Pathophysiology, Clinical Manifestations and Diagnosis of Immune Thrombocytopenia: Contextualization from a Historical Perspective
Abstract
:1. History of Immune Thrombocytopenia and Platelets
2. Pathophysiology of ITP
2.1. Autoantibodies
2.2. T Lymphocytes
2.3. Thrombopoietin
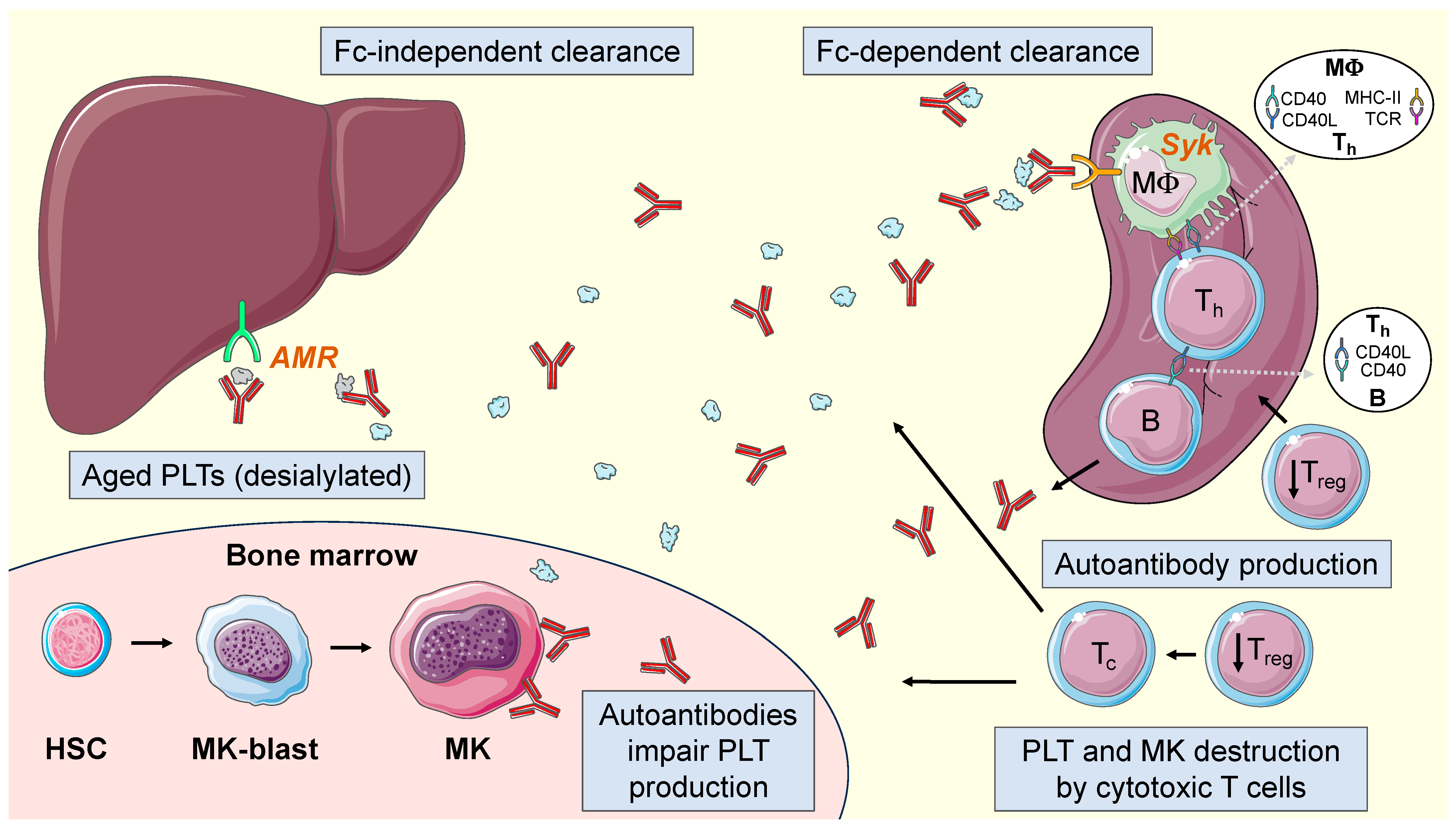
2.4. Current Pathophysiological Model in ITP
3. Terminology
4. Epidemiology
5. Clinical Manifestations
6. Diagnosis
Differential Diagnosis
7. Prognosis
8. Areas for Future Work and Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stasi, R.; Newland, A.C. ITP: A historical perspective. Br. J. Haematol. 2011, 153, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Werlhof, P.G. Disquisitio Medica et Philologica de Variolis et Anthracibus; Sumtibus hHaeredum Nicolai Foersteri et filii: Hannover, Germany, 1735. [Google Scholar]
- Addison, W. On the colourless corpuscles, and on the molecules and cytoblasts in the blood. Lond. Med. Gaz. 1842, 30, 144–148. [Google Scholar]
- Kaushansky, K. Historical review: Megakaryopoiesis and thrombopoiesis. Blood 2008, 111, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Brewer, D.B. Max Schultze (1865), G. Bizzozero (1882) and the discovery of the platelet. Br. J. Haematol. 2006, 133, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Bizzozero, J. Ueber einen neuen Formbestandtheil des Blutes und dessen Rolle bei der Thrombose und der Blutgerinnung. Arch. Pathol. Anat. Physiol. Klin. Med. 1882, 90, 261–332. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Giulio Bizzozero and the discovery of platelets. Leuk. Res. 2007, 31, 1339–1341. [Google Scholar] [CrossRef] [PubMed]
- Brohm, F.; Krauss, E. Ueber Purpura: Innaugural—Dissertation zur Erlangung der Doctorwürde in der Medizin, Chirurgie und Geburtshülfe; Verlag Nicht Ermittelbar: Tübingen, Germany, 1863. [Google Scholar]
- Denys, H. Etudes sur la coagulation du sang dans un cas de purpura avec diminution considerable des plaquettes. La Cell. 1887, 3, 445–462. [Google Scholar]
- Hayem, G. Du Sang et de Ses Alterations Anatomiques; Masson: Paris, France, 1889. [Google Scholar]
- Howell, W.H. Observations upon the occurrence, structure, and function of the giant cells of the marrow. J. Morphol. 1890, 4, 117–130. [Google Scholar] [CrossRef]
- Wright, J.H. The origin and nature of the blood plates. Boston Med. Surg. J. 1906, 23, 643–645. [Google Scholar] [CrossRef]
- Wright, J.H. The histogenesis of the blood platelets. J. Morphol. 1910, 21, 263–278. [Google Scholar] [CrossRef]
- Frank, E. Die essentielle thrombopenie (Konstitutionelle Purpura-Pseudohemophilie), I: Klinische Bild. Berl. Klin. Wochenschr. 1915, 52, 454–458. [Google Scholar]
- Kaznelson, P. Verschwinden der hämorrhagischen Diathese bei einem Falle von “essentieller Thrombopenie” (Frank) nach Milzexstirpation: Splenogene thrombolytische Purpura. Wien. Klin. Wochenschr. 1916, 29, 1451–1454. [Google Scholar]
- Dameshek, W.M.E.B. The megakaryocytes in idiopathic thrombocytopenic purpura, a form of hypersplenism. 1946. Blood 2016, 127, 3. [Google Scholar] [CrossRef]
- Liebman, H.A. Immune thrombocytopenia (ITP): An historical perspective. Hematol. Am. Soc. Hematol. Educ. Program. 2008, 2008, 205. [Google Scholar] [CrossRef] [PubMed]
- Imbach, P.; Kuhne, T.; Signer, E. Historical aspects and present knowledge of idiopathic thrombocytopenic purpura. Br. J. Haematol. 2002, 119, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Harrington, W.J.; Minnich, V.; Hollingsworth, J.W.; Moore, C.V. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J. Lab. Clin. Med. 1951, 38, 1–10. [Google Scholar] [PubMed]
- Evans, R.S.; Takahashi, K.; Duane, R.T.; Payne, R.; Liu, C. Primary thrombocytopenic purpura and acquired hemolytic anemia: Evidence for a common etiology. AMA Arch. Intern. Med. 1951, 87, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Shulman, N.R.; Marder, V.J.; Weinrach, R.S. Similarities between known antiplatelet antibodies and the factor responsible for thrombocytopenia in idiopathic purpura. Physiologic, serologic and isotopic studies. Ann. N. Y. Acad. Sci. 1965, 124, 499–542. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, E.F.; van der Ven, J.T.; Engelfriet, C.P.; von dem Borne, A.E. Specificity of autoantibodies in autoimmune thrombocytopenia. Blood 1982, 59, 23–26. [Google Scholar] [CrossRef]
- Woods, V.L., Jr.; Kurata, Y.; Montgomery, R.R.; Tani, P.; Mason, D.; Oh, E.H.; McMillan, R. Autoantibodies against platelet glycoprotein Ib in patients with chronic immune thrombocytopenic purpura. Blood 1984, 64, 156–160. [Google Scholar] [CrossRef]
- Woods, V.L., Jr.; Oh, E.H.; Mason, D.; McMillan, R. Autoantibodies against the platelet glycoprotein IIb/IIIa complex in patients with chronic ITP. Blood 1984, 63, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Kiefel, V.; Santoso, S.; Weisheit, M.; Mueller-Eckhardt, C. Monoclonal antibody–specific immobilization of platelet antigens (MAIPA): A new tool for the identification of platelet-reactive antibodies. Blood 1987, 70, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.; Tani, P.; Millard, F.; Berchtold, P.; Renshaw, L.; Woods, V.L., Jr. Platelet-associated and plasma anti-glycoprotein autoantibodies in chronic ITP. Blood 1987, 70, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.N.; Moore, J.C.; Warkentin, T.E.; Santos, A.V.; Kelton, J.G. A prospective study of protein-specific assays used to investigate idiopathic thrombocytopenic purpura. Br. J. Haematol. 1999, 104, 442–447. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.; Luiken, G.A.; Levy, R.; Yelenosky, R.; Longmire, R.L. Antibody against megakaryocytes in idiopathic thrombocytopenic purpura. JAMA 1978, 239, 2460–2462. [Google Scholar] [CrossRef] [PubMed]
- Rabellino, E.M.; Levene, R.B.; Leung, L.L.; Nachman, R.L. Human megakaryocytes. II. Expression of platelet proteins in early marrow megakaryocytes. J. Exp. Med. 1981, 154, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Vinci, G.; Tabilio, A.; Deschamps, J.F.; Van Haeke, D.; Henri, A.; Guichard, J.; Tetteroo, P.; Lansdorp, P.M.; Hercend, T.; Vainchenker, W.; et al. Immunological study of in vitro maturation of human megakaryocytes. Br. J. Haematol. 1984, 56, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Nakagawa, P.A.; Williams, S.A.; Schwartz, M.R.; Imfeld, K.L.; Buzby, J.S.; Nugent, D.J. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood 2003, 102, 887–895. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.; Wang, L.; Tomer, A.; Nichol, J.; Pistillo, J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood 2004, 103, 1364–1369. [Google Scholar] [CrossRef]
- Houwerzijl, E.J.; Blom, N.R.; van der Want, J.J.; Esselink, M.T.; Koornstra, J.J.; Smit, J.W.; Louwes, H.; Vellenga, E.; de Wolf, J.T. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenic purpura. Blood 2004, 103, 500–506. [Google Scholar] [CrossRef]
- Semple, J.W.; Bruce, S.; Freedman, J. Suppressed natural killer cell activity in patients with chronic autoimmune thrombocytopenic purpura. Am. J. Hematol. 1991, 37, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Freedman, J. Natural killer cell numbers and activity in patients with chronic autoimmune thrombocytopenic purpura. Blood 1994, 83, 870–871. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Milev, Y.; Cosgrave, D.; Mody, M.; Hornstein, A.; Blanchette, V.; Freedman, J. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: Relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood 1996, 87, 4245–4254. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R.; Cooper, N.; Del Poeta, G.; Stipa, E.; Laura Evangelista, M.; Abruzzese, E.; Amadori, S. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood 2008, 112, 1147–1150. [Google Scholar] [CrossRef]
- Yu, J.; Heck, S.; Patel, V.; Levan, J.; Yu, Y.; Bussel, J.B.; Yazdanbakhsh, K. Defective circulating CD25 regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood 2008, 112, 1325–1328. [Google Scholar] [CrossRef]
- Olsson, B.; Andersson, P.O.; Jernas, M.; Jacobsson, S.; Carlsson, B.; Carlsson, L.M.; Wadenvik, H. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat. Med. 2003, 9, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Zhao, C.; Li, L.; Peng, J.; Hou, M. CD8+ T cells suppress autologous megakaryocyte apoptosis in idiopathic thrombocytopenic purpura. Br. J. Haematol. 2007, 139, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, E.; Cserhati, I.; Tanos, B. Demonstration and some properties of human thrombopoietin in thrombocythaemic sera. Acta Haematol. 1958, 20, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bartley, T.D.; Bogenberger, J.; Hunt, P.; Li, Y.S.; Lu, H.S.; Martin, F.; Chang, M.S.; Samal, B.; Nichol, J.L.; Swift, S.; et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor MpI. Cell 1994, 77, 1117–1124. [Google Scholar] [CrossRef]
- Kuter, D.J.; Beeler, D.L.; Rosenberg, R.D. The purification of megapoietin: A physiological regulator of megakaryocyte growth and platelet production. Proc. Natl. Acad. Sci. USA 1994, 91, 11104–11108. [Google Scholar] [CrossRef]
- Lok, S.; Kaushansky, K.; Holly, R.D.; Kuijper, J.L.; Lofton-Day, C.E.; Oort, P.J.; Grant, F.J.; Heipel, M.D.; Burkhead, S.K.; Kramer, J.M.; et al. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature 1994, 369, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Emmons, R.V.; Reid, D.M.; Cohen, R.L.; Meng, G.; Young, N.S.; Dunbar, C.E.; Shulman, N.R. Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood 1996, 87, 4068–4071. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Kurata, Y.; Tomiyama, Y.; Tahara, T.; Kato, T.; Tadokoro, S.; Shiraga, M.; Honda, S.; Kanakura, Y.; Matsuzawa, Y. Circulating thrombopoietin level in chronic immune thrombocytopenic purpura. Br. J. Haematol. 1996, 93, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Kuter, D.J.; Gernsheimer, T.B. Thrombopoietin and platelet production in chronic immune thrombocytopenia. Hematol. Oncol. Clin. N. Am. 2009, 23, 1193–1211. [Google Scholar] [CrossRef] [PubMed]
- Grozovsky, R.; Begonja, A.J.; Liu, K.; Visner, G.; Hartwig, J.H.; Falet, H.; Hoffmeister, K.M. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat. Med. 2015, 21, 47–54. [Google Scholar] [CrossRef]
- Grozovsky, R.; Giannini, S.; Falet, H.; Hoffmeister, K.M. Regulating billions of blood platelets: Glycans and beyond. Blood 2015, 126, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Consolini, R.; Legitimo, A.; Caparello, M.C. The Centenary of Immune Thrombocytopenia—Part 1: Revising Nomenclature and Pathogenesis. Front. Pediatr. 2016, 4, 102. [Google Scholar] [CrossRef]
- Audia, S.; Mahevas, M.; Samson, M.; Godeau, B.; Bonnotte, B. Pathogenesis of immune thrombocytopenia. Autoimmun. Rev. 2017, 16, 620–632. [Google Scholar] [CrossRef]
- Li, J.; Callum, J.L.; Lin, Y.; Zhou, Y.; Zhu, G.; Ni, H. Severe platelet desialylation in a patient with glycoprotein Ib/IX antibody-mediated immune thrombocytopenia and fatal pulmonary hemorrhage. Haematologica 2014, 99, e61–e63. [Google Scholar] [CrossRef]
- Li, J.; van der Wal, D.E.; Zhu, G.; Xu, M.; Yougbare, I.; Ma, L.; Vadasz, B.; Carrim, N.; Grozovsky, R.; Ruan, M.; et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat. Commun. 2015, 6, 7737. [Google Scholar] [CrossRef]
- Najaoui, A.; Bakchoul, T.; Stoy, J.; Bein, G.; Rummel, M.J.; Santoso, S.; Sachs, U.J. Autoantibody-mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP). Eur. J. Haematol. 2012, 88, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Provan, D.; Semple, J.W. Recent advances in the mechanisms and treatment of immune thrombocytopenia. EBioMedicine 2022, 76, 103820. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, A.; Kapur, R.; Semple, J.W. Pathogenesis and Therapeutic Mechanisms in Immune Thrombocytopenia (ITP). J. Clin. Med. 2017, 6, 16. [Google Scholar] [CrossRef]
- Swinkels, M.; Rijkers, M.; Voorberg, J.; Vidarsson, G.; Leebeek, F.W.G.; Jansen, A.J.G. Emerging Concepts in Immune Thrombocytopenia. Front. Immunol. 2018, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, L.; Yang, G.; Zhuang, Y.; Qian, X.; Zhou, X.; Xiao, D.; Shen, Y. Contributions of T lymphocyte abnormalities to therapeutic outcomes in newly diagnosed patients with immune thrombocytopenia. PLoS ONE 2015, 10, e0126601. [Google Scholar] [CrossRef] [PubMed]
- Sahip, B.; Pamuk, G.E.; Uyanik, M.S.; Pamuk, O.N. Higher interleukin 21 level is predictive of relapse in immune thrombocytopenia. Is it associated with activation of the complement system? Br. J. Haematol. 2016, 173, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Uzun, G.; Bakchoul, T. Primary Immune Thrombocytopenia: Novel Insights into Pathophysiology and Disease Management. J. Clin. Med. 2021, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, M.; Fortuna, S.; Rodeghiero, F. Heterogeneity of terminology and clinical definitions in adult idiopathic thrombocytopenic purpura: A critical appraisal from a systematic review of the literature. Haematologica 2008, 93, 98–103. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef]
- Terrell, D.R.; Beebe, L.A.; Vesely, S.K.; Neas, B.R.; Segal, J.B.; George, J.N. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am. J. Hematol. 2010, 85, 174–180. [Google Scholar] [CrossRef]
- Grimaldi-Bensouda, L.; Nordon, C.; Michel, M.; Viallard, J.F.; Adoue, D.; Magy-Bertrand, N.; Durand, J.M.; Quittet, P.; Fain, O.; Bonnotte, B.; et al. Immune thrombocytopenia in adults: A prospective cohort study of clinical features and predictors of outcome. Haematologica 2016, 101, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Moulis, G.; Palmaro, A.; Montastruc, J.L.; Godeau, B.; Lapeyre-Mestre, M.; Sailler, L. Epidemiology of incident immune thrombocytopenia: A nationwide population-based study in France. Blood 2014, 124, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, P.E.; Hall, S.A.; Feudjo-Tepie, M.; Mitrani-Gold, F.S.; Logie, J. The incidence of idiopathic thrombocytopenic purpura among adults: A population-based study and literature review. Eur. J. Haematol. 2009, 83, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Hedman, A.; Henter, J.I.; Hedlund, I.; Elinder, G. Prevalence and treatment of chronic idiopathic thrombocytopenic purpura of childhood in Sweden. Acta Paediatr. 1997, 86, 226–227. [Google Scholar] [CrossRef]
- Palau, J.; Sancho, E.; Herrera, M.; Sanchez, S.; Mingot, M.E.; Upegui, R.I.; Rodriguez Salazar, M.J.; de la Cruz, F.; Fernandez, M.C.; Gonzalez Lopez, T.J.; et al. Characteristics and management of primary and other immune thrombocytopenias: Spanish registry study. Hematology 2017, 22, 484–492. [Google Scholar] [CrossRef]
- Sarpatwari, A.; Bennett, D.; Logie, J.W.; Shukla, A.; Beach, K.J.; Newland, A.C.; Sanderson, S.; Provan, D. Thromboembolic events among adult patients with primary immune thrombocytopenia in the United Kingdom General Practice Research Database. Haematologica 2010, 95, 1167–1175. [Google Scholar] [CrossRef]
- Chandan, J.S.; Thomas, T.; Lee, S.; Marshall, T.; Willis, B.; Nirantharakumar, K.; Gill, P. The association between idiopathic thrombocytopenic purpura and cardiovascular disease: A retrospective cohort study. J. Thromb. Haemost. 2018, 16, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Enger, C.; Bennett, D.; Forssen, U.; Fogarty, P.F.; McAfee, A.T. Comorbidities in patients with persistent or chronic immune thrombocytopenia. Int. J. Hematol. 2010, 92, 289–295. [Google Scholar] [CrossRef]
- Norgaard, M.; Severinsen, M.T.; Lund Maegbaek, M.; Jensen, A.O.; Cha, S.; Sorensen, H.T. Risk of arterial thrombosis in patients with primary chronic immune thrombocytopenia: A Danish population-based cohort study. Br. J. Haematol. 2012, 159, 109–111. [Google Scholar] [CrossRef]
- Severinsen, M.T.; Engebjerg, M.C.; Farkas, D.K.; Jensen, A.O.; Norgaard, M.; Zhao, S.; Sorensen, H.T. Risk of venous thromboembolism in patients with primary chronic immune thrombocytopenia: A Danish population-based cohort study. Br. J. Haematol. 2011, 152, 360–362. [Google Scholar] [CrossRef]
- Yusuf, H.R.; Hooper, W.C.; Beckman, M.G.; Zhang, Q.C.; Tsai, J.; Ortel, T.L. Risk of venous thromboembolism among hospitalizations of adults with selected autoimmune diseases. J. Thromb. Thrombolysis 2014, 38, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Zoller, B.; Li, X.; Sundquist, J.; Sundquist, K. Risk of pulmonary embolism in patients with autoimmune disorders: A nationwide follow-up study from Sweden. Lancet 2012, 379, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Swan, D.; Newland, A.; Rodeghiero, F.; Thachil, J. Thrombosis in immune thrombocytopenia—Current status and future perspectives. Br. J. Haematol. 2021, 194, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Roman, M.T.; Fernandez-Bello, I.; Jimenez-Yuste, V.; Martin-Salces, M.; Arias-Salgado, E.G.; Rivas Pollmar, M.I.; Justo Sanz, R.; Butta, N.V. Procoagulant profile in patients with immune thrombocytopenia. Br. J. Haematol. 2016, 175, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Liu, Q.; Zhao, H.G.; Peng, J.; Ni, H.; Hou, M.; Jansen, A.J.G. Low platelet count as risk factor for infections in patients with primary immune thrombocytopenia: A retrospective evaluation. Ann. Hematol. 2018, 97, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.L.; Reese, J.A.; Watson, S.I.; Vesely, S.K.; Bolton-Maggs, P.H.; George, J.N.; Terrell, D.R. Fatigue in adult patients with primary immune thrombocytopenia. Eur. J. Haematol. 2011, 86, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Hill, Q.A.; Newland, A.C. Fatigue in immune thrombocytopenia. Br. J. Haematol. 2015, 170, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.L.; Sanz, M.A.; Vicente, V.; Grupo Español de PTI (GEPTI). Guidelines of the Spanish ITP Group for the diagnosis, treatment and follow-up of patients with immune thrombopenia. Med. Clin. 2021, 157, 191–198. [Google Scholar] [CrossRef]
- Mingot-Castellano, M.E.; Canaro Hirnyk, M.; Sanchez-Gonzalez, B.; Alvarez-Roman, M.T.; Barez-Garcia, A.; Bernardo-Gutierrez, A.; Bernat-Pablo, S.; Bolanos-Calderon, E.; Butta-Coll, N.; Caballero-Navarro, G.; et al. Recommendations for the Clinical Approach to Immune Thrombocytopenia: Spanish ITP Working Group (GEPTI). J. Clin. Med. 2023, 12, 6422. [Google Scholar] [CrossRef]
- Lozano, M.L.; Revilla, N.; Gonzalez-Lopez, T.J.; Novelli, S.; Gonzalez-Porras, J.R.; Sanchez-Gonzalez, B.; Bermejo, N.; Perez, S.; Lucas, F.J.; Alvarez, M.T.; et al. Real-life management of primary immune thrombocytopenia (ITP) in adult patients and adherence to practice guidelines. Ann. Hematol. 2016, 95, 1089–1098. [Google Scholar] [CrossRef]
- Uthman, I.; Godeau, B.; Taher, A.; Khamashta, M. The hematologic manifestations of the antiphospholipid syndrome. Blood Rev. 2008, 22, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Provan, D.; Arnold, D.M.; Bussel, J.B.; Chong, B.H.; Cooper, N.; Gernsheimer, T.; Ghanima, W.; Godeau, B.; Gonzalez-Lopez, T.J.; Grainger, J.; et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019, 3, 3780–3817. [Google Scholar] [CrossRef]
- Moulis, G.; Comont, T.; Germain, J.; Sommet, A.; Lapeyre-Mestre, M.; Beyne-Rauzy, O.; Adoue, D.; CARMEN Investigators Group. Significance of antinuclear antibodies in primary immune thrombocytopenia: Results of the CARMEN registry. Blood Adv. 2020, 4, 1974–1977. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, P.; Duncan, J.; Connell, N.; Cooper, N.; Lim, W.; Rodeghiero, F.; Tomiyama, Y.; Grace, R.F.; Bakchoul, T.; Arnold, D.M.; et al. International survey on Helicobacter pylori testing in patients with immune thrombocytopenia: Communication of the platelet immunology scientific and standardization committee. J. Thromb. Haemost. 2021, 19, 287–296. [Google Scholar] [CrossRef]
- Gasbarrini, A.; Franceschi, F.; Tartaglione, R.; Landolfi, R.; Pola, P.; Gasbarrini, G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet 1998, 352, 878. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R.; Sarpatwari, A.; Segal, J.B.; Osborn, J.; Evangelista, M.L.; Cooper, N.; Provan, D.; Newland, A.; Amadori, S.; Bussel, J.B. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: A systematic review. Blood 2009, 113, 1231–1240. [Google Scholar] [CrossRef]
- Jarque, I.; Andreu, R.; Llopis, I.; De la Rubia, J.; Gomis, F.; Senent, L.; Jimenez, C.; Martin, G.; Martinez, J.A.; Sanz, G.F.; et al. Absence of platelet response after eradication of Helicobacter pylori infection in patients with chronic idiopathic thrombocytopenic purpura. Br. J. Haematol. 2001, 115, 1002–1003. [Google Scholar] [CrossRef] [PubMed]
- Vrbensky, J.R.; Moore, J.E.; Arnold, D.M.; Smith, J.W.; Kelton, J.G.; Nazy, I. The sensitivity and specificity of platelet autoantibody testing in immune thrombocytopenia: A systematic review and meta-analysis of a diagnostic test. J. Thromb. Haemost. 2019, 17, 787–794. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B.; Liebman, H.A.; Luning Prak, E.T. The ITP syndrome: Pathogenic and clinical diversity. Blood 2009, 113, 6511–6521. [Google Scholar] [CrossRef]
- Arnold, D.M.; Nazy, I.; Clare, R.; Jaffer, A.M.; Aubie, B.; Li, N.; Kelton, J.G. Misdiagnosis of primary immune thrombocytopenia and frequency of bleeding: Lessons from the McMaster ITP Registry. Blood Adv. 2017, 1, 2414–2420. [Google Scholar] [CrossRef]
- Noris, P.; Schlegel, N.; Klersy, C.; Heller, P.G.; Civaschi, E.; Pujol-Moix, N.; Fabris, F.; Favier, R.; Gresele, P.; Latger-Cannard, V.; et al. Analysis of 339 pregnancies in 181 women with 13 different forms of inherited thrombocytopenia. Haematologica 2014, 99, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Moulis, G.; Germain, J.; Comont, T.; Brun, N.; Dingremont, C.; Castel, B.; Arista, S.; Sailler, L.; Lapeyre-Mestre, M.; Beyne-Rauzy, O.; et al. Newly diagnosed immune thrombocytopenia adults: Clinical epidemiology, exposure to treatments, and evolution. Results of the CARMEN multicenter prospective cohort. Am. J. Hematol. 2017, 92, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Heitink-Polle, K.M.J.; Uiterwaal, C.; Porcelijn, L.; Tamminga, R.Y.J.; Smiers, F.J.; van Woerden, N.L.; Wesseling, J.; Vidarsson, G.; Laarhoven, A.G.; de Haas, M.; et al. Intravenous immunoglobulin vs. observation in childhood immune thrombocytopenia: A randomized controlled trial. Blood 2018, 132, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Heitink-Polle, K.M.; Nijsten, J.; Boonacker, C.W.; de Haas, M.; Bruin, M.C. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: A systematic review and meta-analysis. Blood 2014, 124, 3295–3307. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Maegbaek, M.L.; Norgaard, M. Twenty-year mortality of adult patients with primary immune thrombocytopenia: A Danish population-based cohort study. Br. J. Haematol. 2014, 166, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.R.; Kuo, H.C.; Huang, W.C.; Huang, Y.F.; Chiou, Y.H.; Chang, Y.H.; Nong, B.R. Incidence, clinical characteristics, and associated diseases in patients with immune thrombocytopenia: A nationwide population-based study in Taiwan. Thromb. Res. 2018, 164, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Portielje, J.E.; Westendorp, R.G.; Kluin-Nelemans, H.C.; Brand, A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood 2001, 97, 2549–2554. [Google Scholar] [CrossRef]
- Schifferli, A.; Moulis, G.; Godeau, B.; Leblanc, T.; Aladjidi, N.; Michel, M.; Leverger, G.; Elalfy, M.; Grainger, J.; Chitlur, M.; et al. Adolescents and young adults with newly diagnosed primary immune thrombocytopenia. Haematologica 2023, 108, 2783–2793. [Google Scholar] [CrossRef]
| Year | Author | Discovery |
|---|---|---|
| 1735 | Werlhof [2] | “Morbus maculosus hemorrhagicus” (ITP) identified |
| 1841 | Addison [3] | The first-time platelets were referred to (“extremely minute…granules”) |
| 1865 | Schultze [5] | First accurate description of platelets |
| 1882 | Bizzozero [6] | Introduced the name “platelets” Role of platelets in hemostasis |
| 1883 | Brohm [8] | Association between thrombocytopenia and the Werlhof syndrome |
| 1889 | Hayem [10] | First platelet count documenting thrombocytopenia in this purpura |
| 1890 | Howell [11] | Description of megakaryocytes |
| 1906 | Wright [12,13] | Platelets as fragments of the cytoplasm of megakaryocytes |
| 1915 | Frank [14] | A toxic substance produced by the spleen causes thrombocytopenia |
| 1916 | Kaznelson [15] | Increased platelet destruction in the spleen First splenectomy |
| 1946 | Dameshek and Miller [16] | Increase in the total number of megakaryocytes in the bone marrow. Most of these cells did not produce platelets |
| 1951 | Harrington et al. [19] | “Humoral factor” (Immune etiology) |
| 1951 | Evans et al. [20] | Anti-platelet autoantibodies |
| 1958 | Kelemen et al. [41] | The first to coin the term “thrombopoietin” |
| 1965 | Shulman et al. [21] | The humoral factor causing thrombocytopenia is an immunoglobulin G |
| 1978 | McMillan et al. [28] | Anti-megakaryocyte autoantibodies |
| 1982 | Van Leeuwen et al. [22] | Anti-glycoprotein autoantibodies |
| 1991 | Semple et al. [34] | T cell abnormalities |
| 1994 | Bartley et al. [42] Kuter et al. [43] Lok et al. [44] | Purification and cloning of thrombopoietin |
| 1996 | Emmons et al. [45] Kosugi et al. [46] | TPO levels in ITP are low compared to central thrombocytopenia |
| 2003 | Chang et al. [31] | Anti-megakaryocyte autoantibodies |
| 2004 | McMillan et al. [32] | Anti-megakaryocyte autoantibodies |
| 2004 | Houwezijl et al. [33] | Megakaryocytic abnormalities |
| 2015 | Grozovsky et al. [48,49] | Desialylated platelets are removed from the circulation in hepatocytes via AMR |
| Mandatory |
| Patient history, family history, physical examination, CBC and reticulocytes, peripheral blood smear, basic coagulation test, liver function, folic acid and vitamin B12, immunoglobulin levels, serology (HIV, HBV, HCV) |
| Recommended |
| Anti-phospholipid antibodies, anti-thyroid antibodies and thyroid function, antinuclear antibodies, serum proteinogram |
| Tests of potential utility |
| Anti-glycoprotein antibodies, reticulated/immature platelet fraction, H. pylori, bone marrow examination (in selected patients), Viral PCR for EBV, CMV, and parvovirus, blood group and Rh, direct antiglobulin test, pregnancy test |
| Tests of unproven or uncertain benefit |
| TPO levels, bleeding time, serum complement |
| Bone marrow diseases, including myelodysplastic syndromes, leukemias, other neoplasms, metastatic disease, aplastic anemia, myelofibrosis, and Gaucher disease |
| Liver disease (including cirrhosis or portal hypertension) |
| Hereditary thrombocytopenia |
| Secondary immune thrombocytopenia, due to infections (HIV, HCV, HBV, H. pylori), autoimmune disorders/immunodeficiency (CVID, systemic lupus erythematosus, hyperthyroidism or APS), malignancy (for example, lymphoproliferative disorders) |
| Splenomegaly (Hypersplenism) |
| Drugs including heparin, alemtuzumab, PD-1 inhibitors, abciximab, valproate, alcohol abuse, quinine use, exposure to environmental toxins or chemotherapy, herbal products |
| Other microangiopathic disorders (DIC, TTP, HUS), Evans syndrome |
| Recent transfusions (post-transfusion purpura) and vaccinations |
| Others: vitamin B12 or folate deficiency, pregnancy, giant hemangiomas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Carballeira, D.; Bernardo, Á.; Caro, A.; Soto, I.; Gutiérrez, L. Pathophysiology, Clinical Manifestations and Diagnosis of Immune Thrombocytopenia: Contextualization from a Historical Perspective. Hematol. Rep. 2024, 16, 204-219. https://doi.org/10.3390/hematolrep16020021
Martínez-Carballeira D, Bernardo Á, Caro A, Soto I, Gutiérrez L. Pathophysiology, Clinical Manifestations and Diagnosis of Immune Thrombocytopenia: Contextualization from a Historical Perspective. Hematology Reports. 2024; 16(2):204-219. https://doi.org/10.3390/hematolrep16020021
Chicago/Turabian StyleMartínez-Carballeira, Daniel, Ángel Bernardo, Alberto Caro, Inmaculada Soto, and Laura Gutiérrez. 2024. "Pathophysiology, Clinical Manifestations and Diagnosis of Immune Thrombocytopenia: Contextualization from a Historical Perspective" Hematology Reports 16, no. 2: 204-219. https://doi.org/10.3390/hematolrep16020021
APA StyleMartínez-Carballeira, D., Bernardo, Á., Caro, A., Soto, I., & Gutiérrez, L. (2024). Pathophysiology, Clinical Manifestations and Diagnosis of Immune Thrombocytopenia: Contextualization from a Historical Perspective. Hematology Reports, 16(2), 204-219. https://doi.org/10.3390/hematolrep16020021






