Acute Promyelocytic Leukemia and Brugada Syndrome: A Report on the Safety of Arsenic Trioxide/All-Trans-Retinoic Acid Therapy
Abstract
1. Introduction
2. Materials and Methods
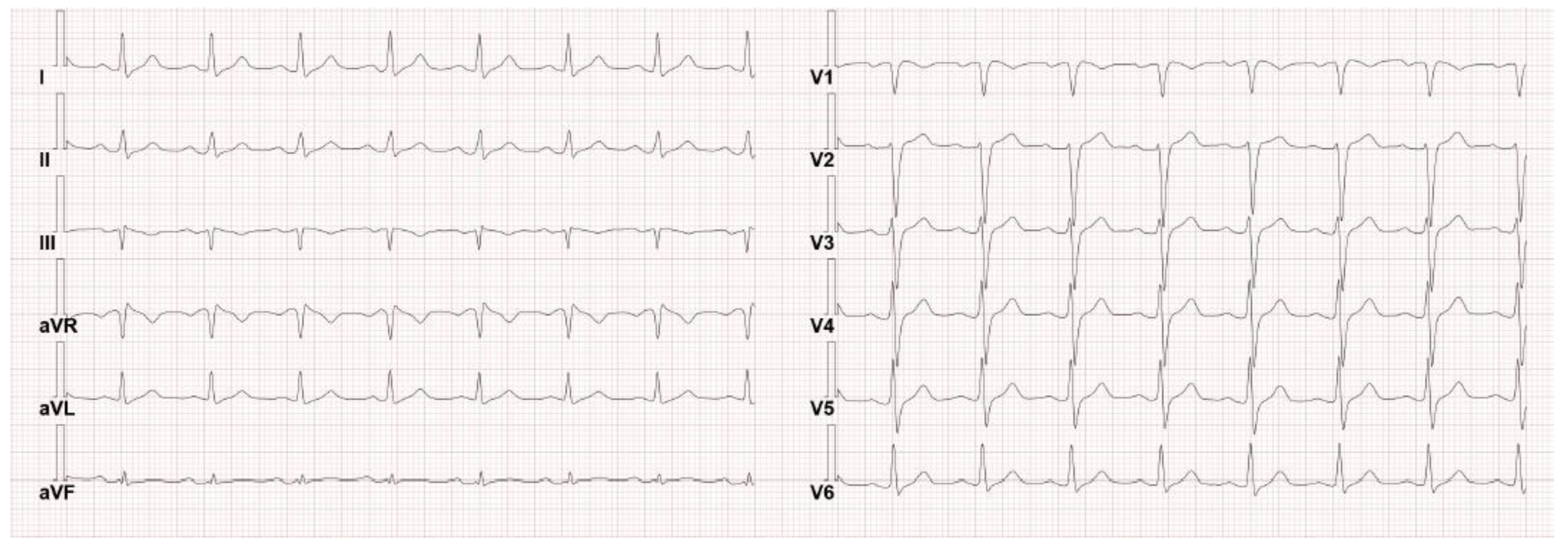
3. Case Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Vutthikraivit, W.; Rattanawong, P.; Putthapiban, P.; Sukhumthammarat, W.; Vathesatogkit, P.; Ngarmukos, T.; Thakkinstian, A. Worldwide Prevalence of Brugada Syndrome: A Systematic Review and Meta-Analysis. Acta Cardiol. Sin. 2018, 34, 267–277. [Google Scholar] [CrossRef] [PubMed]
- BrugadaDrugs.org|Safe Drug Use and the Brugada Syndrome. Available online: https://www.brugadadrugs.org/ (accessed on 12 January 2023).
- Matsuo, K.; Akahoshi, M.; Nakashima, E.; Suyama, A.; Seto, S.; Hayano, M.; Yano, K. The prevalence, incidence and prognostic value of the Brugada-type electrocardiogram: A population-based study of four decades. J. Am. Coll. Cardiol. 2001, 38, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Gehi, A.K.; Duong, T.D.; Metz, L.D.; Gomes, J.A.; Mehta, D. Risk stratification of individuals with the Brugada electrocardiogram: A meta-analysis. J. Cardiovasc. Electrophysiol. 2006, 17, 577–583. [Google Scholar] [CrossRef]
- Liquori, A.; Ibañez, M.; Sargas, C.; Sanz, M.Á.; Barragán, E.; Cervera, J. Acute Promyelocytic Leukemia: A Constellation of Molecular Events around a Single PML-RARA Fusion Gene. Cancers 2020, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Vitaliano-Prunier, A.; Halftermeyer, J.; Ablain, J.; De Reynies, A.; Peres, L.; Le Bras, M.; Metzger, D.; De Thé, H. Clearance of PML/RARA-bound promoters suffice to initiate APL differentiation. Blood 2014, 124, 3772–3780. [Google Scholar] [CrossRef]
- Ablain, J.; Lallemand-Breittenbach, V.; de Thé, H. PML/RARA as the Master Driver of Acute Promyelocytic Leukemia Pathogenesis and Basis for Therapy Response. EHA Libr. Available online: https://library.ehaweb.org/eha/2013/18th/97244/hugues.de.th.pml-rara.as.the.master.driver.of.acute.promyelocytic.leukemia.html?f=menu%3D6%2Abrowseby%3D8%2Asortby%3D2%2Atopic%3D6009 (accessed on 13 January 2023).
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Thomas, X. Acute Promyelocytic Leukemia: A History over 60 Years—From the Most Malignant to the most Curable Form of Acute Leukemia. Oncol. Ther. 2019, 7, 33–65. [Google Scholar] [CrossRef]
- Heuser, M.; Ofran, Y.; Boissel, N.; Mauri, S.B.; Craddock, C.; Janssen, J.; Wierzbowska, A.; Buske, C. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 697–712. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Acute Myeloid Leukemia (Version 2.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf (accessed on 11 January 2023).
- Gatta, G.; Capocaccia, R.; Botta, L.; Mallone, S.; De Angelis, R.; Ardanaz, E.; Comber, H.; Dimitrova, N.; Leinonen, M.K.; Siesling, S.; et al. Burden and centralised treatment in Europe of rare tumours: Results of RARECAREnet-a population-based study. Lancet Oncol. 2017, 18, 1022–1039. [Google Scholar] [CrossRef]
- Dinmohamed, A.G.; Visser, O. Incidence of acute promyelocytic leukemia across Europe: Results of RARECAREnet—A population-based study. Stem Cell Investig. 2019, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.A.; Grimwade, D.; Tallman, M.S.; Lowenberg, B.; Fenaux, P.; Estey, E.H.; Naoe, T.; Lengfelder, E.; Büchner, T.; Döhner, H.; et al. Management of acute promyelocytic leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2009, 113, 1875–1891. [Google Scholar] [CrossRef] [PubMed]
- Sgherza, N.; Russo Rossi, A.V.; Colonna, P.; Carluccio, P.; Delia, M.; Specchia, G. Use of tyrosine kinase inhibitors in a patient with Brugada syndrome and chronic myeloid leukemia. Int. J. Hematol. 2013, 98, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, E.; Fujisaki, T.; Minamoto, Y.; Aoki, K.; Yokota, E. Brugada syndrome occurring after autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Rinsho Ketsueki 2004, 45, 481–483. [Google Scholar]
- Das, A.; Gupta, A.; Das, S. ST segment elevations in a patient with neutropenic fever. Eur. J. Intern. Med. 2017, 40, e7–e8. [Google Scholar] [CrossRef]
- Kwon, H.-M.; Kim, S.-H.; Park, S.-U.; Rhim, J.-H.; Park, H.-S.; Kim, W.-J.; Nam, G.-B. Propofol for implantable cardioverter defibrillator implantation in patients with Brugada syndrome. Pacing Clin. Electrophysiol. PACE 2018, 41, 656–660. [Google Scholar] [CrossRef]
- Martorano, P.P.; Barboni, E.; Buscema, G.; Di Rienzo, A. A novel anaesthetical approach to patients with brugada syndrome in neurosurgery. Case Rep. Anesthesiol. 2013, 2013, 280826. [Google Scholar] [CrossRef]
- Inamura, M.; Okamoto, H.; Kuroiwa, M.; Hoka, S. General anesthesia for patients with Brugada syndrome. A report of six cases. Can. J. Anaesth. J. Can. Anesth. 2005, 52, 409–412. [Google Scholar] [CrossRef]
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.-T.; et al. Posaconazole vs. Fluconazole or Itraconazole Prophylaxis in Patients with Neutropenia. N. Engl. J. Med. 2007, 356, 348–359. [Google Scholar] [CrossRef]
- Afacan Ozturk, H.B.; Albayrak, M.; Maral, S.; Aras, M.R.; Yilmaz, F.; Akyol, P.; Tiglioglu, M.; Malkan, U.Y. Hypercalcemia associated with the interaction between all trans retinoic acid and posaconazole in an acute promyelocytic leukemia case. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2021, 27, 2027–2029. [Google Scholar] [CrossRef]
- Barbey, J.T.; Pezzullo, J.C.; Soignet, S.L. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 3609–3615. [Google Scholar] [CrossRef] [PubMed]
- Roboz, G.J.; Ritchie, E.K.; Carlin, R.F.; Samuel, M.; Gale, L.; Provenzano-Gober, J.L.; Curcio, T.J.; Feldman, E.J.; Kligfield, P.D. Prevalence, management, and clinical consequences of QT interval prolongation during treatment with arsenic trioxide. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Yim, R.; Kumana, C.R.; Tse, E.; Kwong, Y.L. Oral arsenic trioxide, all-trans retinoic acid, and ascorbic acid maintenance after first complete remission in acute promyelocytic leukemia: Long-term results and unique prognostic indicators. Cancer 2020, 126, 3244–3254. [Google Scholar] [CrossRef]
- Aribi, A.; Kantarjian, H.M.; Estey, E.H.; Koller, C.A.; Thomas, D.A.; Kornblau, S.M.; Faderl, S.H.; Laddie, N.M.; Garcia-Manero, G.; Cortes, J.E. Combination therapy with arsenic trioxide, all-trans retinoic acid, and gemtuzumab ozogamicin in recurrent acute promyelocytic leukemia. Cancer 2007, 109, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
| Analyses on Peripheral Blood | |
| Complete blood count | WBC 0.87 × 103/μL (neutrophils 0.47 × 103/μL, lymphocytes 0.36 × 103/μL, monocytes 0.04 × 103/μL, eosinophils 0 × 103/μL, basophils 0 × 103/μL), PLTs 86 × 103/μL confirmed on citrate, hemoglobin 12.4 mg/dL, MCV 93 fl, hematocrit 36%. |
| Blood tests | PT ratio 1.11, aPTT ratio 0.85 and fibrinogen 489 mg/dL, creatinine 0.9 mg/dL (eGFR 94 mL/min), sodium 138.6 mmol/L, potassium 4.45 mmol/L, calcium 2.37 mmol/L, glucose 121 mg/dL, AST 28 U/L, ALT 64 U/L, GGT 41 U/L, ALP 61 U/L, total bilirubin 0.7 mg/dL, LDH 214 U/L, uric acid 5.6 mg/dL, C-reactive protein (6.34 mg/dL), erythrocyte sedimentation rate 93 mm/h, iron 55 μg/dL, transferrin 204 mg/dL, ferritin 522 ng/mL, folic acid 16.6 ng/mL, vitamin B12 > 2000 pg/mL, D-dimer 14,187 ng/mL. Negative serological tests for HBV, HCV, HIV, EBV, and CMV. |
| Analyses on Bone Marrow | |
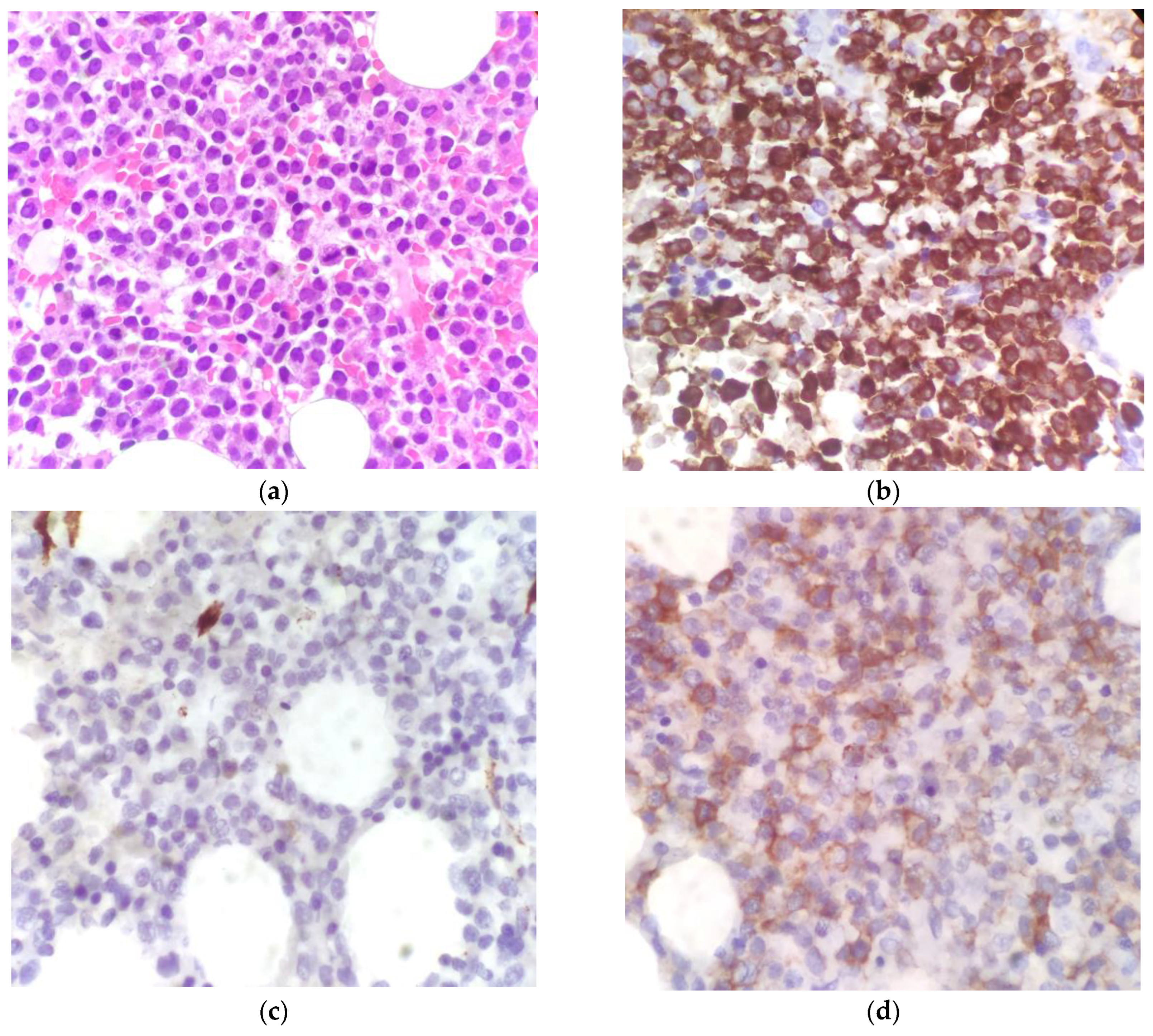
| Flow cytometry | Big granular myeloid cells CD11b−, CD11c−, CD13+, CD33+, CD56−, CD117+, HLA-DR−, TdT−, and MPO+ amounting to 85% of cells, compatible with promyelocytes. |
| Histology | Cellularity of 80% to 90% characterized by an immature-looking myeloid population with oval nuclei and hypergranular eosinophilic cytoplasm. At the immunohistochemical assay, the cells showed intense and widespread positivity for MPO and weaker positivity for CD117, and they were negative for CD34, CD56, and TdT. |
| Molecular and cytogenetic analyses | Normal male karyotype at the cytogenetical analysis; presence of t(15;17) at the fluorescence in-situ hybridization (FISH); and PML-RARA fusion gene identified at the RT-PCR. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosati, G.; Camerlo, S.; Dalmazzo, M.; Padrini, M.; Busana, T.T.; De Gobbi, M.; Fornari, A.; Morotti, A. Acute Promyelocytic Leukemia and Brugada Syndrome: A Report on the Safety of Arsenic Trioxide/All-Trans-Retinoic Acid Therapy. Hematol. Rep. 2023, 15, 440-447. https://doi.org/10.3390/hematolrep15030045
Rosati G, Camerlo S, Dalmazzo M, Padrini M, Busana TT, De Gobbi M, Fornari A, Morotti A. Acute Promyelocytic Leukemia and Brugada Syndrome: A Report on the Safety of Arsenic Trioxide/All-Trans-Retinoic Acid Therapy. Hematology Reports. 2023; 15(3):440-447. https://doi.org/10.3390/hematolrep15030045
Chicago/Turabian StyleRosati, Giorgio, Sofia Camerlo, Matteo Dalmazzo, Melissa Padrini, Tiziano Tommaso Busana, Marco De Gobbi, Alessandro Fornari, and Alessandro Morotti. 2023. "Acute Promyelocytic Leukemia and Brugada Syndrome: A Report on the Safety of Arsenic Trioxide/All-Trans-Retinoic Acid Therapy" Hematology Reports 15, no. 3: 440-447. https://doi.org/10.3390/hematolrep15030045
APA StyleRosati, G., Camerlo, S., Dalmazzo, M., Padrini, M., Busana, T. T., De Gobbi, M., Fornari, A., & Morotti, A. (2023). Acute Promyelocytic Leukemia and Brugada Syndrome: A Report on the Safety of Arsenic Trioxide/All-Trans-Retinoic Acid Therapy. Hematology Reports, 15(3), 440-447. https://doi.org/10.3390/hematolrep15030045







