing Immunosuppressive Regimens. Transplantation 2022, 106, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Delaporta, P.; Terpos, E.; Solomou, E.E.; Gumeni, S.; Nitsa, E.; Apostolakou, F.; Kyriakopoulou, D.; Ntanasis-Stathopoulos, I.; Papassotiriou, I.; Trougakos, I.P.; et al. Immune response and adverse events after vaccination against SARS-CoV-2 in adult patients with transfusion-dependent thalassaemia. Br. J. Haematol. 2022, 197, 576–579. [Google Scholar] [CrossRef]
- Carsetti, R.; Agrati, C.; Pinto, V.M.; Gianesin, B.; Gamberini, R.; Fortini, M.; Barella, S.; Denotti, R.; Perrotta, S.; Casale, M.; et al. Premature aging of the immune system affects the response to SARS-CoV-2 mRNA vaccine in β-thalassemia: Role of an additional dose. Blood 2022, 140, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, E.; Marziali, M.; Preziosi, A.; Berardelli, E.; Losardo, A.A.; Ribersani, R.; Pugliese, P.; Farina, A.; Mancini, P.; Angeloni, A. Humoral immune response to Comirnaty (BNT162b2) SARS-Cov2 mRNA vaccine in Thalassemia Major patients. Microbes Infect. 2022, 24, 104976. [Google Scholar] [CrossRef]
- Zurlo, M.; Nicoli, F.; Proietto, D.; Dallan, B.; Zuccato, C.; Cosenza, L.C.; Gasparello, J.; Papi, C.; d’Aversa, E.; Borgatti, M.; et al. Effects of Sirolimus treatment on patients with β-Thalassemia: Lymphocyte immunophenotype and biological activity of memory CD4+ and CD8+ T cells. J. Cell. Mol. Med. 2023, 27, 353–364. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).