Abstract
Cytoreductive surgery (CRS) is the cornerstone of treating advanced ovarian cancer. Approximately 60–70% of patients with advanced ovarian cancer will have involvement in the upper abdomen or the supracolic compartment of the abdominal cavity. Though the involvement of this region results in poorer survival compared, complete cytoreduction benefits overall survival, making upper-abdominal cytoreduction an essential component of CRS for advanced ovarian cancer. The upper abdomen constitutes several vital organs and large blood vessels draped with the parietal or visceral peritoneum, common sites of disease in ovarian cancer. A surgeon treating advanced ovarian cancer should be well versed in upper-abdominal cytoreduction techniques, including diaphragmatic peritonectomy and diaphragm resection, lesser omentectomy, splenectomy with or without distal pancreatectomy, liver resection, cholecystectomy, and suprarenal retroperitoneal lymphadenectomy. Other procedures such as clearance of the periportal region, Glisson’s capsulectomy, clearance of the superior recess of the lesser sac, and Morrison’s pouch are essential as these regions are often involved in ovarian cancer. This manuscript covers the surgical anatomy of the upper abdomen, the techniques and therapeutic rationale of upper-abdominal cytoreduction, and specific measures for perioperative management of these patients. The main focus is the description of various peritonectomies and regional lymphadenectomies.
1. Introduction
Epithelial ovarian cancer (EOC) continues to be diagnosed in an advanced stage in over 70% of patients, and approximately 60–70% of these patients will have involvement in the upper abdomen or the supracolic compartment of the abdominal cavity [1,2,3]. Over time, upper-abdominal cytoreductive surgery (CRS) has become an essential component of the skill set of surgical and gynecological oncologists treating advanced EOC [4,5]. The most common surgical procedures to clear the tumor from the upper abdomen include diaphragmatic peritonectomy and diaphragm resection, lesser omentectomy, splenectomy with or without distal pancreatectomy, liver resection, cholecystectomy, and suprarenal retroperitoneal lymphadenectomy. Other procedures such as clearance of the periportal region, Glisson’s capsulectomy, clearance of the superior recess of the lesser sac, and Morrison’s pouch are essential as these regions are often involved in ovarian cancer [6].
Advanced EOC is a peritoneal disease that often has a miliary or plaque-like morphology and is not detected on preoperative imaging [7]. A thorough exploration of the abdominal cavity to identify all disease sites is essential to avoid missing disease in some areas and thus perform what could be termed a ‘pseudo-complete’ cytoreduction [8,9]. There is limited information about the patterns of peritoneal dissemination or disease distribution in the scientific literature, which increases the dependence on surgical exploration and visual inspection performed during surgery [10]. The upper abdomen, in particular, could be challenging to explore for surgeons unfamiliar with the anatomy.
In this manuscript, we discuss the therapeutic rationale, the surgical anatomy of the upper abdomen, and technical details of the common and uncommon procedures performed during upper-abdominal cytoreductive surgery (CRS) and perioperative management of patients. The focus is chiefly on description of the techniques of peritonectomies and the lymphadenectomies performed in this region.
2. Benefit of Upper-Abdominal Cytoreduction
The benefit of upper-abdominal cytoreduction in patients undergoing CRS as part of first-line therapy for advanced EOC has been established [11,12]. Chi et al. reviewed 378 stage IIIC and IV EOC patients, of which 168 patients had what was considered standard surgery during that era (group 1) and 210 had extensive upper-abdominal surgery (group 2), including procedures such as diaphragm peritonectomy and/or resection, splenectomy, distal pancreatectomy, partial liver resection, cholecystectomy, and porta hepatis tumor resection [11]. The proportion of patients having a complete gross resection increased from 11% to 27% without a significant increase in morbidity. The median overall-survival (OS) significantly improved in group 2 compared to group 1 (54 months vs. 43 months, p = 0.03). These investigators suggested that incorporating extensive upper-abdominal procedures was associated with increased optimal cytoreduction rates and significantly improved survival [11]. Harter et al. performed an analysis on 396 patients with stages IIB–IV EOC. They found that as more aggressive surgical procedures-bowel resection, diaphragm resection, splenectomy, and liver resection were performed, the rates of complete cytoreduction increased from 33% to 62%. Consequently, the OS increased (26 months vs. 37 months vs. 45 months for three different periods, p < 0.003) [13]. Ren et al. compared 116 patients undergoing radical surgery in the upper abdomen with 237 patients undergoing standard surgery in which only nodules larger than 1 cm were resected in the upper abdomen. The median progression-free survival (PFS) and OS were significantly higher in the radical surgery group compared to the standard surgery group (PFS: 19.5 vs. 13.3 months, HR: 0.61; 95% CI: 0.46–0.80, p < 0.001; OS: not reached vs. 39.3 months, HR: 0.47; 95% CI: 0.30–0.72, p < 0.001) [14]. Aggressive upper-abdominal surgery, thus, has a survival benefit in advanced EOC [15,16,17].
The role of such extensive and aggressive surgery in recurrent ovarian cancer is unclear. Recently, two randomized trials showed a benefit in OS with the addition of secondary CRS to systemic chemotherapy (SC) in managing first platinum-sensitive recurrence compared to SC alone, while another trial showed no benefit [18,19,20]. The criteria used to select patients in some of these trials do not predict the benefit from surgery but the probability of a complete cytoreduction. Thus, which patients benefit most from secondary CRS remains unknown. Some good prognostic factors are a long platinum-free interval, no ascites, and single versus multiple sites of recurrence [21]. Patients with the limited disease at recurrence determined by a PCI < 10 or involvement of 1–2 regions of the peritoneal cavity experience a more prolonged overall survival. Perhaps these are the patients that benefit most from secondary CRS [21,22]. Most patients with upper-abdominal recurrence will have disseminated disease, and the benefit of secondary CRS in these patients is undefined [23,24]. Upper-abdominal cytoreduction is safe and feasible for recurrent disease, but the survival benefit has not been demonstrated yet. At the same time, there is no direct evidence to say that it does not have a role. Hence, such procedures should be undertaken only in selected patients after discussion in multidisciplinary team meetings.
3. Pattern of Peritoneal Dissemination and Involvement of the Upper Abdomen
In EOC, the early formation of ascites (stage 1C) leads to the redistribution of cancer cells in the peritoneal cavity and greater involvement of some regions that are dependent regions of the peritoneal cavity or have a greater concentration of lymphatics [25,26]. The pelvis, greater omentum, right paracolic regions, and right diaphragm have a greater propensity for the disease [6]. Both translymphatic and transmesothelial spread is seen in ovarian cancer [26]. The disease distribution in the peritoneal cavity could be best described as a ‘widespread cancer’ distribution and not a ‘random proximal distribution’ as expected in a high-grade malignancy (Figure S1 in the Supplementary Materials) [27]. There are few studies on the incidence of involvement of the upper abdomen in ovarian cancer. In a retrospective study of 214 patients by Sehouli et al., the diaphragm was involved in 44% of patients with stages I–IV of EOC [25]. Patients with serous histology (p < 0.001), ascites (p = 0.070), and lymph node metastases (p = 0.030) had a higher incidence of involvement in the upper abdomen. Upper abdomen involvement was associated with a significantly lower rate of complete cytoreduction [28]. In another prospective study of 134 patients undergoing interval CRS, region 1 was involved in 77.8%, region 2 in 57%, and region 3 in 53.3% during surgery [29]. The disease was confirmed on pathology in 59.2% in region 1, 48.1% in region 2, and 34% in region 3. Thus, the overall incidence of disease in the upper abdomen is high in patients undergoing primary and interval CRS. In another study that included 44 patients with EOC, the umbilical round ligament was involved in 36.3%, the lesser omentum in 20.4%, and the falciform ligament in 4.5% [30].
The high incidence of disease in the upper regions, especially in those with stage III-C and above, merits a thorough exploration of these regions to identify and resect all disease sites. In a study of 134 patients undergoing interval CRS, involvement of the greater omentum was associated with the presence of disease in regions 1 and 2 in over 80% and region 3 in over 70% of patients [29].
In a prospective multicentric study, it was shown that serous cancer involved the lower regions (regions 5, 6, and 7 according to the description in Sugarbaker’s peritoneal cancer index first) followed by the middle regions (0, 4, 8—mainly the omentum) and then the upper regions (1, 2, 3) [31]. None of the patients had involvement in the upper regions without the involvement of the lower regions. Thus, disease in the upper abdomen should be expected and looked for in patients in whom the lower and middle regions are both involved by disease.
3.1. Lymph Node Involvement
Local lymphatics regularly recycle the peritoneal fluid [32]. This leads to the transfer of free-floating cells to these lymphatics, leading to lymph node metastases and subsequent distant metastases [32]. In addition, infiltration of the sub-peritoneal layer can lead to lymphatic and lymph node involvement. Experimental studies have shown that the lymphatic drainage of the peritoneum occurs mainly through the visceral peritoneum and goes first to the retroperitoneal nodes (superior mesenteric, periportal, and celiac groups) and then via the thoracic duct to the mediastinal nodes [33,34]. Thus, these nodes could be involved secondary to peritoneal disease in the upper or lower abdomen or both. Lymph from the parietal peritoneum also goes to the mediastinum via the transdiaphragmatic lymphatics, which results in the involvement of the supradiaphragmatic nodes (the sub-xiphoid nodes and cardiophrenic angle nodes) [35].
3.2. Parenchymal Metastases
Parenchymal metastases that could involve the spleen or the liver are rare. In most cases, the disease is on the surface with infiltration of the underlying parenchyma and is thus peritoneal disease. In a study of 338 patients undergoing primary CRS, the incidence of parenchymal liver metastases was 0.88% [24]. In another study of 1211 patients undergoing CRS, of the 21 patients with parenchymal liver metastases, 3 patients with advanced ovarian cancer underwent primary CRS [36]. Tanner et al. reported parenchymal splenic metastases in 3.4% of 576 patients undergoing primary CRS and 20.8% with splenectomy. Parenchymal splenic metastases were associated with a significantly lower overall survival [37].
4. Surgical Anatomy of the Upper Abdomen
The upper abdomen could be defined in two ways. Considering the anatomy of the peritoneal cavity and its spaces, the supracolic compartment could be regarded as the ‘upper abdomen’ [38]. The peritoneal cancer index devised by Paul Sugarbaker divides the abdominal cavity into 13 anatomical regions, of which regions 1, 2, and 3 could jointly be considered the upper-abdominal regions [39].
4.1. Peritoneal Ligaments and Spaces
The upper-abdominal cavity or supracolic compartment comprises several ligaments and spaces [40]. The falciform and triangular ligaments are the suspensory ligaments of the liver that bind the bare area. The hepatoduodenal ligament (containing the common bile duct, hepatic artery, and portal vein) and the gastrohepatic ligament (which includes the left gastric artery and the coronary vein) form the lesser omentum [40]. The gastrosplenic ligament connects the greater curve of the stomach to the spleen and contains the short gastric vessels. The splenorenal ligament has the pancreatic tail. Ovarian cancer could involve any of these structures and the surgeon should be familiar with their anatomy.
The supramesocolic compartment is divided into the left and right subphrenic spaces by the falciform ligament on the anterior aspect of the liver. The sub-hepatic space, including the lesser sac, is located under the liver. The right subphrenic space is located under the right diaphragm; it communicates inferiorly with the right paracolic space and is separated from the left subphrenic space by the falciform ligament [41]. The right sub-hepatic space continues medially through the foramen of Winslow (Epiploic foramen) to the lesser sac (Bursa Omentalis) [41].
The lesser sac is a potentially large cavity with various recesses communicating with the left subphrenic space cranially and into the greater omentum caudally. The lesser sac contains a superior recess (located above the peritoneal reflection of the left gastric artery) close to the caudate lobe and a larger inferior recess between the stomach and the pancreatic body. The superior and inferior recesses are separated by a peritoneal fold that accompanies the left gastric artery. Sometimes, the inferior recess communicates with a potential space between the leaves of the greater omentum [41].
4.2. Anatomical Boundaries from the Surgical Viewpoint
The peritoneal regions lying in each region and their boundaries are described in Table 1. More details can be found online in the PROMISE internet application (www.e-promise.org). [42]

Table 1.
Boundaries of peritoneal regions lying in the upper abdomen from the surgical viewpoint.
4.3. Regional Nodes
Several nodal stations in the upper abdomen could be involved in advanced ovarian cancer. Some of these nodes are involved secondary to the primary tumor as part of retroperitoneal lymphadenopathy and these include the suprarenal retroperitoneal or paraaortic nodes such as the superior mesenteric and celiac axis nodes. The other nodes are the omental nodes along the lesser and greater curves, the left gastric nodes, periportal nodes, and pericardiac nodes. The supradiaphragmatic nodes often dissected during upper-abdominal cytoreduction include the sub-xiphoid or cardiophrenic angle nodes, costophrenic lymph nodes, and paracaval nodes (Figure S2 in the Supplementary Materials) [45]. It is unclear whether these nodal stations are regional nodes or distant metastases, as their involvement has not been classified in the TNM and FIGO classifications [25,46].
5. Preoperative Imaging
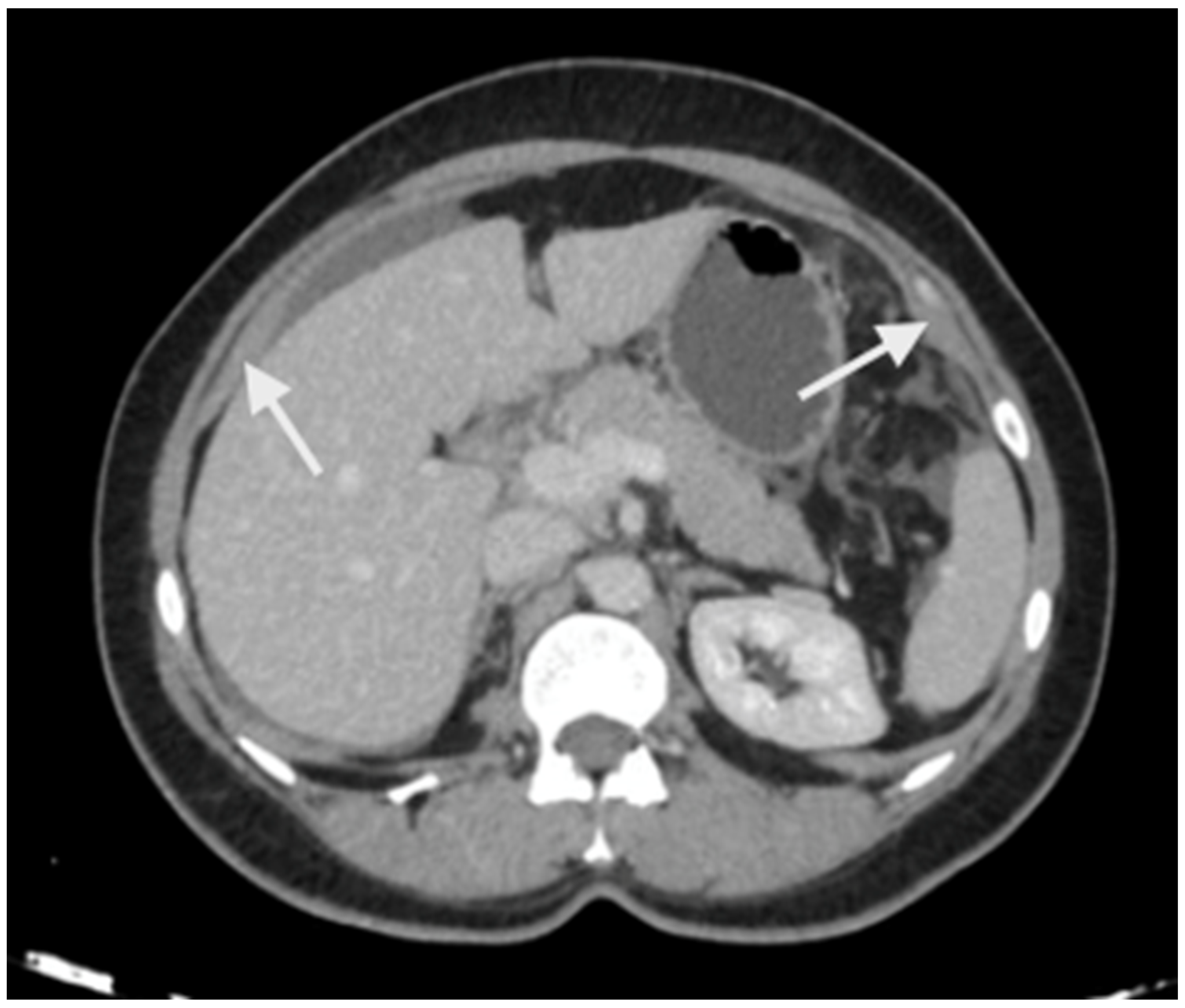
Imaging has two significant roles: the first is the detection of limited disease and all sites of disease in the upper abdomen, and the second is predicting resectability. The most common study is a contrast-enhanced CT scan of the thorax, abdomen, and pelvis [47]. Several investigators have shown that a CT scan underestimates the extent of peritoneal disease [48,49,50]. The spread of peritoneal disease is usually reported using the peritoneal cancer index (PCI). The accuracy in predicting the extent of upper-abdominal disease is not studied separately; the overall accuracy ranges from 15 to 80% [48,49,50]. Peritoneal disease can present as a thickening and/or enhancement of the parietal peritoneum (the subphrenic peritoneum) or discrete nodular deposits (Figure 1).
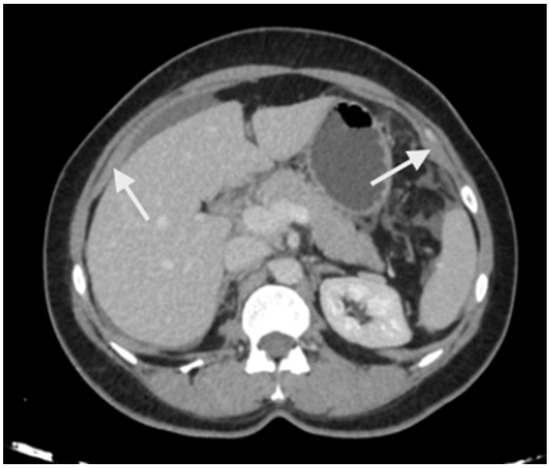
Figure 1.
Peritoneal thickening and enhancement (white arrows) as seen on a contrast-enhanced CT scan suggestive of the presence of disease.
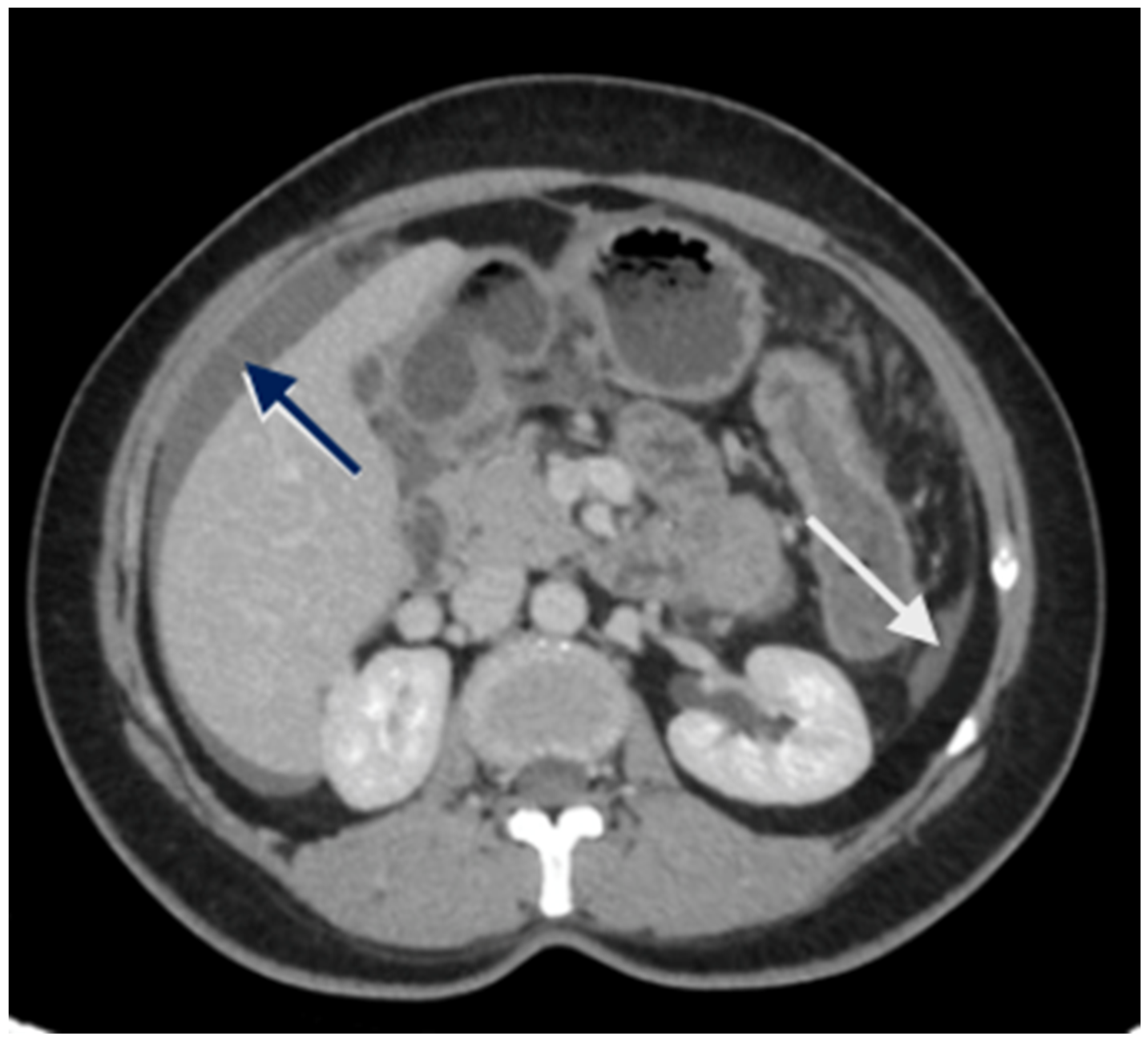
Loculated fluid collections adjacent to the liver, indentation of the liver surface, and fluid in the sub-hepatic region are some pre-emptive signs of underlying peritoneal disease (Figure 2). A contrast-enhanced MRI of the abdomen and pelvis with a dedicated protocol for the detection of peritoneal disease is being used at several centers and has shown to be more accurate in predicting the disease extent [51,52].
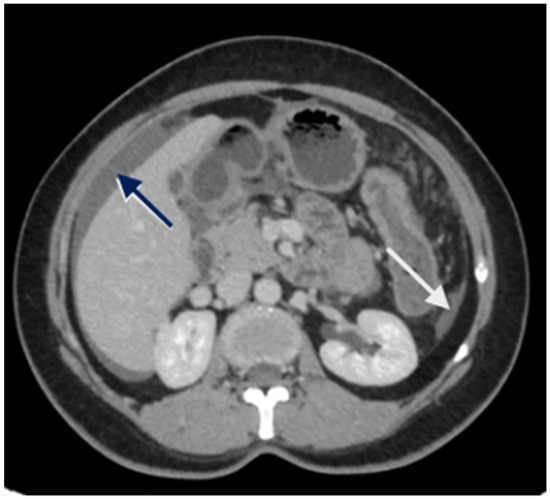
Figure 2.
CT scan showing nodular peritoneal deposit on the subphrenic peritoneum (white arrow); loculated fluid collection (blue arrow).
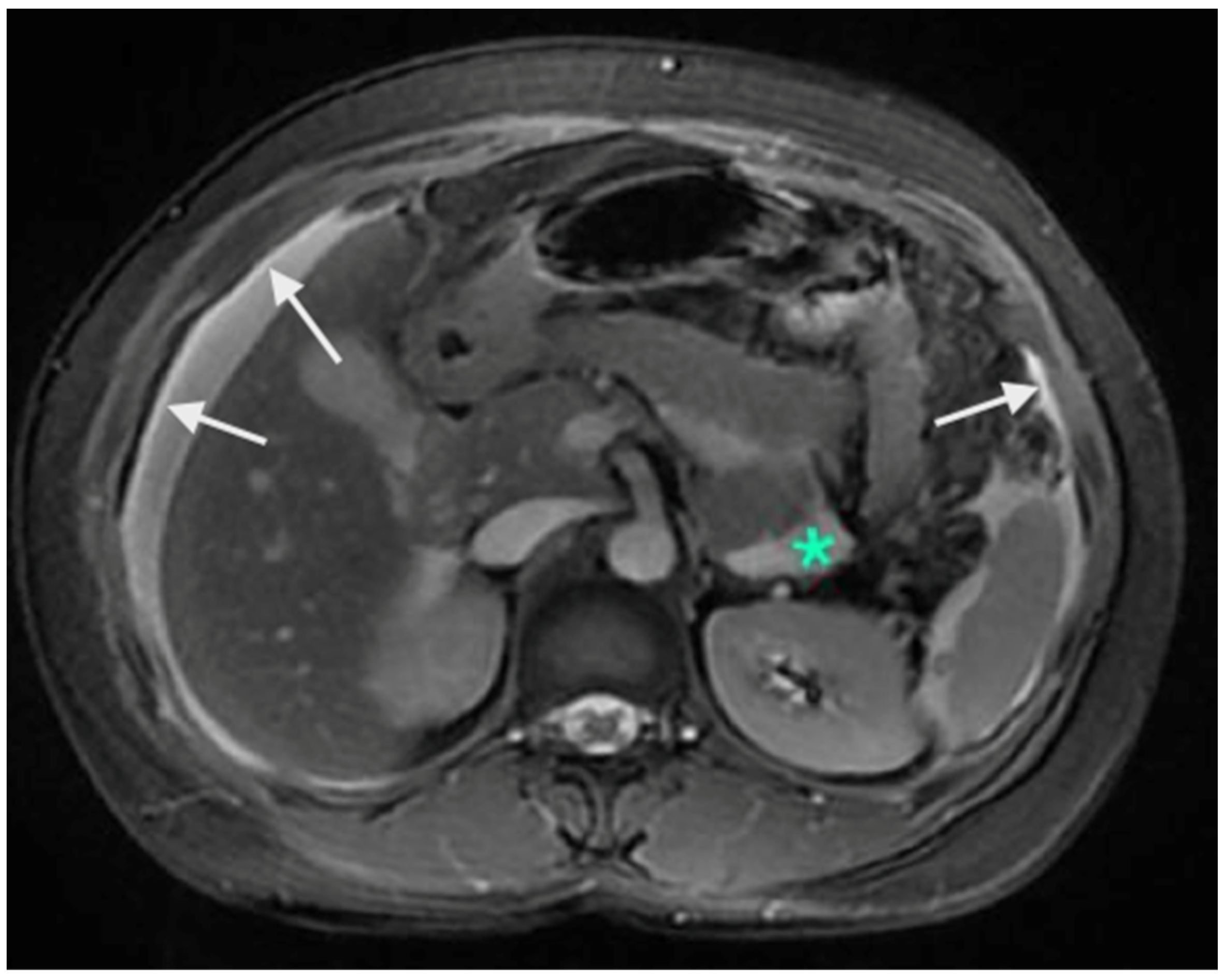
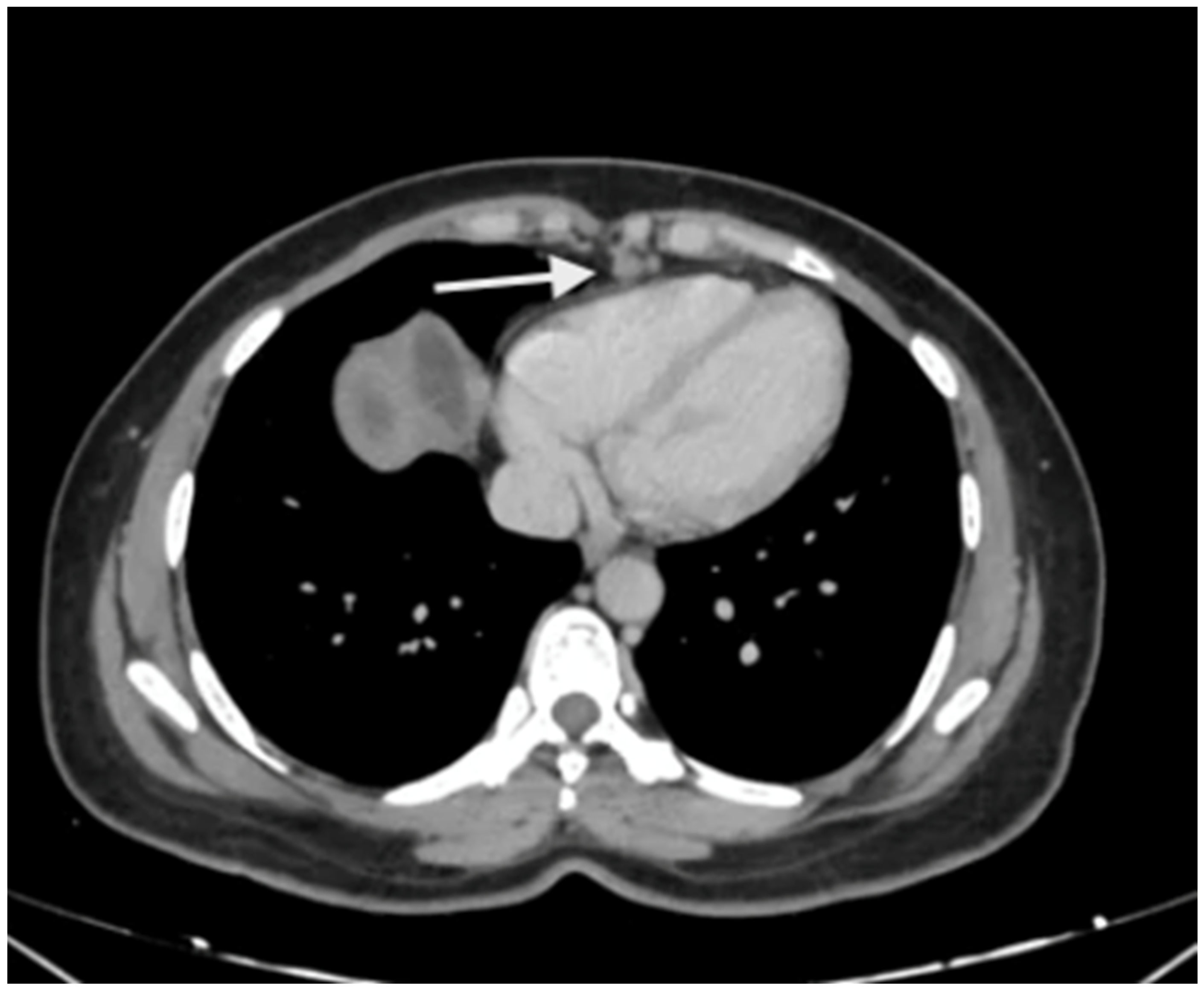
There are no studies evaluating the performance in the upper abdomen, but subtle peritoneal thickening and nodularity are more accurately detected on MRI compared to a CT scan (Figure 3) [53]. The regional lymph node stations described above should be studied on imaging to identify the nodal disease (Figure 4). Considering disease in the upper abdomen alone, extensive disease in the gastrocolic ligament infiltrating the head of the pancreas, extensive infiltration of the stomach, disease infiltrating the porta hepatis, and diffuse and massive involvement of the subphrenic region are pointers of extensive disease that is not amenable to a complete cytoreduction. No one finding can be considered in isolation, and it is the overall disease extent and the general condition of the patient that are considered while deciding to proceed with CRS or not. Though many surgeons perform a staging laparoscopy to select patients for CRS, these obvious signs of inoperability on imaging could avoid an unnecessary surgical procedure [54].
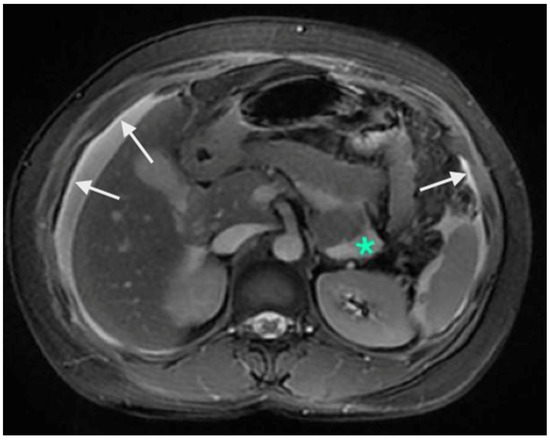
Figure 3.
Contrast-enhanced MRI showing A: enhancement of the peritoneum (white arrows). These changes are picked up earlier on MRI compared to a CT scan. The green * shows a fluid collection without any nodularity or thickening and is not scored as a peritoneal deposit.
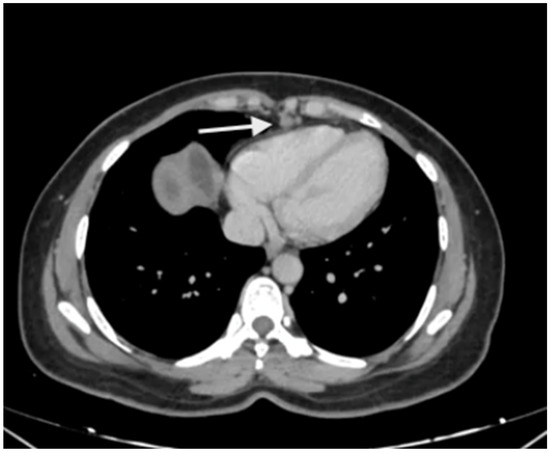
Figure 4.
Enlarged supradiaphragmatic nodes in a patient with advanced epithelial ovarian cancer with upper-abdominal disease. Based on the morphology, they appeared to be metastatic and this was confirmed on pathology after resection of these nodes during CRS.
6. Technical Aspects of Upper-Abdominal Cytoreduction
The preoperative preparation is similar to that for cytoreductive surgery [55]. The patient is positioned supine with the legs placed in lithotomy position to allow access to the perineum. The legs should be supported as described previously to avoid nerve damage and compartment syndrome [55]. The presence of disease in the omentum is predictive of disease in one or more regions of the upper abdomen in over 50% of patients [29]. The goal of surgery is a CC-0 resection or complete cytoreduction and these procedures should only be undertaken in patients whom a complete cytoreduction is feasible [56]. The incision should extend from the xiphoid to the pubis in all patients with advanced ovarian cancer. Resection of the xiphoid itself may not be necessary to obtain adequate exposure though some surgeons prefer to resect it routinely [57].
6.1. Energy Devices
In the initial description of peritonectomy procedures by Paul Sugarabker, a ball tip cautery at very high voltage is used to create a margin of heat necrosis along the line of resection and thereby minimize tumor implantation at the surgical site [58]. Other surgeons use a flat-tipped monopolar cautery at regular voltage for these procedures. This is supplemented with the use of bipolar cautery for cauterizing bleeders close to critical structures and performing hepatic parenchymal resection (when required). Energy devices such as the harmonic scalpel and ligasure could be used to divide the omental attachment along the stomach and for taking down the bowel mesentery when indicated. Some surgeons use a bipolar scissors for performing the peritonectomies and visceral resections [59]. A variety of other devices have been described in literature and their use depends on the local availability and surgeon’s preference and comfort [60].
Seror et al. described the use of plasma jet for removing small nodules over the diaphragm [61]. We perform formal peritonectomies as described below which obviates the need to use such devices for removing small tumor nodules. The plasma jet could be used to remove small nodules over the stomach serosa and thus, avoid a gastrectomy.
6.2. Right Subphrenic Peritonectomy
The peritonectomy begins anteriorly, stripping the peritoneum off the posterior surface of the anterior abdominal wall and moving towards the right. There could be adhesions between the liver surface and the subphrenic peritoneum due to the tumor or the following chemotherapy response (Figure 5). The adhesions can be divided, and the liver mobilized simultaneously to visualize all the diseases in the region. This may not be possible without stripping Glisson’s capsule off the liver if the disease is extensive or infiltrating Glisson’s capsule and the underlying parenchyma. The preferred approach is to strip the peritoneum off the diaphragm and then come on to the liver.
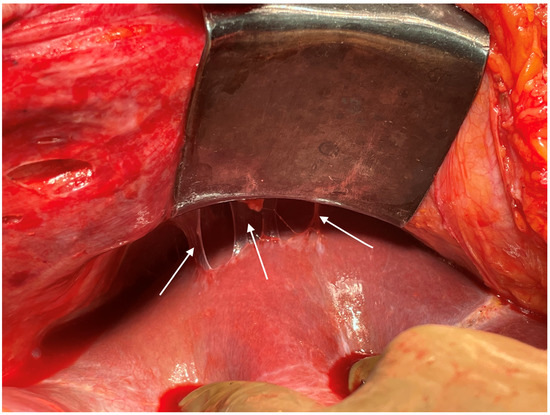
Figure 5.
Disease in the right subphrenic region. Adhesions between the subphrenic peritoneum and liver capsule, which is indicative of disease on the right subphrenic peritoneum, liver capsule or both.
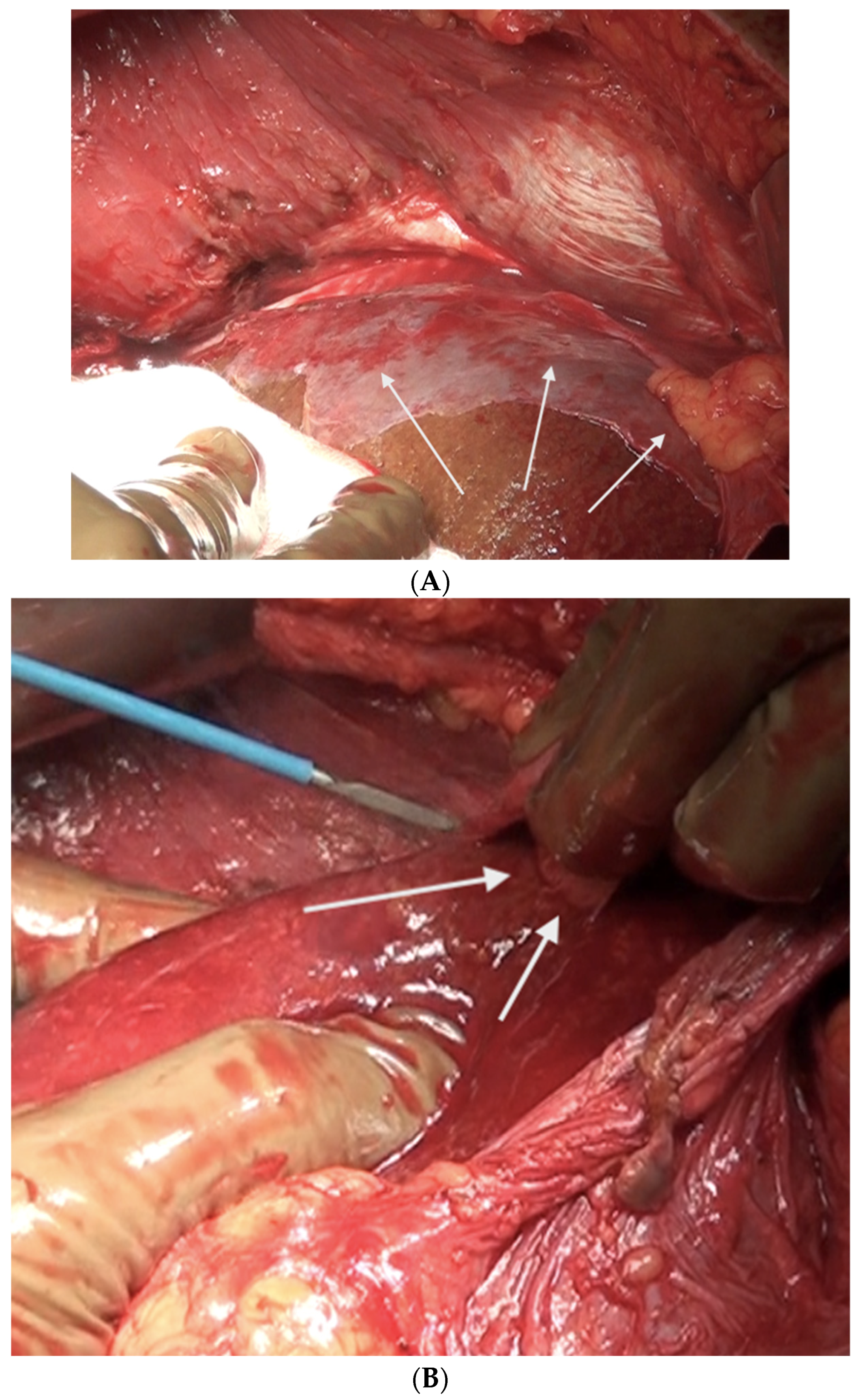
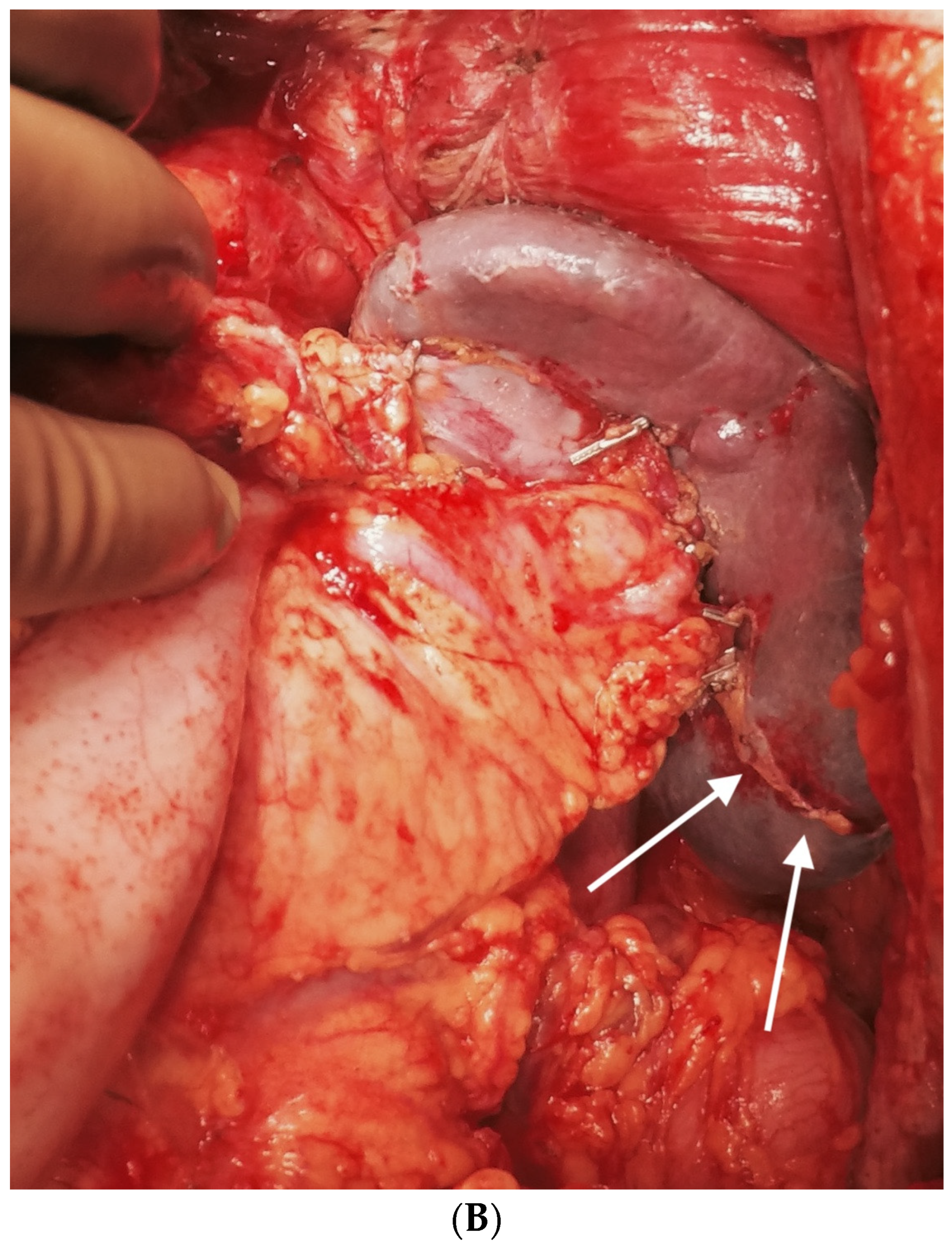
The liver is wholly mobilized at a later stage when all of the subphrenic peritoneum has been dissected off the diaphragm (Figure 6A,B).
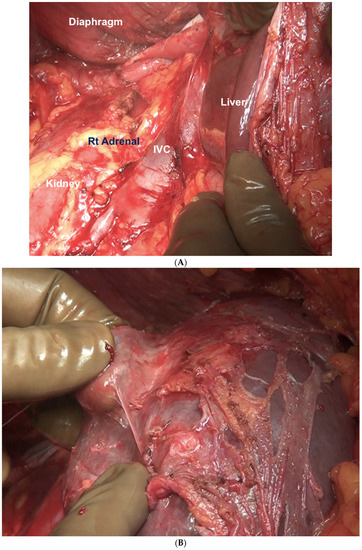
Figure 6.
(A) The liver is completely mobilized, exposing the IVC. The subphrenic peritoneum has been reflected onto the liver. (B) Stripping of Glisson’s capsule begins.
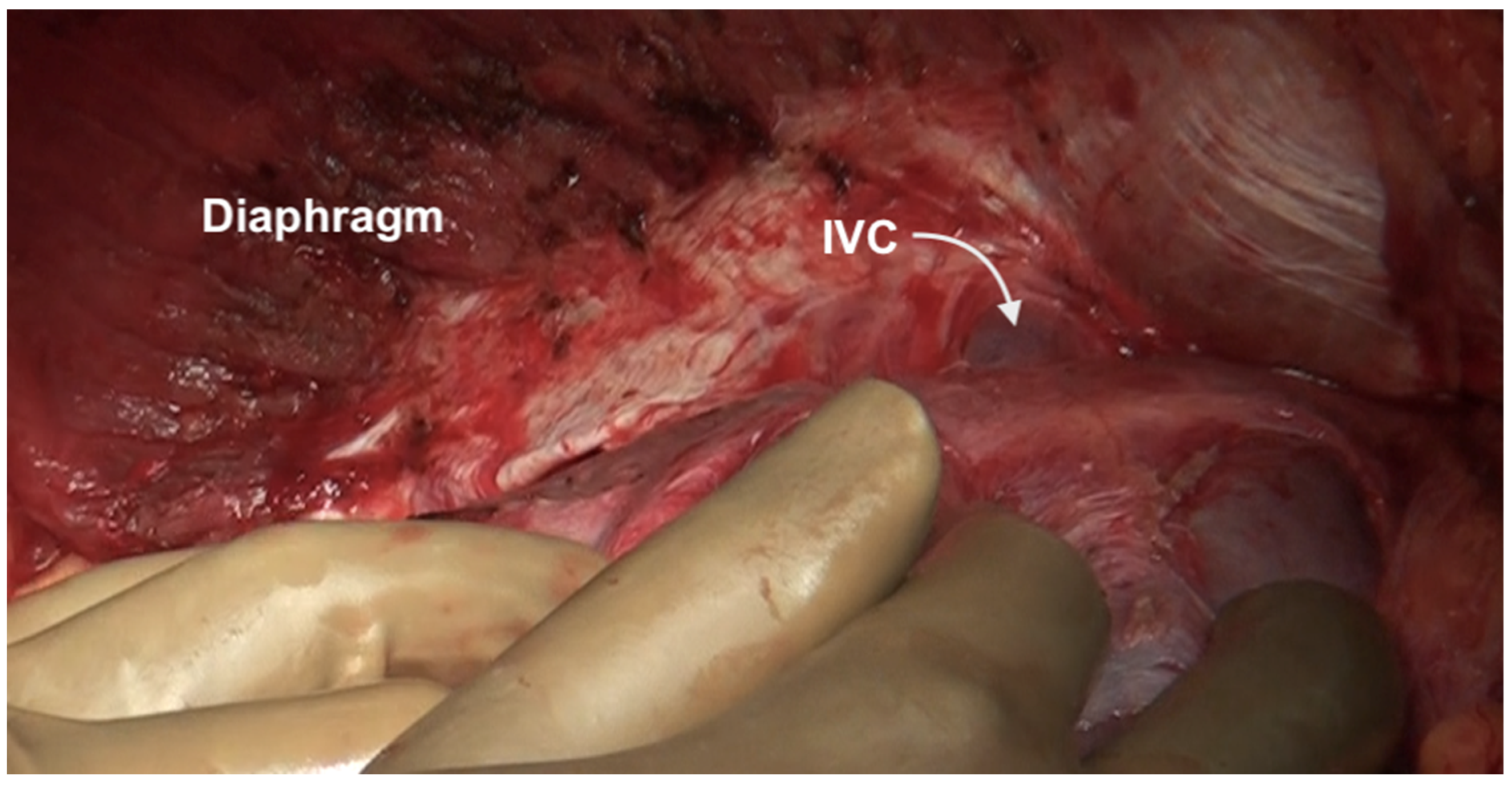
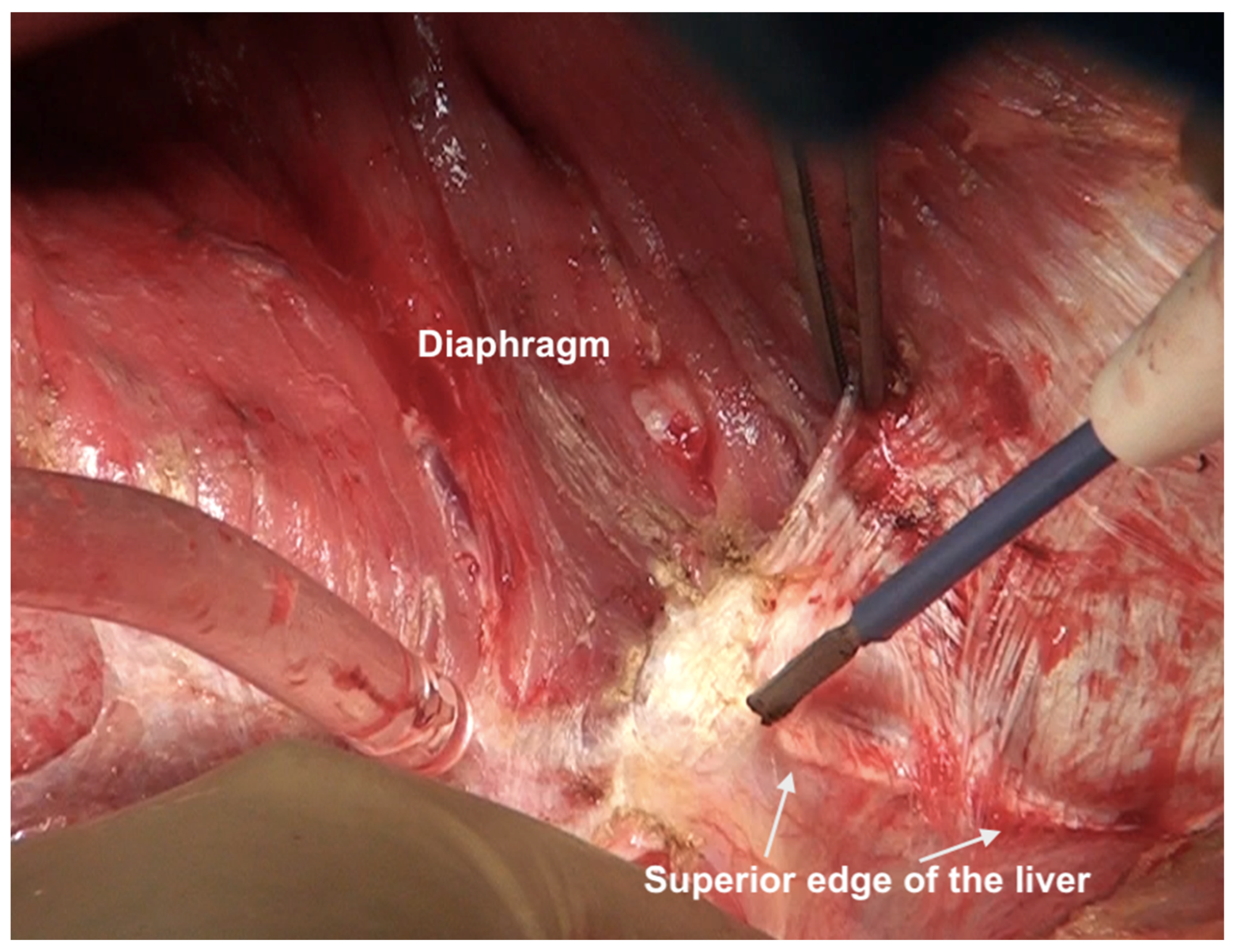
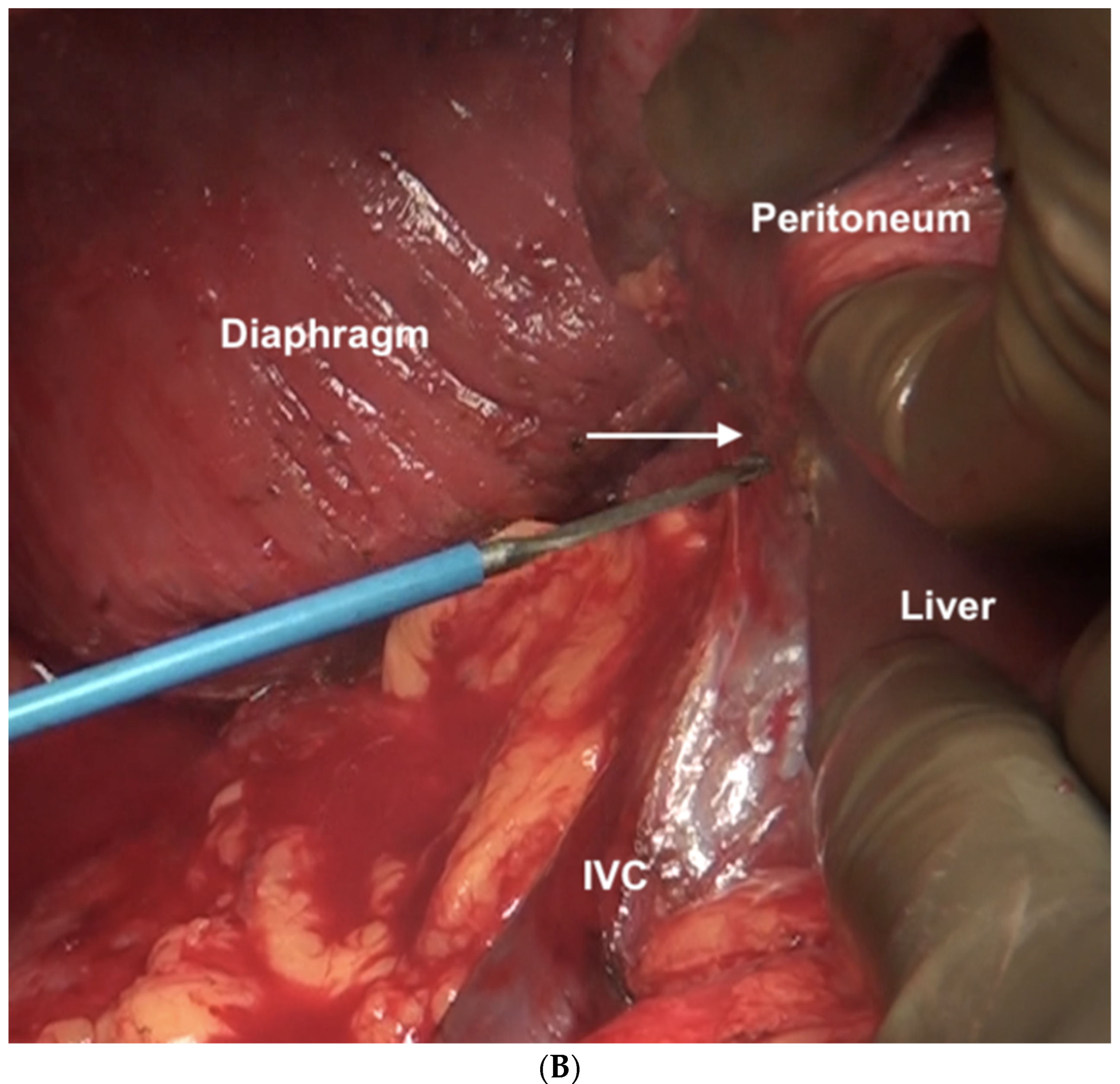
The falciform ligament is divided at its superior attachment to the abdominal wall till the xiphoid is reached. Inferiorly, it is divided at its hepatic attachment till the posterior peritoneal reflection on the liver is reached. The peritoneum is divided where it attaches to the superior surface of the liver, exposing the IVC and right hepatic vein (Figure 7) [44]. The peritoneal deposits often infiltrate the underlying diaphragmatic muscle in part or full thickness. However, the peritoneum overlying the IVC can be stripped off easily due to a layer of loose areolar tissue between the peritoneum and the IVC. Caution is recommended as sometimes there is extensive disease right to the right lateral border of the IVC. The right hepatic vein needs to be safeguarded. The tumor rarely encases the vein itself. Some surgeons prefer to take vascular control before performing such resections [3]. The dissection on the superior edge of the liver continues laterally till the right triangular ligament is reached (Figure 8). The peritoneum is stripped off the right dome till the superior edge of the liver is reached. If the deposits infiltrate the diaphragm, a layer of the underlying muscle is removed along with the peritoneum (Figure 9).
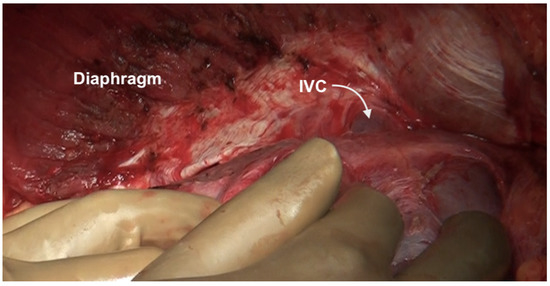
Figure 7.
The suprahepatic IVC is visualized. The peritoneum has completely been dissected off the diaphragm and reflected onto the liver. The loose areolar tissue around the IVC is preserved.
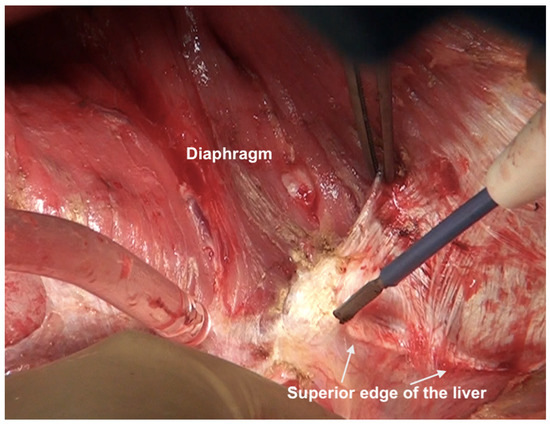
Figure 8.
Dissection lateral to the IVC along the superior edge of the liver. Here, the diaphragmatic peritoneum is fused with Glisson’s capsule and the dissection proceeds from the diaphragm onto the liver. The capsule will be stripped off after the diaphragmatic peritonectomy is complete and the right lobe has been completely mobilized. The IVC itself has not been exposed at this point.
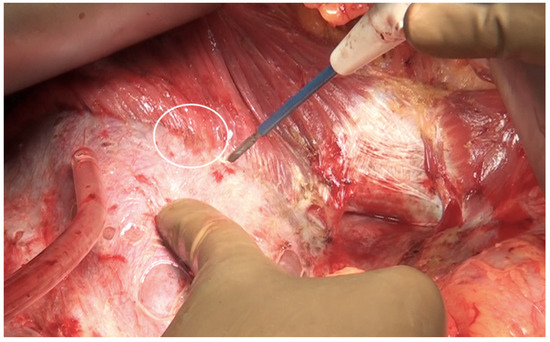
Figure 9.
The peritoneum being stripped off the right dome of the diaphragm with a thin layer of the underlying muscle.
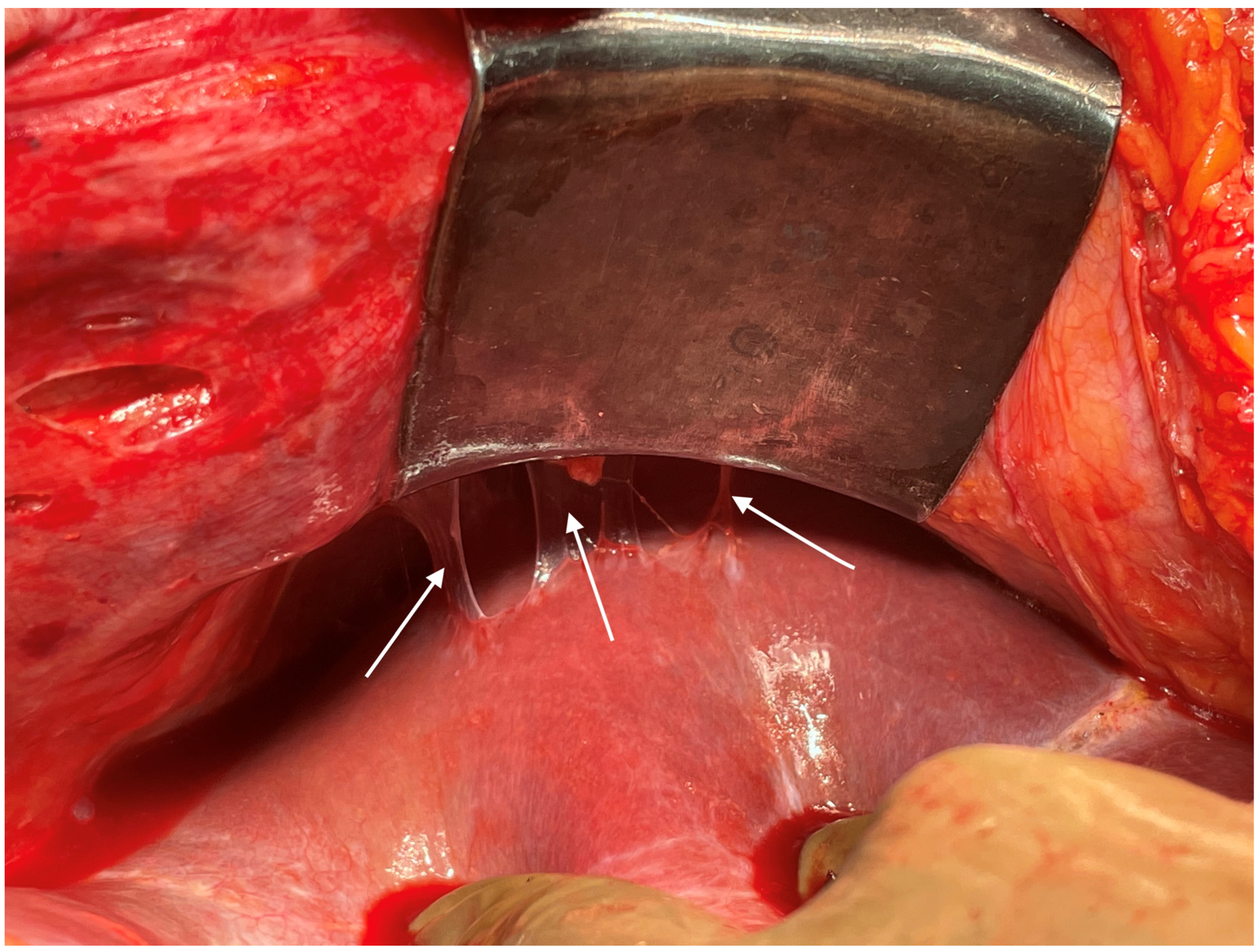
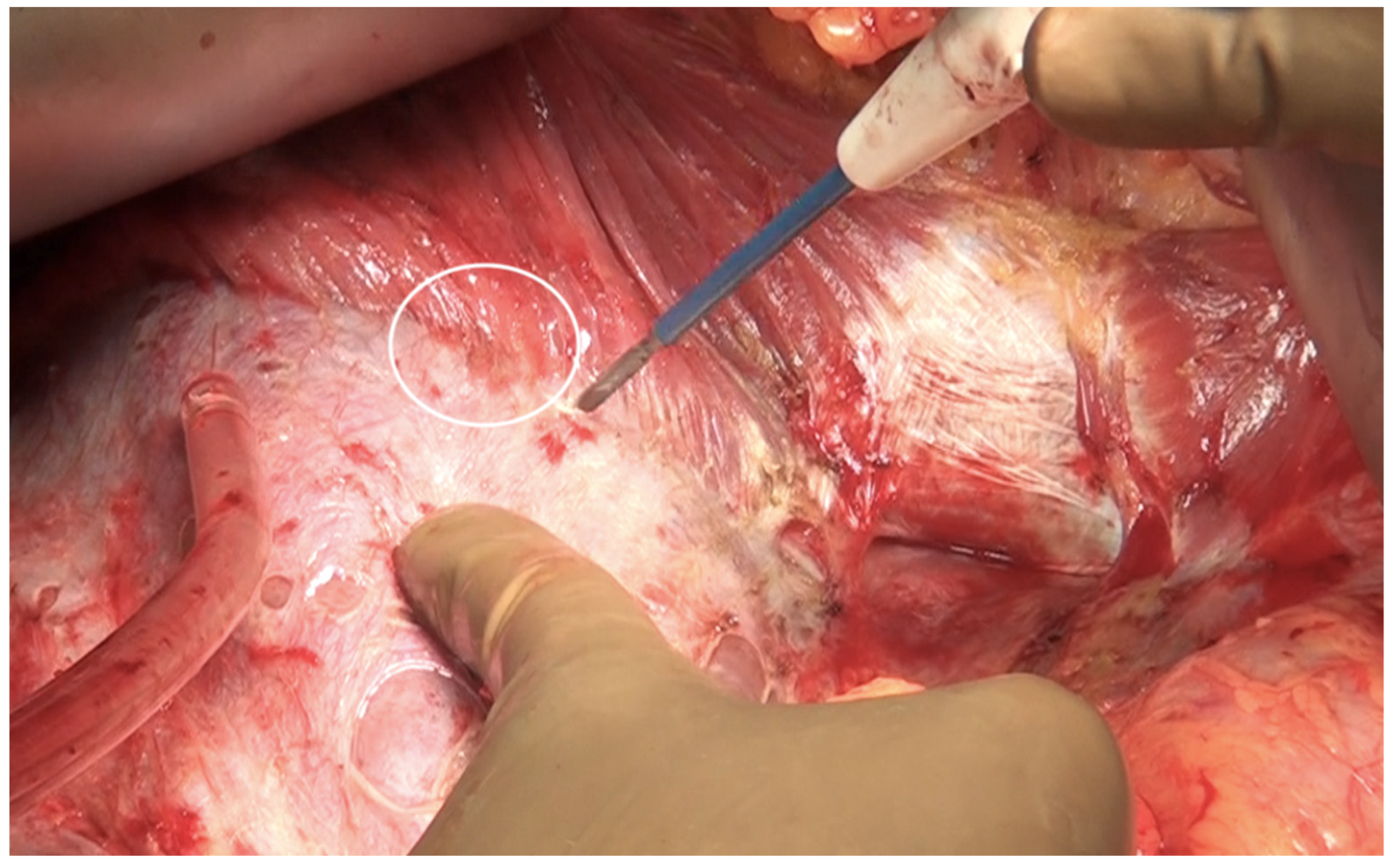
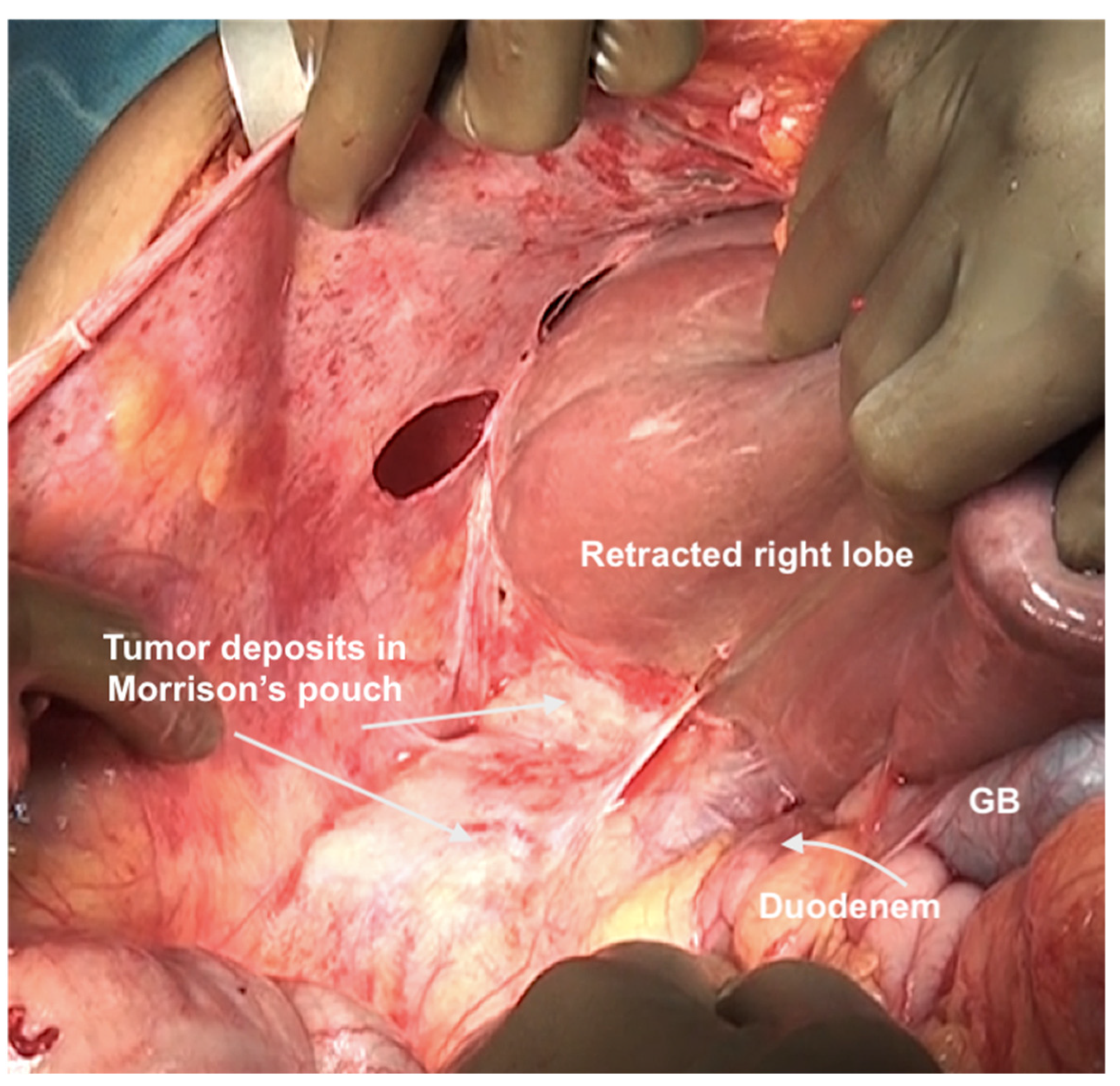
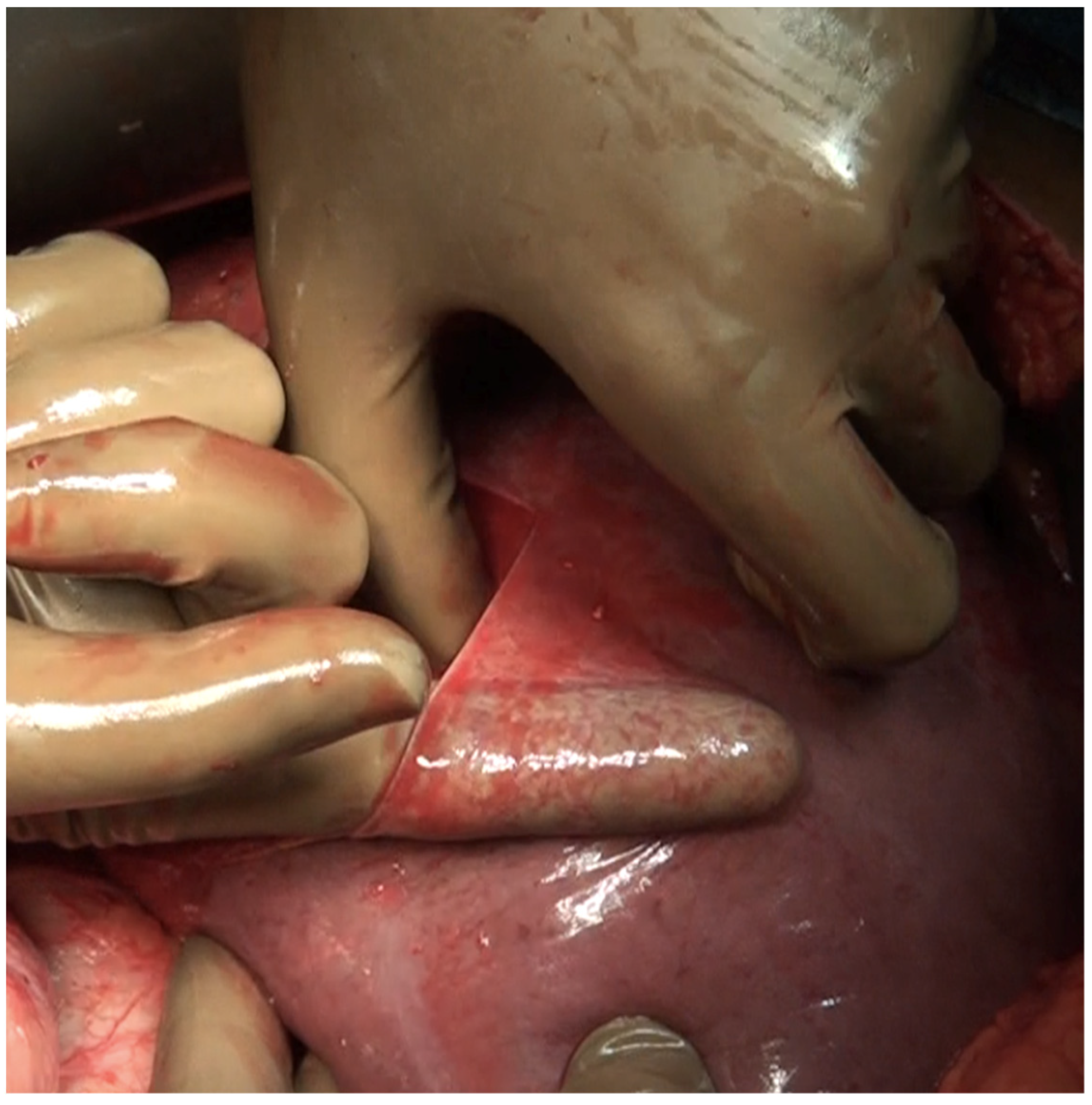
Small blood vessels supplying the diaphragm should be cauterized while the main blood supply to the diaphragm should be preserved as far as possible [43]. When there is full thickness involvement, the diaphragm is divided, and the extent of the diaphragm to be resected is determined by bimanual palpation and direct inspection of the pleural surface of the diaphragm. A full-thickness resection of the diaphragm is then performed depending on the extent of involvement. The diaphragm could be divided using any of the energy devices described above. The defect in the diaphragm is usually repaired only after the right upper quadrant peritonectomy is completed. It is repaired using a heavy absorbable of non-absorbable sutures depending on the preference of the surgeon. Continuous or interrupted sutures could be applied. Some surgeons use a stapler to close the diaphragm rent or perform a diaphragm resection, we do not perform it [62]. It is important to suck out any air, fluid or blood clots in the pleural space before closing the rent. If HIPEC is planned, then it could be repaired before or after the procedure. Very rarely, the defect is too large to be closed primarily or the mobility of the remaining diaphragm is restricted due to adhesions in the pleural space. A polypropylene mesh could be used to repair the defect [63]. The right triangular ligament is excised completely, which also facilitates retraction of the liver to the left, exposing the right inferolateral surface in contact with the retrohepatic IVC. The fold of the peritoneum that gets reflected from the lateral border of segments 6 and 7 onto the diaphragm and covers the IVC is a common site of disease that may be missed if the liver is not mobilized completely. If the liver surface and diaphragm are palpated without mobilizing the liver, disease in this region will be missed, especially in patients with a limited upper-abdominal disease or those who have responded to chemotherapy. At this point, the peritoneal attachment to segments 6 and 7 is divided to expose the IVC completely. Alternatively, the Morrison’s pouch (Figure 10) is dissected first, and this attachment is taken down from below upwards.
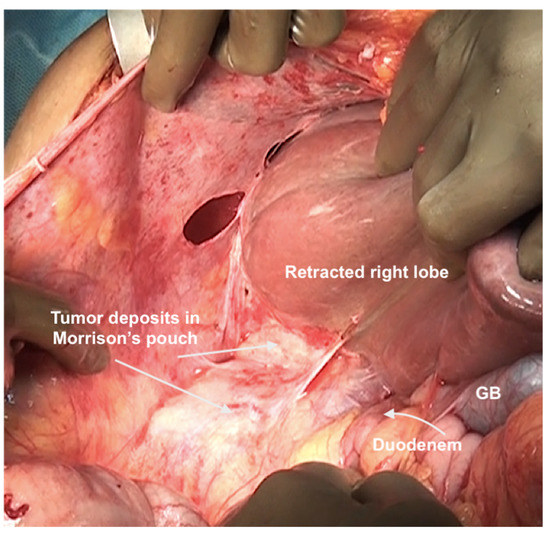
Figure 10.
Tumor deposits in the Morrison’s pouch. This disease cannot be visualized unless the liver is mobilized.
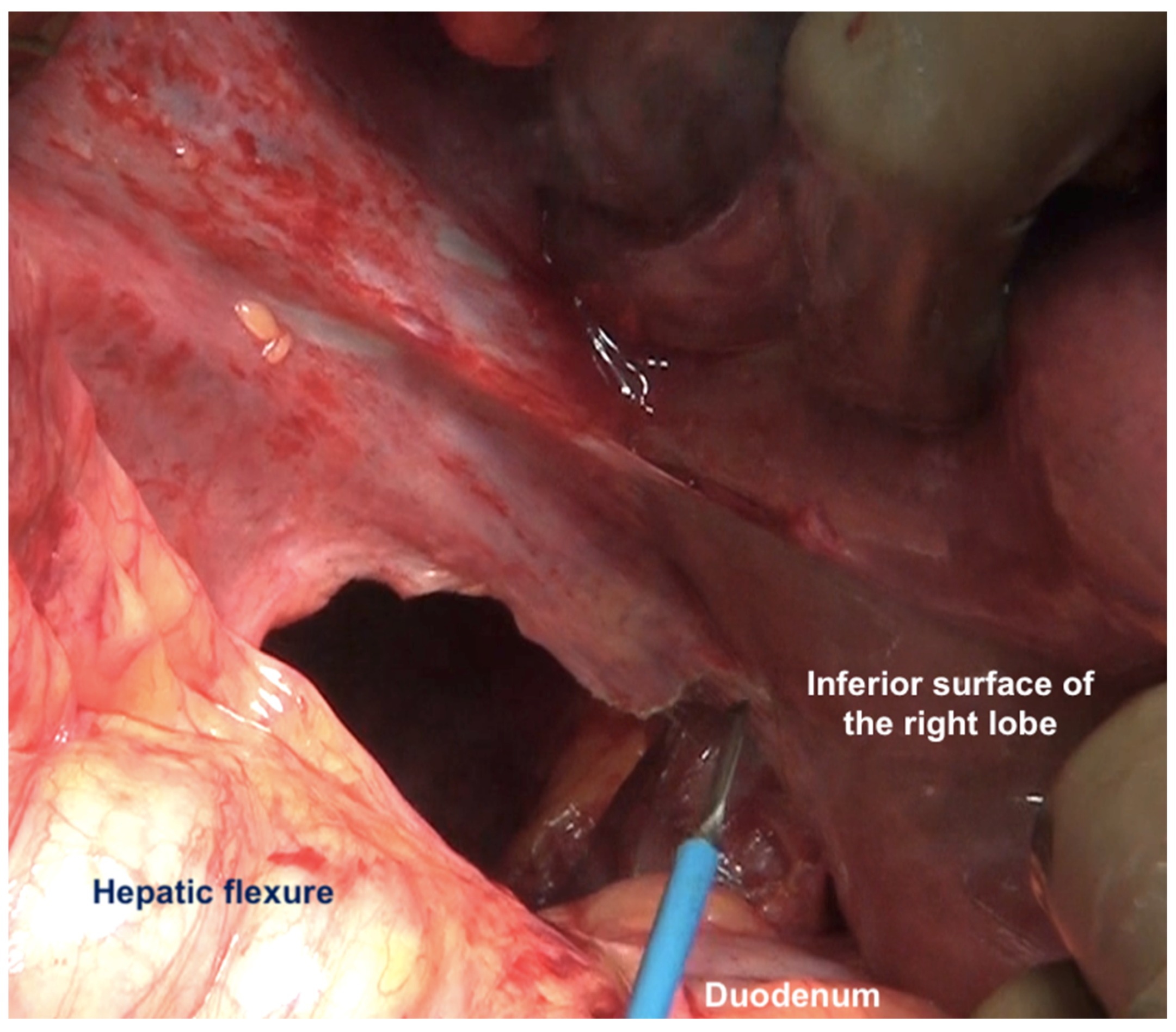
The peritoneal reflection along the lateral border of the duodenum is divided continuing the division along the line of Toldt and onto the peritoneum overlying the right kidney (Figure 11).
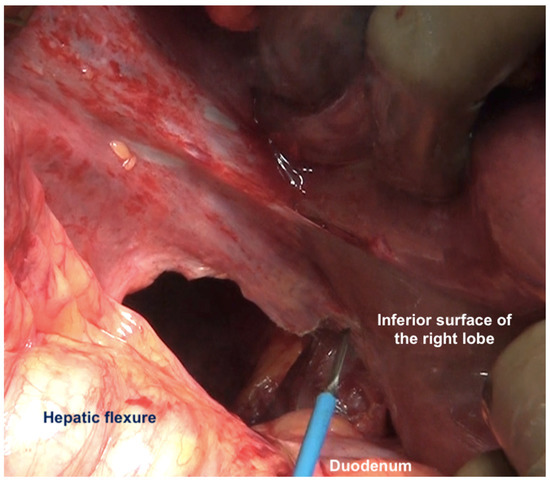
Figure 11.
The peritoneum has been divided along the lateral border of second part of the duodenum. Superiorly, the dissection continues along the lateral edge of the hepatoduodenal ligament and then along the inferior surface of the right lobe (as seen in this image). Inferiorly, it continues along the white line of Todt, where the antero-parietal peritonectomy ends.
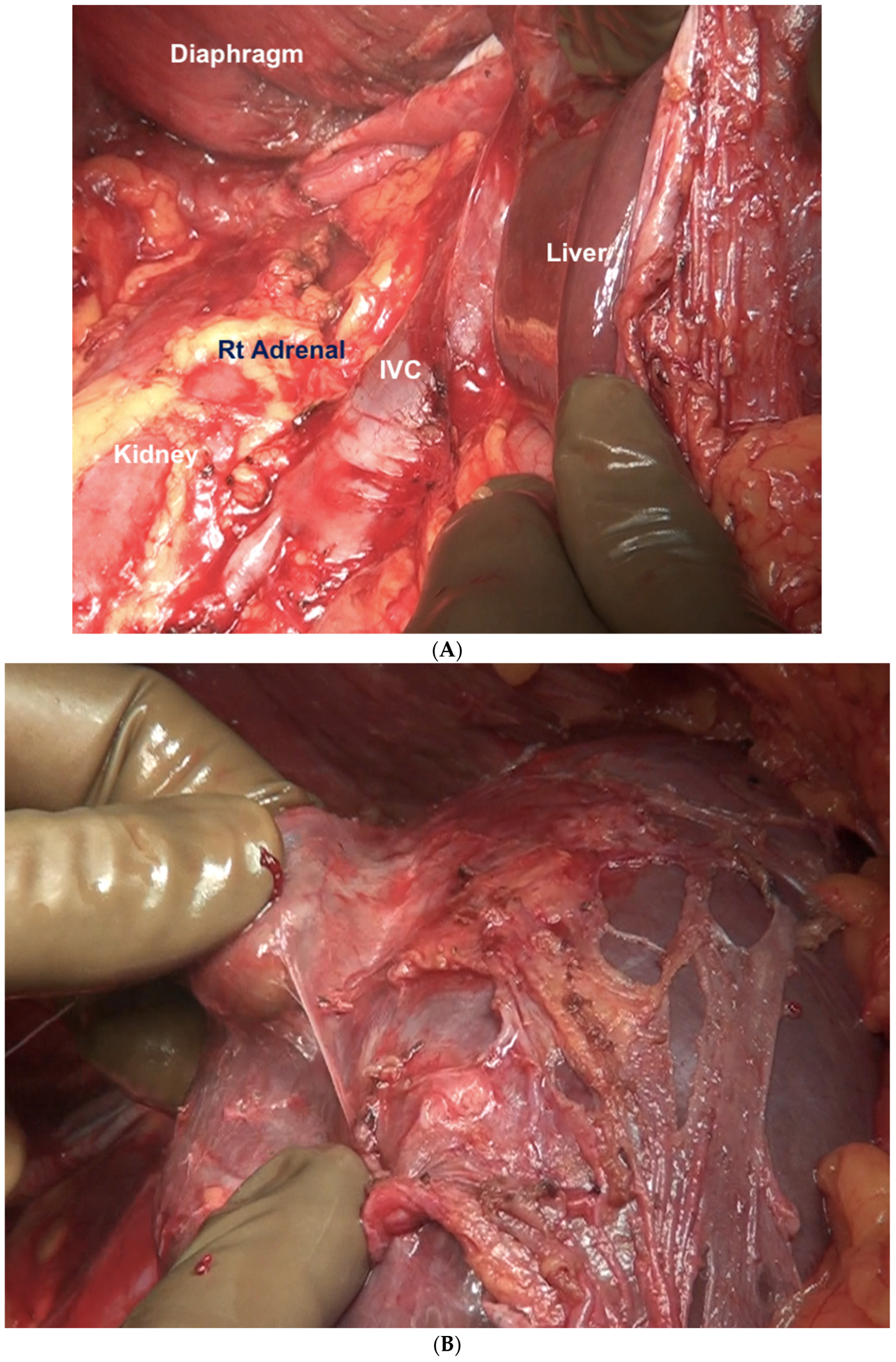
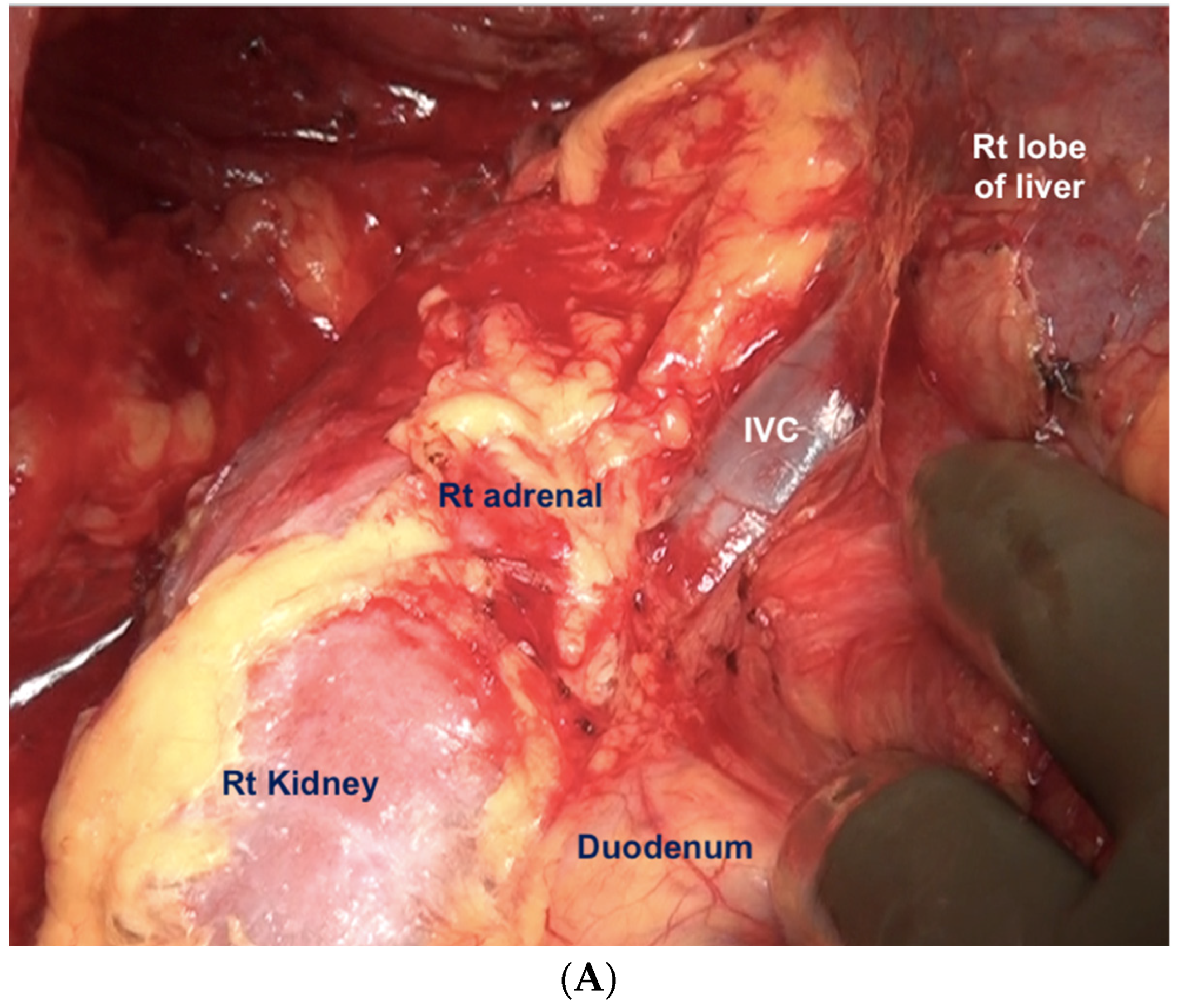
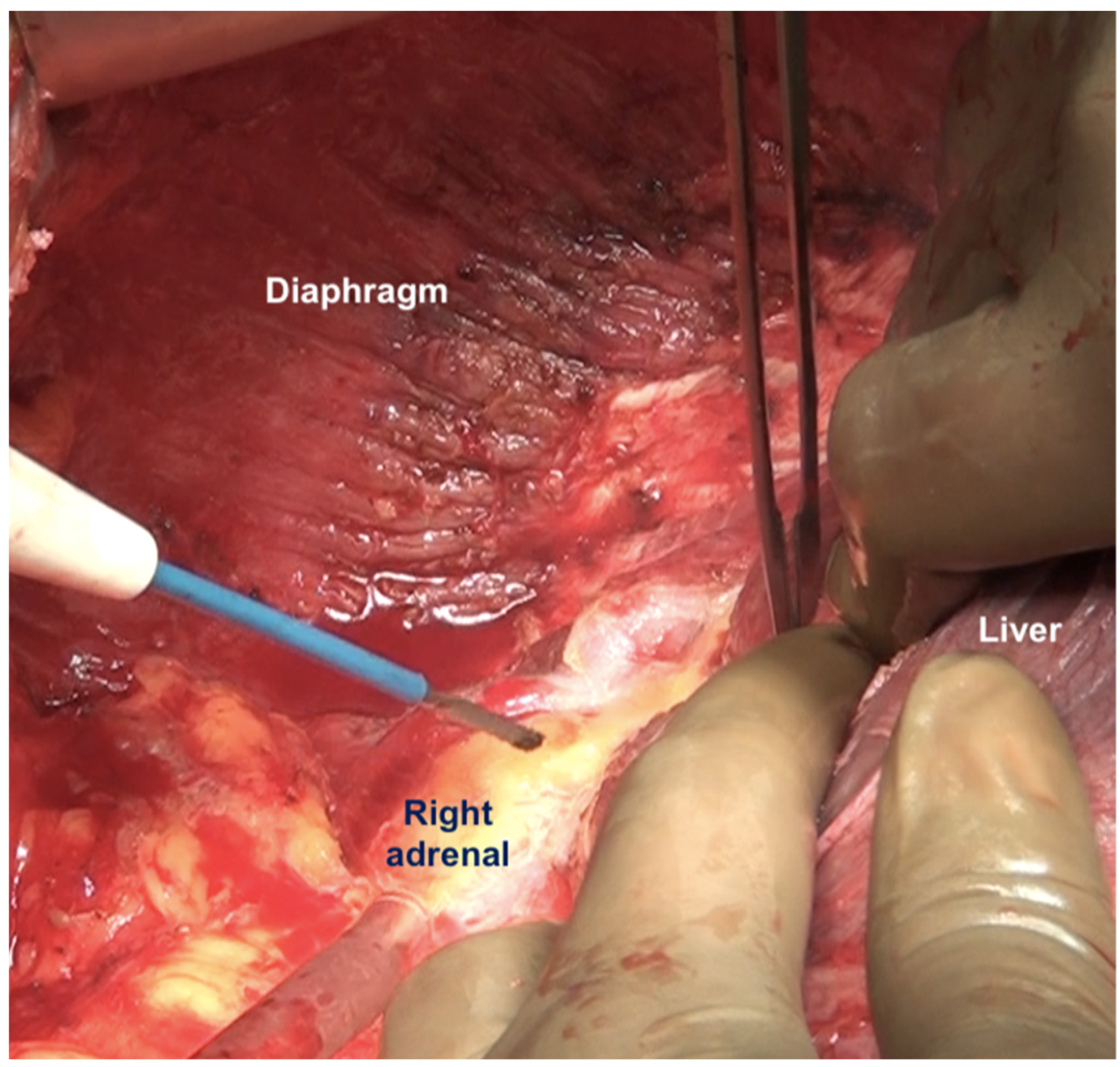
Superiorly, the division continues along the lateral edge of the hepatoduodenal ligament and onto the inferior surface of the liver. The diaphragmatic dissection is completed laterally and the peritoneum is stripped off the pararenal fat to complete clearance of the Morrison’s pouch (Figure 12). The right adrenal should be safeguarded at the medial end, and injury to the adrenal vein should be avoided (Figure 13). The peritoneal attachment on the inferior and inferolateral surfaces of segment 6 and along the inferolateral surface of segment 7 is then taken down to complete the resection of the right upper quadrant peritoneum.
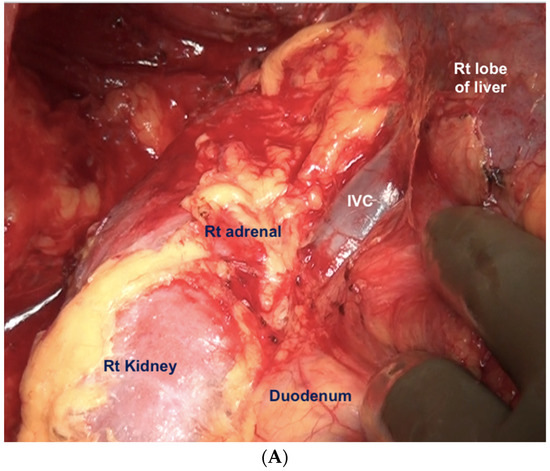
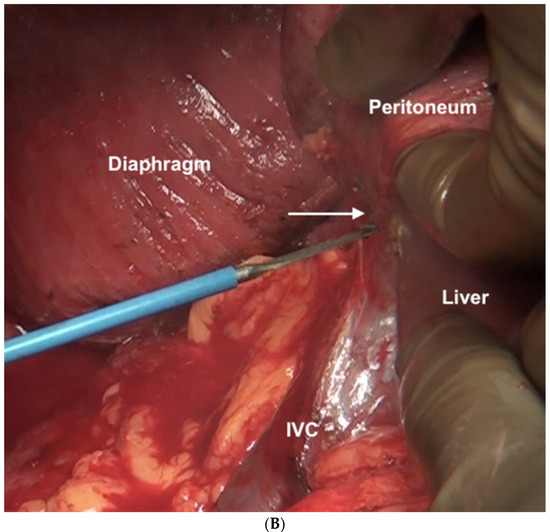
Figure 12.
(A) Dissection of the Morrison’s pouch is complete with the underlying structures exposed including the sub-hepatic inferior vena cava. (B) The peritoneum is reflected onto the liver and dissected off it in continuity with Glisson’s capsule.
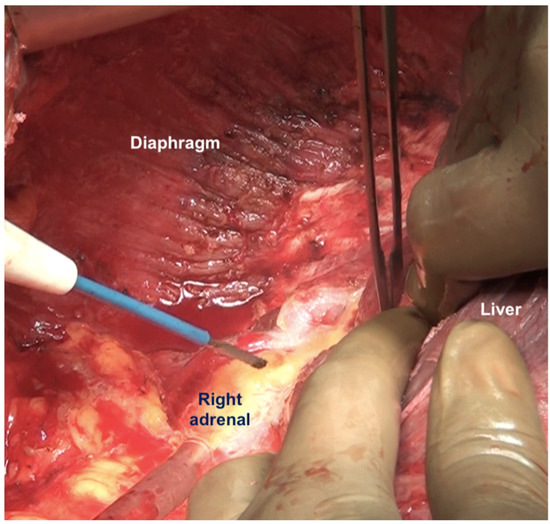
Figure 13.
The peritoneum being dissected off the right adrenal gland. Dissecting in a deeper plane could lead to injury to the adrenal vein and should be avoided.
Regarding the extent of diaphragmatic surgery, we perform stripping of the entire diaphragm on that side if there is diaphragmatic involvement and not a limited stripping or only focal resection as performed by some surgeons [64,65].
6.3. Glissonectomy
Tumor deposits on Glisson’s capsule can be destroyed with electrocautery. Alternatively, a digital glissonectomy, as described by Glehen et al., can be performed. It has the advantage of addressing occult disease in this region [57]. The capsule resection is performed only in the regions involved, using finger dissection alone or combining it with a bipolar scissor dissection (Figure 14). The capsule is incised with scissors or electrocautery at a distance of 1–2 cm from the tumor deposits. The plane between the liver and the capsule is identified, and the capsule overlying that particular surface/lobe or segment is resected. This dissection can be performed even in the case of infiltrative deposits, but care should be taken not to damage the underlying parenchyma. All anatomical scissuras should be explored carefully to remove peritoneal tumor deposits. When possible, Pringle’s maneuver is performed to minimize blood loss. After the capsule is removed, the liver surface is covered and packed with mops to control the bleeding.
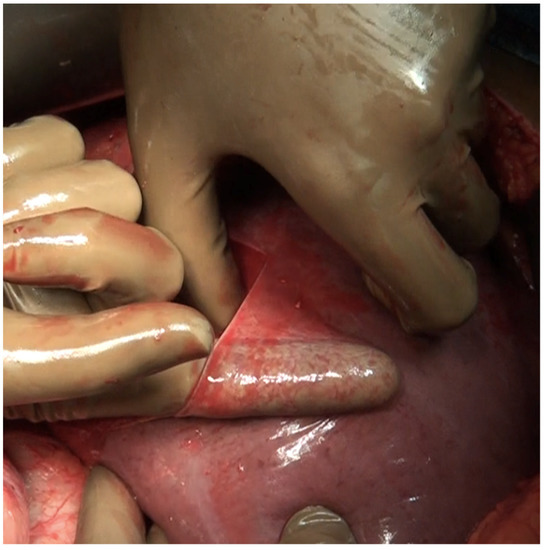
Figure 14.
Digital glissonectomy over the right lobe of the liver in progress.
The digital dissection should be easy to perform, and if resistance is encountered, it indicates either an entry into a “false plane” or parenchymal infiltration by the tumor [66]. Continuing dissection in the false plane could lead to significant hemorrhage and should be avoided. In some regions such as the region around the superior edge of segments 7 and 8 the capsule if more firmly adherent to the liver (Figure 15).
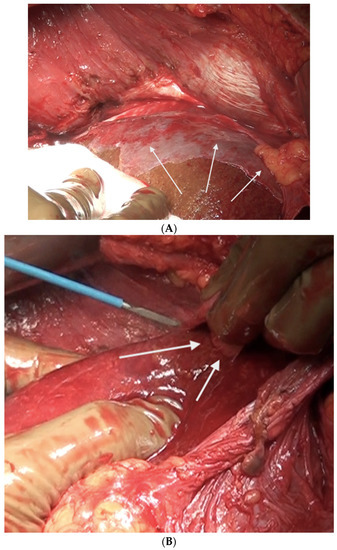
Figure 15.
(A) Part of the capsule adherent to the superior surface and edge of segments 7 and 8 not removed in continuity. (B) This portion was excised with electrocautery.
This region could be dissected separately if resistance is encountered while stripping the capsule. When the parenchyma is infiltrated, the finger dissection should be done following the edge of the tumor to prevent as minor parenchymal damage as possible. Alternatively, non-anatomical liver resection is performed [3]. The most common sites of disease are the right superior and lateral surfaces of the liver to the right of the falciform, the left superior surface to the left of the falciform, and the inferior surfaces. Isolated deposits of tumor can be electrovaporated or dissected. At the end of the CRS, a second look is performed to confirm and/or achieve thorough haemostasias and biliostasis [66].
6.4. Left Subphrenic Peritonectomy and Central Diaphragmatic Peritonectomy
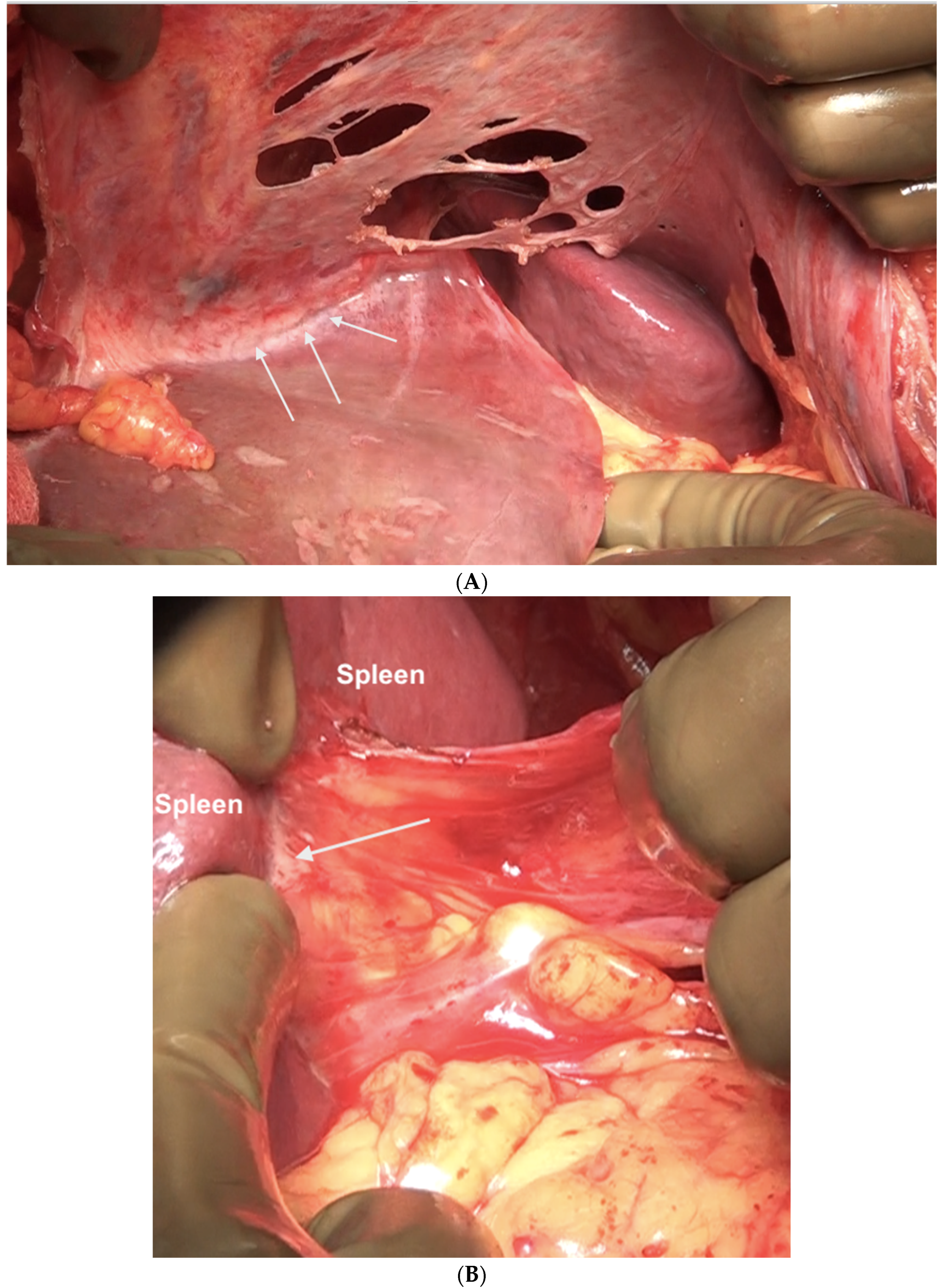
The common disease sites in the left upper quadrant and the central region are on the left lobe surface and the adjacent diaphragmatic peritoneum, the left dome of the diaphragm, the splenic capsule, at the splenic hilum, and in the gastrosplenic ligament (Figure 16).
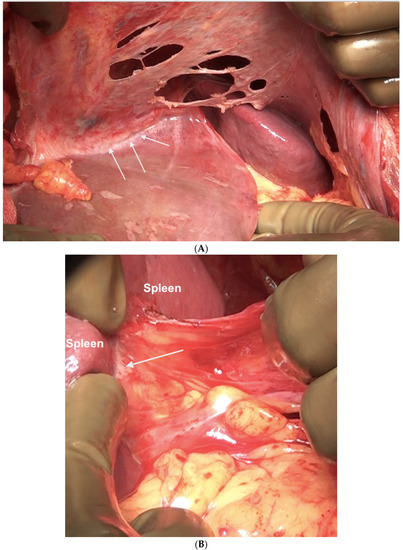
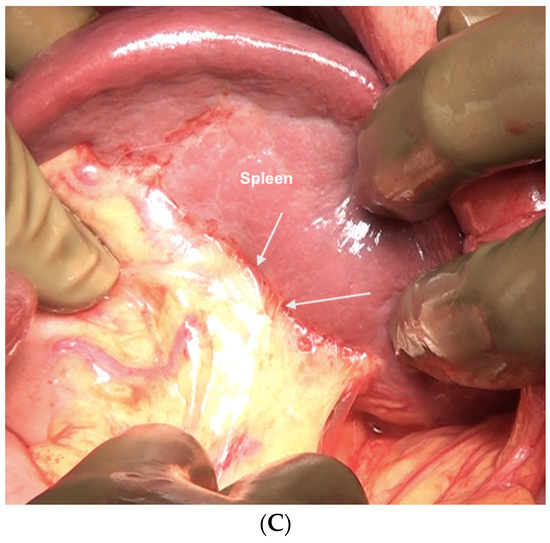
Figure 16.
Sites of disease in region 3 in a patient who has received neoadjuvant chemotherapy. (A) Along the superior edge of the left lobe. (B) On the posterior surface of the spleen along the peritoneal attachment. (C) Anteriorly, the omentum is adherent at the hilum indicative of disease that has responded to chemotherapy. On palpation, tumor nodules were identified. All sites of disease were confirmed on pathology.
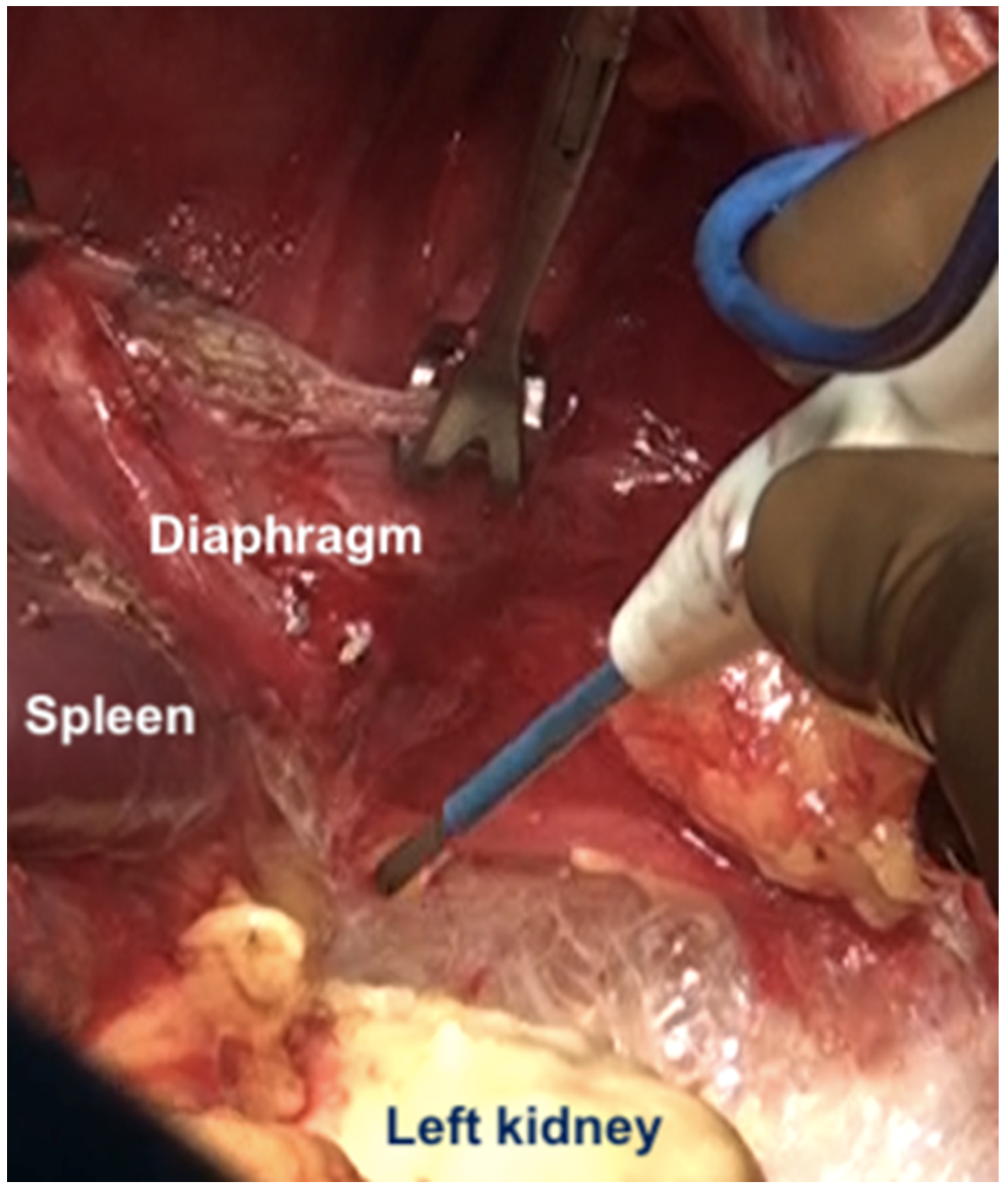
The dissection begins at the edge of the abdominal incision dissecting the epigastric fat and peritoneum off the posterior sheath and then off the left hemidiaphragm. The left side is relatively easier to strip than the right side as there is no intervening lobe of the liver. The same principles as described for the right side are followed. Once the dome has been stripped, dissection continues over Gerota’s fascia (anterior renal fascia which is a fibroconnective tissue layer surrounding the kidney) and then onto the posterior surface of the spleen [44]. The peritoneum is dissected off the left adrenal and the superior half of the peri-renal fat. The splenic flexure of the colon is then mobilized, and the colon retracted inferiorly. The process can be facilitated by carrying the dissection posterior to the kidney and mobilizing it which makes it easier to dissect the reflection of the peritoneum from the diaphragm onto Gerota’s fascia (Figure 17).
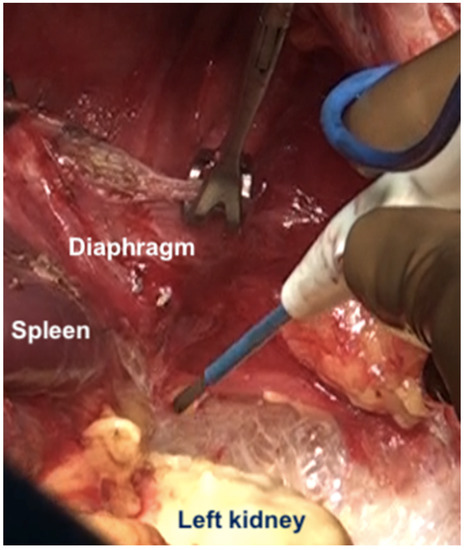
Figure 17.
The kidney is mobilized to facilitate dissection at the posterior part of the diaphragmatic cupola and along the posterior surface of the spleen.
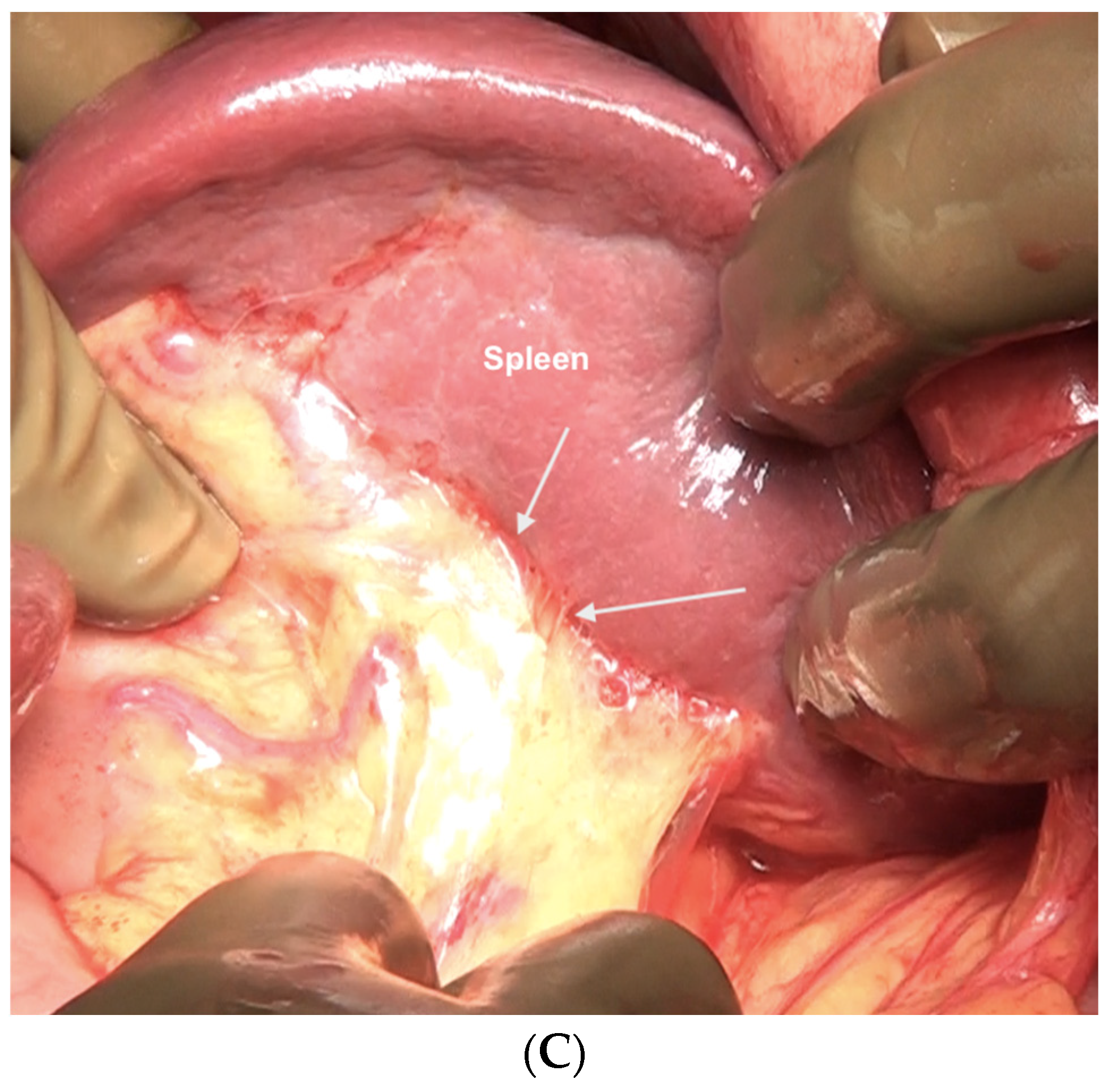
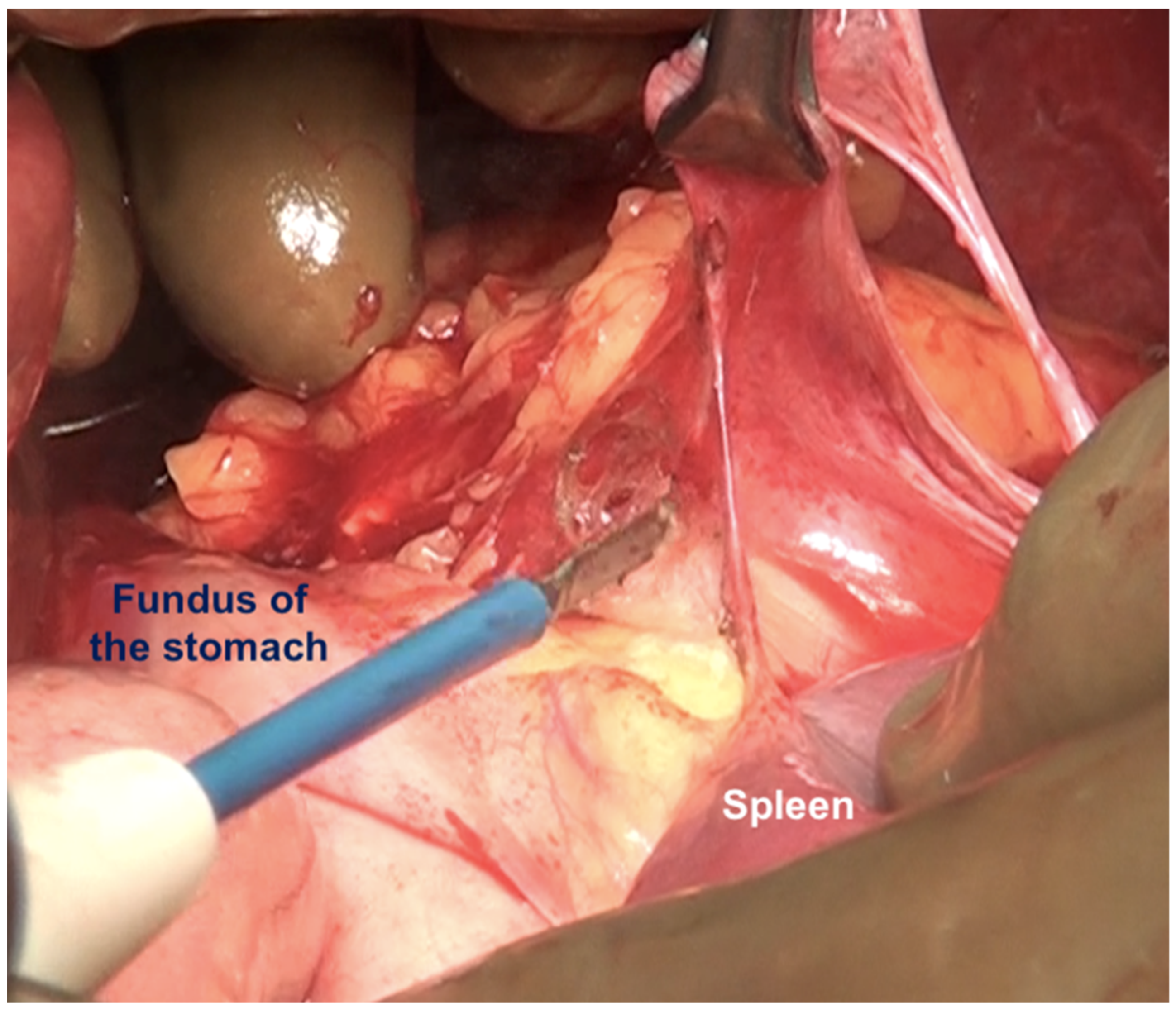
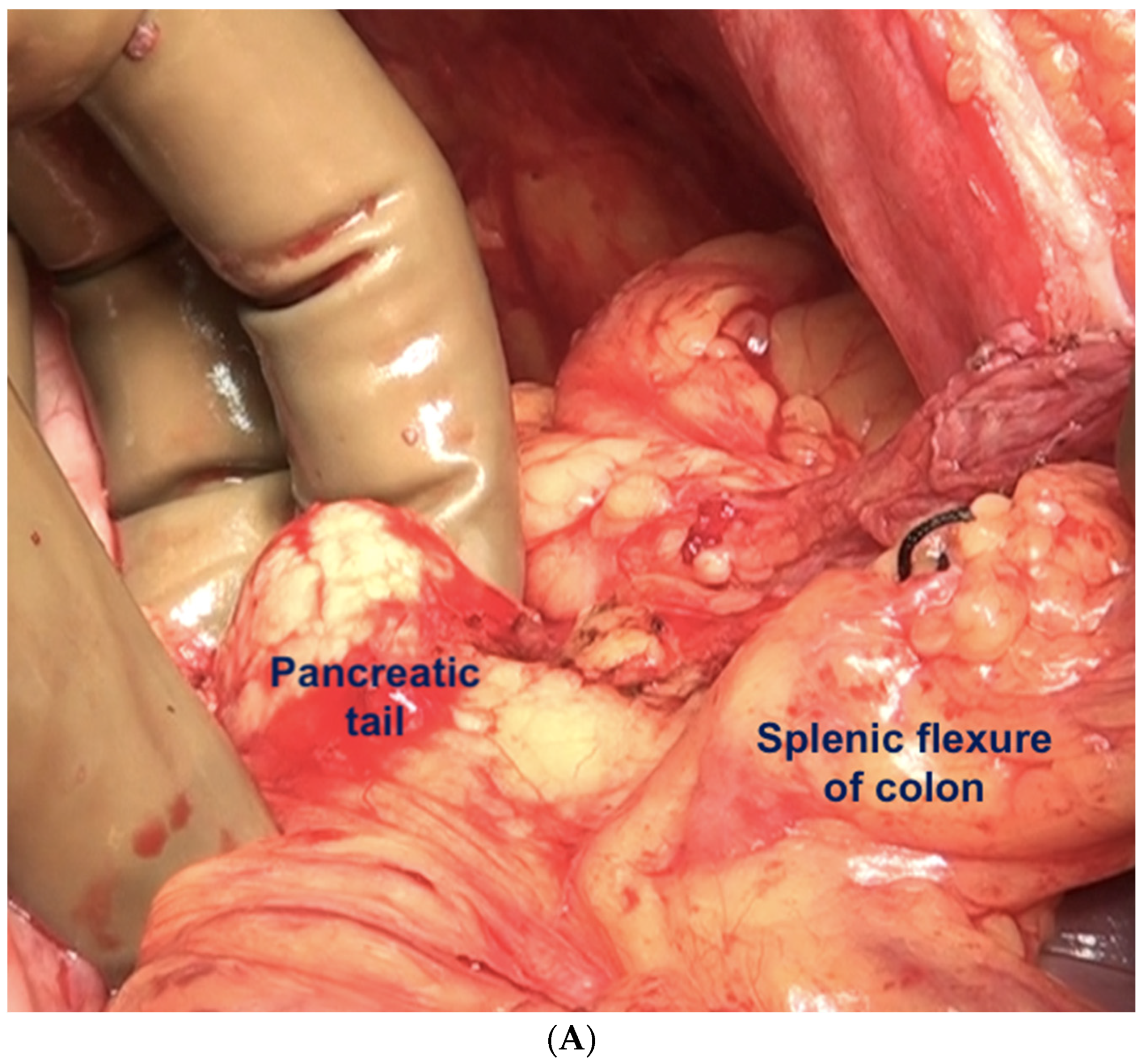
If the splenectomy is not planned, the dissection stops the posteroinferior and posterolateral border of the spleen. The splenic hilum should be exposed posteriorly to complete the subphrenic peritonectomy. If a splenectomy is planned, then the splenic vessels could be ligated and divided after retracting the spleen anteriorly, or the pedicle could be approached anteriorly [44]. The anterior approach may be complex when there is an extensive disease at the hilum. The central dissection begins to the left of the falciform ligament, stripping the peritoneum off the central diaphragm and excising the left triangular ligament. The peritoneum overlying the central tendinous portion of the diaphragm is relatively thin, and the pericardial cavity accidental opening by inexperienced surgeons is common. Such a pericardial defect should be repaired immediately. Second, the left hepatic vein that is encountered while mobilizing the left lobe can have a tortuous course in some patients and needs to be safeguarded. The dissection continues over the abdominal esophagus, the greater curvature of the stomach, and onto the spleen. If a splenectomy is not planned, the short gastric vessels must be safeguarded. The peritoneum over the short gastric vessels and to the left of the fundus should, however, be carefully resected (Figure 18). The tail of the pancreas should also be safeguarded (Figure 19).
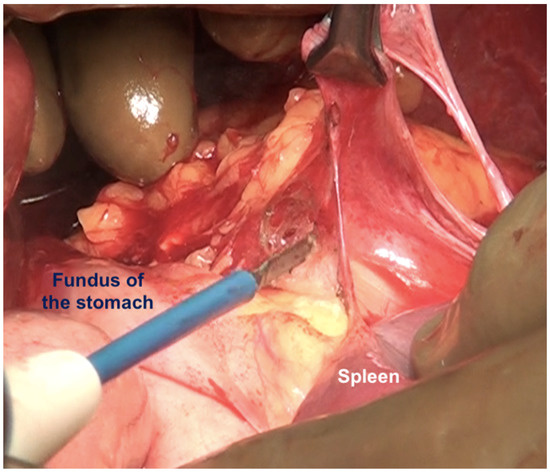
Figure 18.
Dissection of this peritoneum continues laterally and over the short gastric vessels if the spleen is preserved. In this patient, a splenectomy was performed.
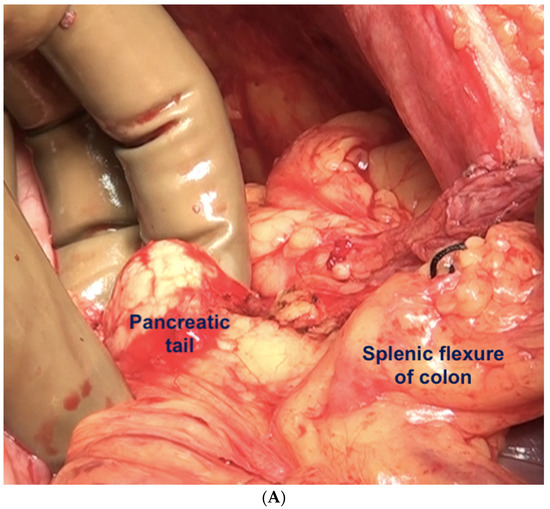
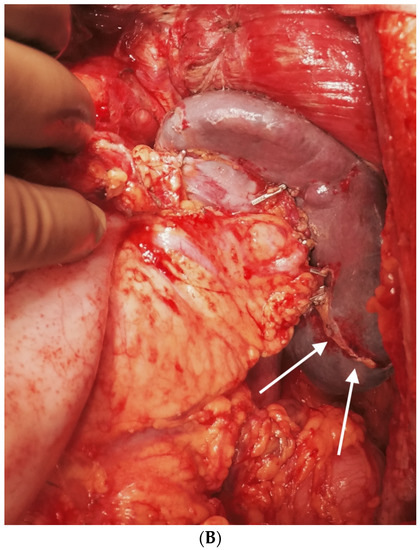
Figure 19.
(A) The left upper quadrant peritonectomy and splenectomy is complete with no damage to the tail of the pancreas. (B) Spleen preserving left upper quadrant peritonectomy. The omental attachment on the spleen has been completely divided (white arrows).
6.5. Lesser Omentectomy and Hepatoduodenal Ligament Clearance
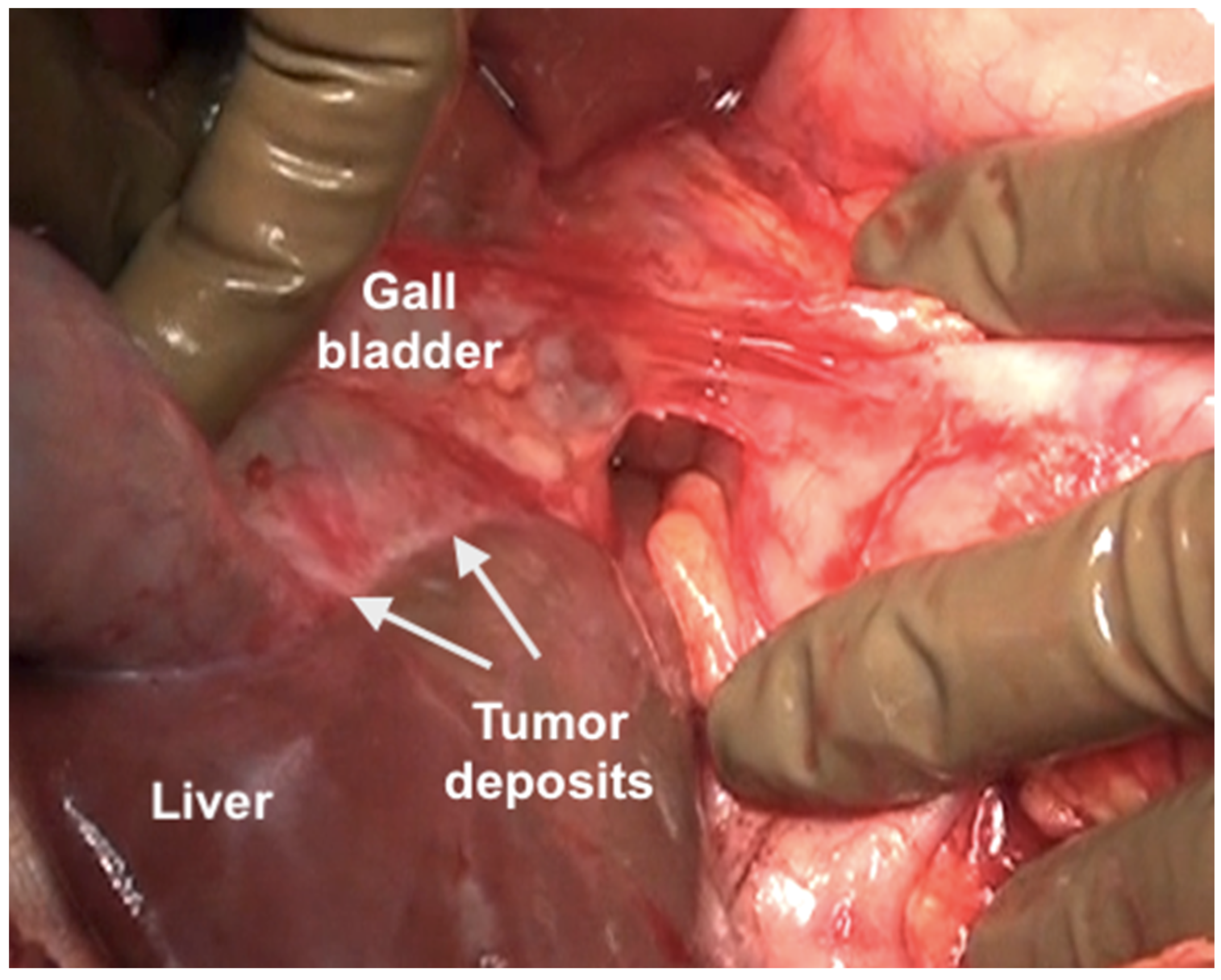
The clearance of the hepatoduodenal ligament begins with a cholecystectomy [43]. The peritoneal fold covering the gall bladder is a common disease site (Figure 20).
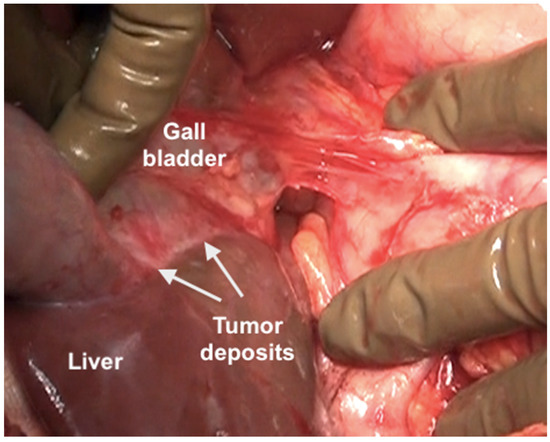
Figure 20.
Tumor deposits on the serosa of the gall bladder. The peritoneal fold between the gall bladder and the duodenum has to be divide to visualize this disease.
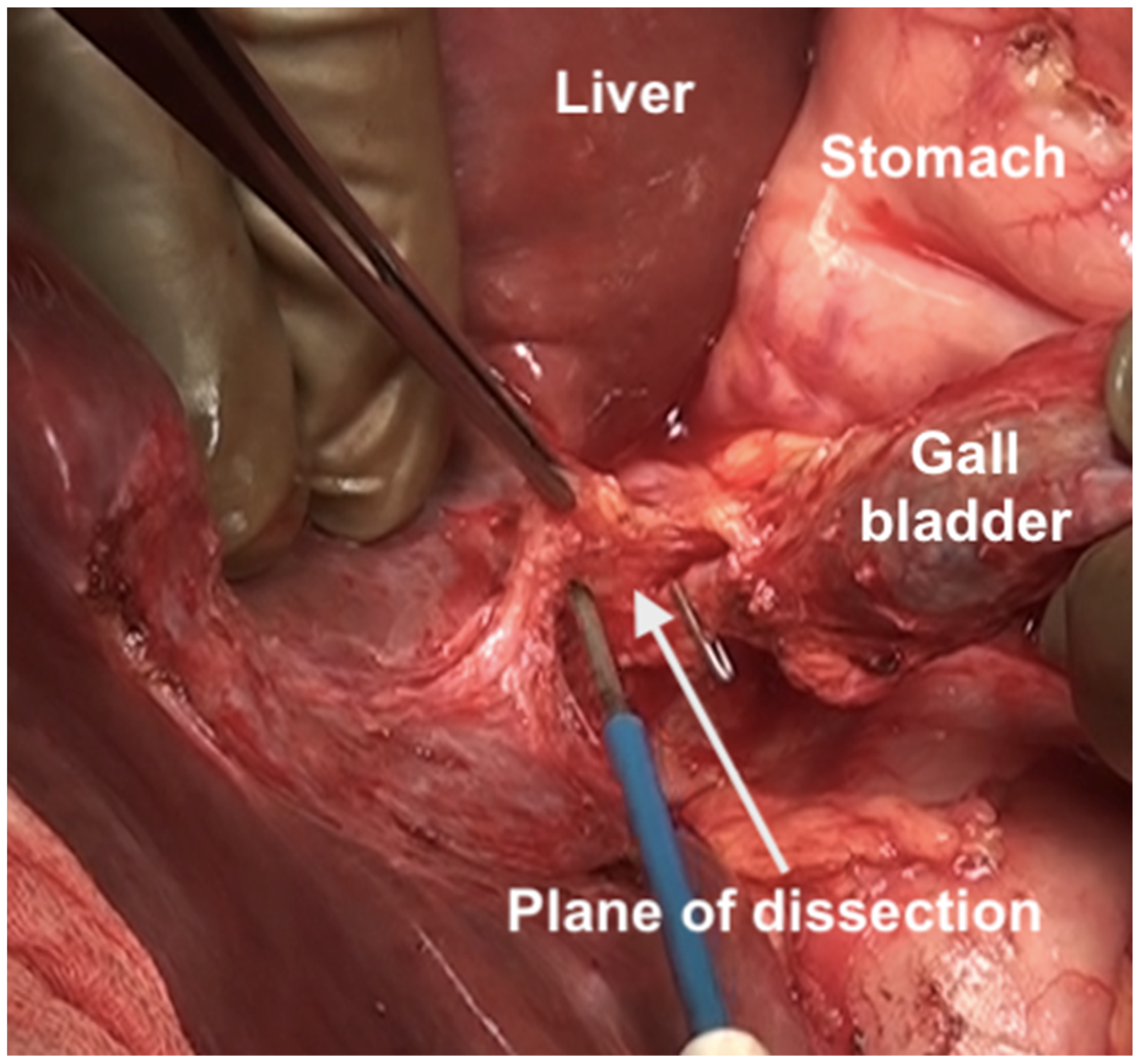
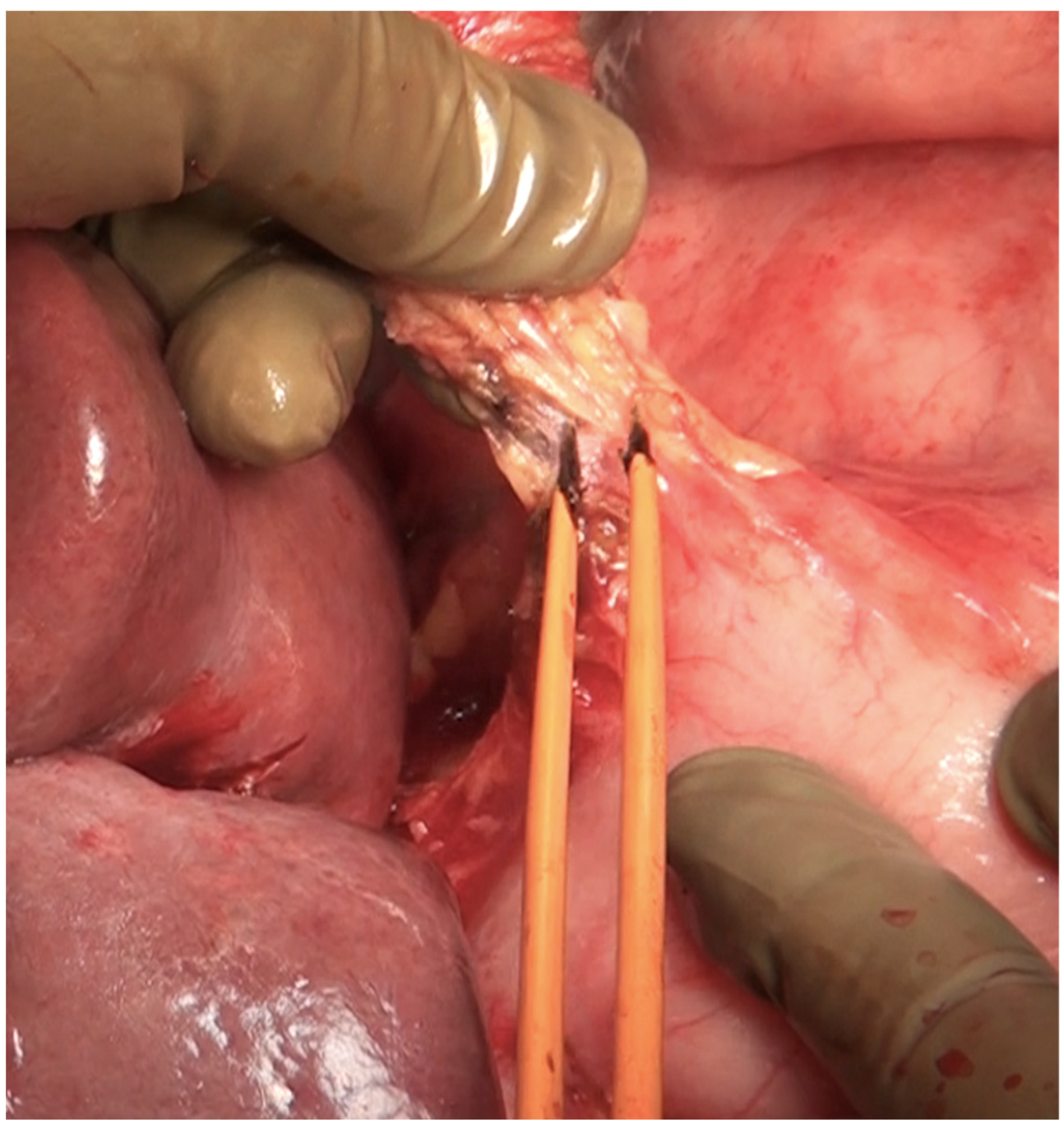
The fundus first approaches the gall bladder by dissecting the cystic artery and the cystic duct and dividing them. The peritoneum over the gall bladder is maintained intact and then retracted over the portal structures, which are carefully dissected using blunt or monopolar or bipolar dissection (Figure 21) [67]. When there is an extensive disease, the cystic duct can be used to identify the correct plane. The fat around the portal structures should be preserved (Figure 22). Variations in the anatomy of the portal structures should be considered. The lesser omentum could be removed in continuity or separately.
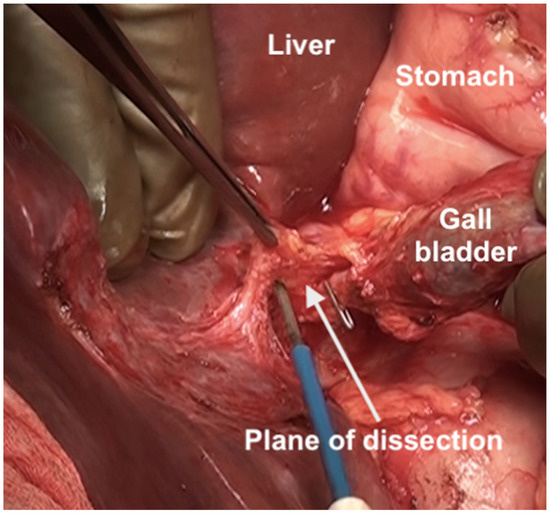
Figure 21.
Resection of the hepatoduodenal ligament in progress.
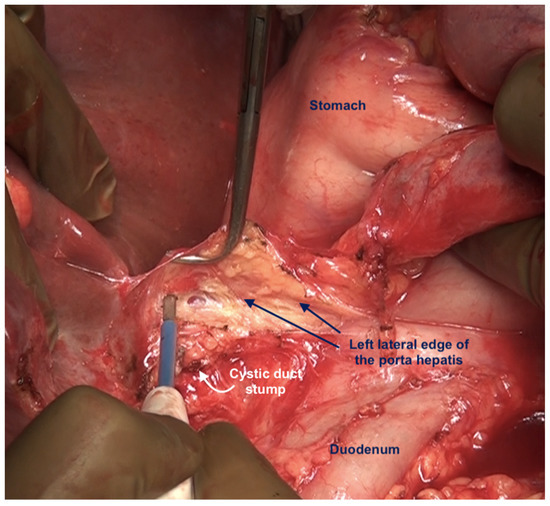
Figure 22.
The porta hepatis after complete dissection of the anterior layer of the hepatoduodenal ligament. The fat around the portal structures is preserved.
The hepatogastric ligament is divided at its attachment to the caudate lobe. An accessory or aberrant left hepatic artery may be present in this area which needs to be identified and preserved. If the artery is embedded in the tumor and or its preservation prevents clear exposure of the omental bursa, the artery can be divided close to the liver [68]. The right gastric artery should be preserved as far as possible.
Superior clearance of the hepatogastric ligament requires mobilization of the left lateral segment of the liver by dividing the left triangular ligament. It is retracted to the right to expose the ligament completely. Along the lesser curve, blunt and sharp dissection is performed. The vagal fibers and left gastric vessels should be preserved as far as possible (Figure 23). This is important if the gastroepiploic arch has been divided. The tumor over the surface of the caudate lobe may be electro-evaporated or excised [68].
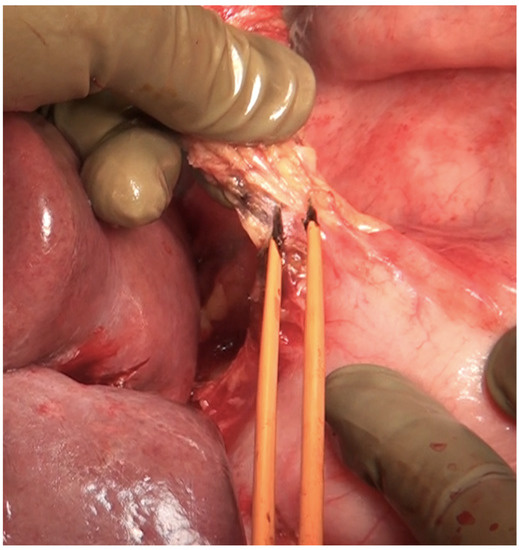
Figure 23.
Dissection of the lesser omentum using bipolar electrocautery. The neurovascular structures along the lesser curve are preserved as far as possible.
6.6. Foramen of Winslow and the Posterior Layer of the Hepatoduodenal Ligament
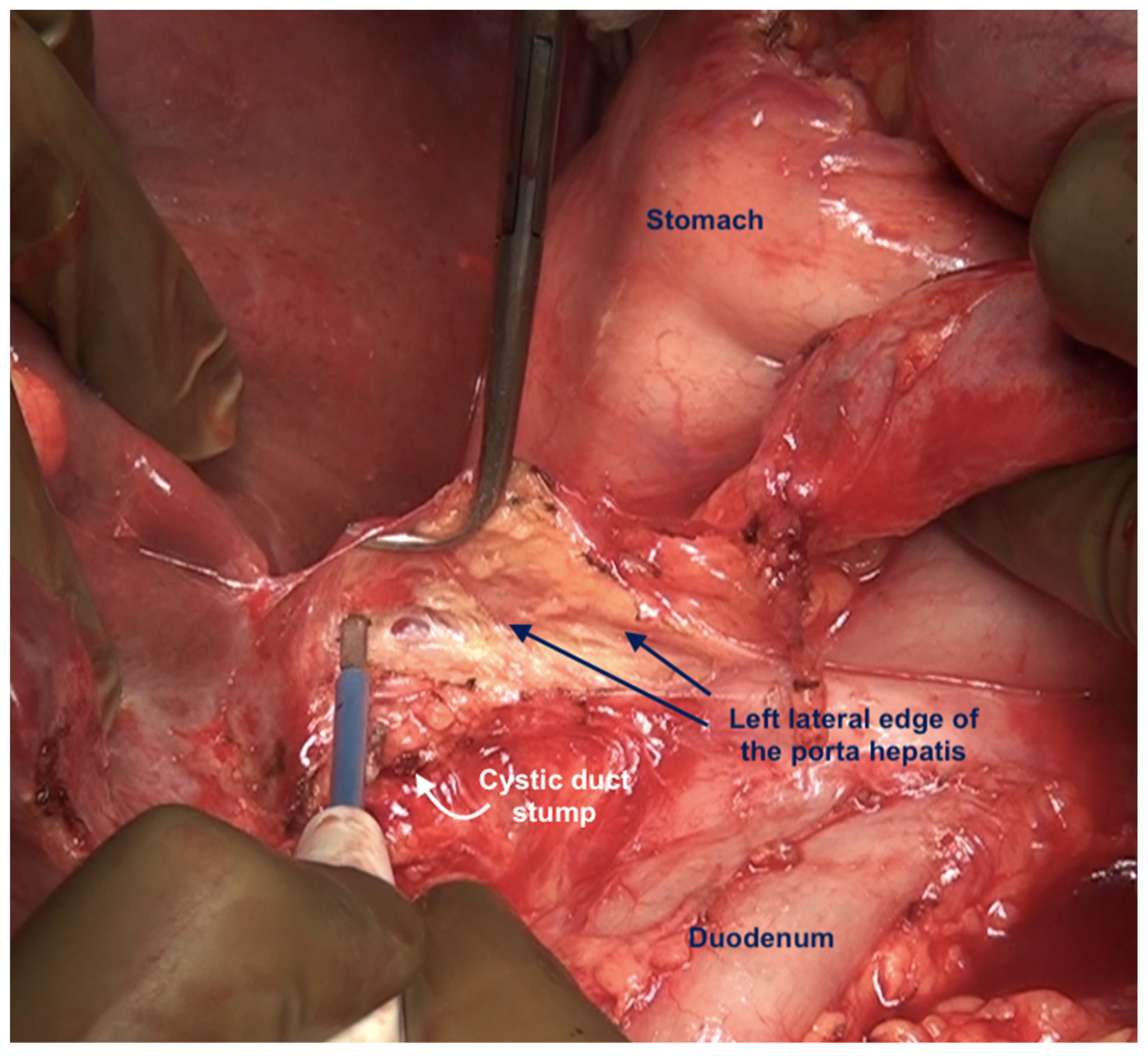
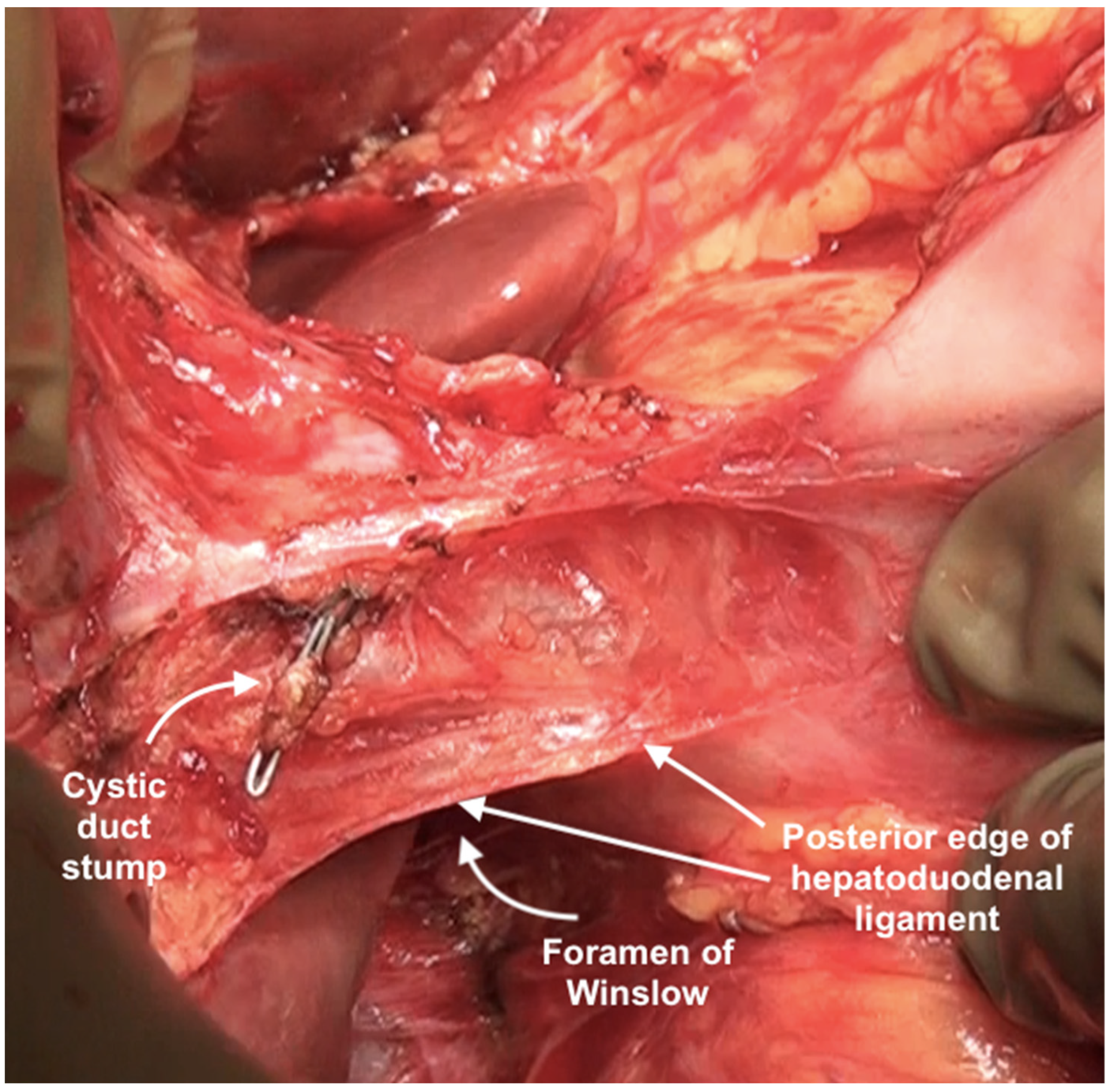
The posterior layer of the hepatoduodenal ligament covering the portal structures and the foramen of Winslow are two other sites of disease in the upper abdomen, especially in patients with extensive disease (Figure 24). The posterior layer of the hepatoduodenal ligament forms the anterior boundary of the foramen of Winslow, which is also bounded by the peritoneum covering the IVC posteriorly, the junction of the right and left caudate lobes of the liver superiorly, and by the peritoneum covering the first portion of the duodenum inferiorly [67]. Tumor nodules in this region may not be visible unless looked for. Whereas tumor on the IVC can be easily visualized, the posterior part of the porta should be palpated to determine the presence of disease. Sugarbaker et al. have also described this site as a potential for incomplete cytoreduction in patients with mucinous appendiceal neoplasms [69].
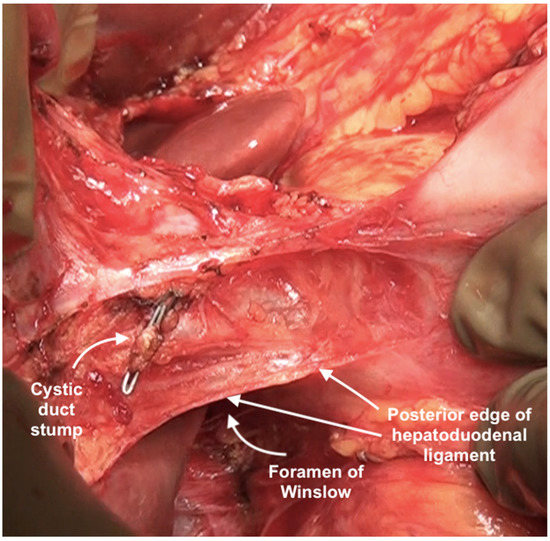
Figure 24.
Foramen of Winslow and the posterior layer of the hepatoduodenal ligament after removal of the anterior layer and the gall bladder.
When there are few nodules, the peritoneum can be removed from both the anterior surface of the IVC and the posterior surface of the porta using blunt or sharp dissection or a combination of both [55]. The peritoneum is divided at its attachment to the liver, safeguarding the portal structures. When there is a more bulky disease, Kocher’s maneuver (mobilization of the duodenum and the pancreas) can be used to provide better exposure to the Foramen of Winslow, as described by Sugarbaker et al. [67]. This procedure opens up the retroperitoneal space with the risk of retroperitoneal tumor implantation. Thus, it should be undertaken only when a complete cytoreduction has been obtained in all other regions. Rare complications that could arise are acute pancreatitis due to rotation of the pancreatic head and capsular injury [67].
6.7. Superior Recess of the Lesser Sac
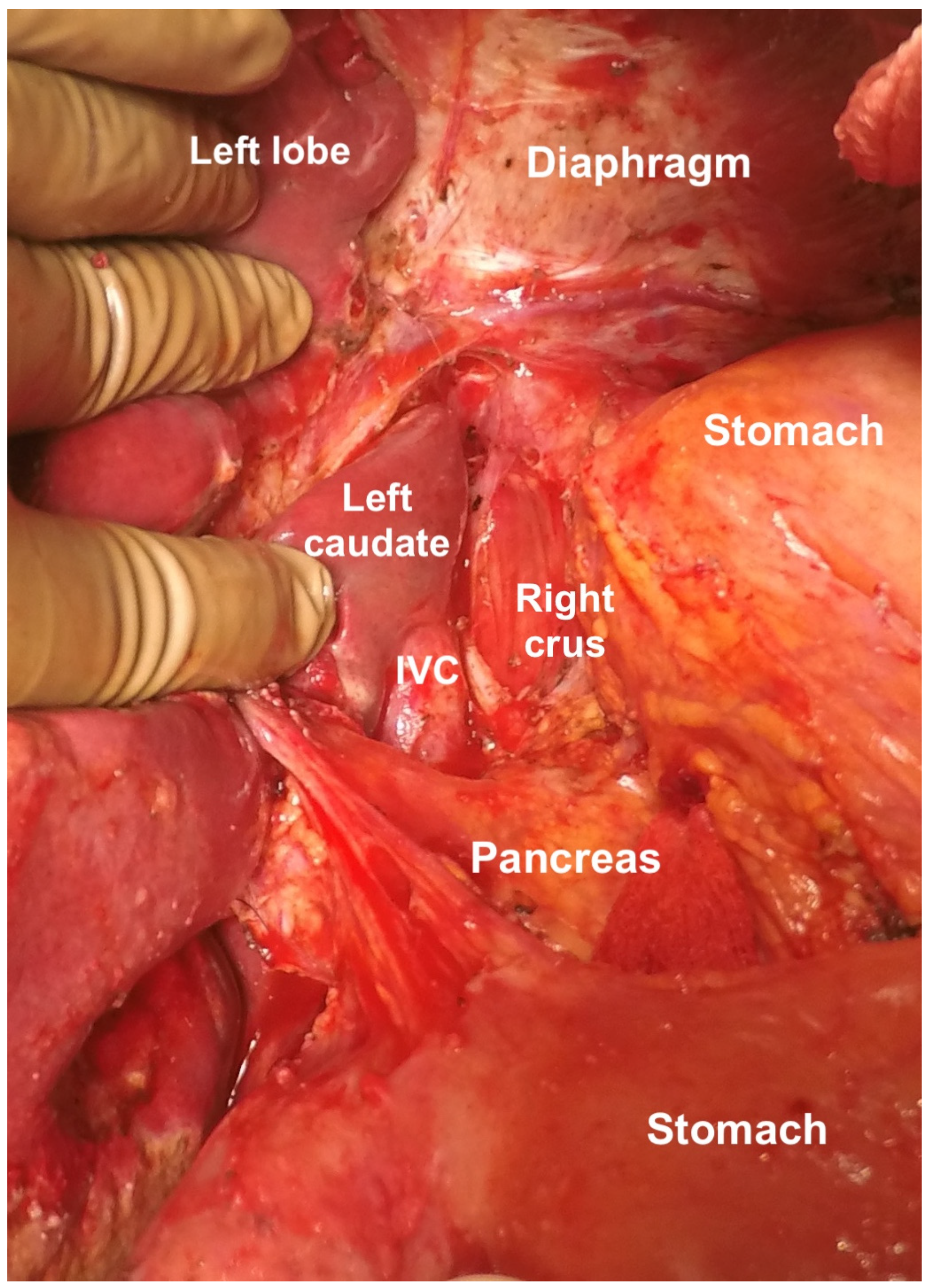
The caudate lobe is retracted to expose the floor of the superior recess of the lesser sac. Tumor deposits on the posterior surface of the caudate lobe and the anterior surface of the inferior vena cava are removed (Figure 25) [44]. Medially, the crus of the diaphragm is cleared off the tumor [44]. A combination of blunt and bipolar dissection is used for the same. Care is taken to protect the right phrenic artery [55]. Then, the peritoneum at the lesser curvature of the stomach is stripped from the vascular arcade. The dissection proceeds with caution to avoid traumatizing the right and left gastric arteries, the left gastric vein, and the anterior vagal nerve [55].
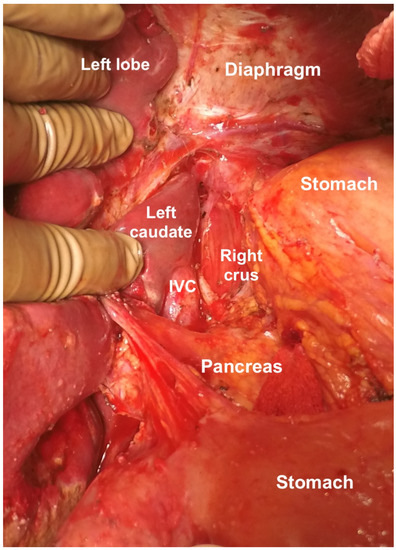
Figure 25.
Clearance of the superior recess of the lesser sac. The caudate lobe has been retracted to the right to expose the IVC.
6.8. Inferior Recess of the Lesser Sac
This is removed in continuity with the greater omentum. The anterior layer of the peritoneum covering the transverse mesocolon, the peritoneum overlying the pancreas, and the fold connecting the superior border of the pancreas to the lesser curve are resected. The peritoneum anterior to the pancreas may be left behind without tumor deposits [44]. However, the posterior layer of the lesser sac between the stomach and pancreas should be exposed to rule out tumor involvement and resected if it has tumor deposits [44].
6.9. Umbilical Round Ligament
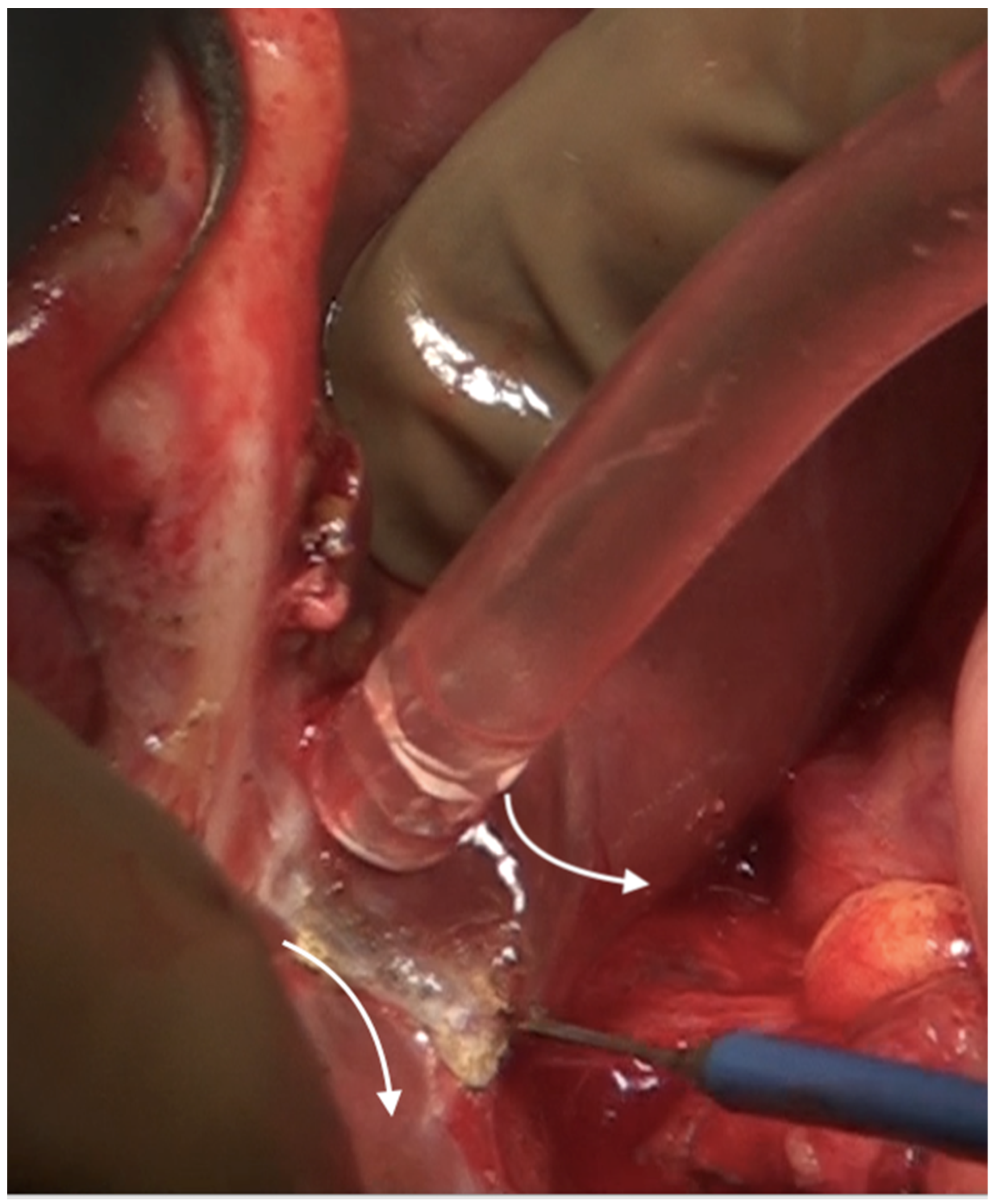
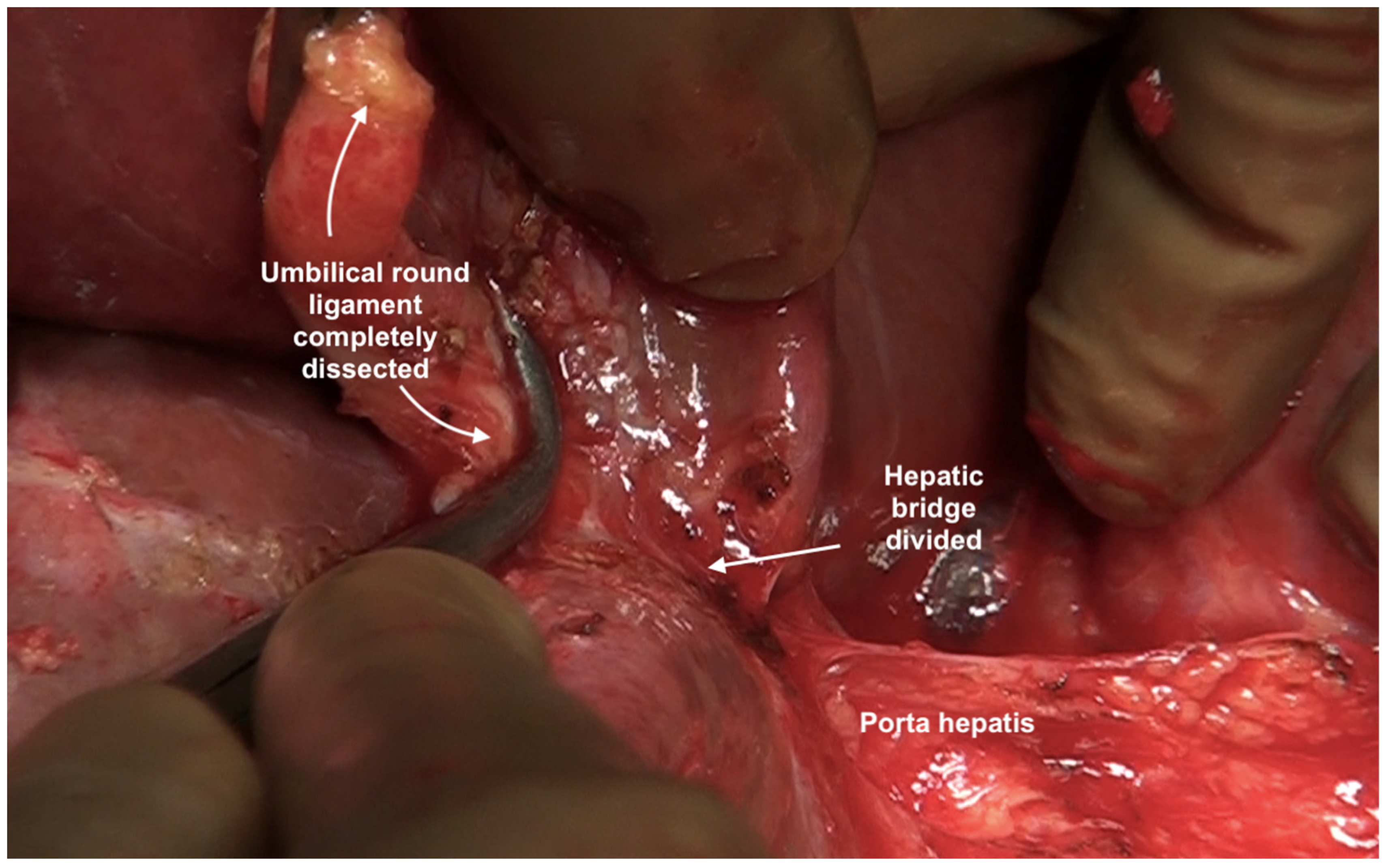
The umbilical ligament needs to be removed completely. It may be surrounded by a variable amount of hepatic parenchyma in the umbilical fissure. Sugarbaker has referred to this bridge as “pont hepatique”; in some cases, the liver tissue is absent, i.e., an absent or open hepatic bridge [70]. To clear deposits in the peritoneum surrounding the umbilical ligament, the hepatic bridge must be divided to expose the full length of the ligament (Figure 26). The left hepatic artery or one of its branches may be at risk of injury during the stripping of the peritoneum in the umbilical fissure, and special care needs to be taken to avoid this (Figure 27) [70].
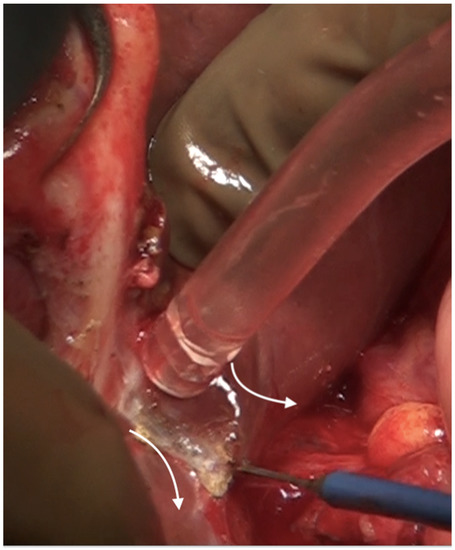
Figure 26.
The hepatic bridge has been divided to expose the entire length of the umbilical round ligament.
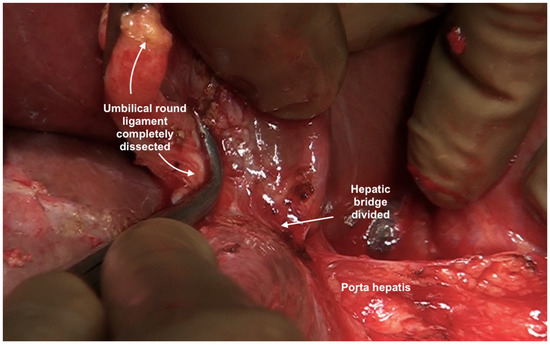
Figure 27.
The umbilical round ligament completely dissected. Posterior and inferior dissection should be performed with caution to avoid injury to the portal structures.
6.10. The Peritoneum Overlying the Pancreas
The gastrocolic ligament is excised to expose the anterior surface of the pancreas. If tumor deposits are found, the peritoneum is resected using blunt and sharp dissection. Dissection usually begins at the inferior border of the pancreas till the superior border where the splenic vessels lie [70]. Injury to the splenic vessels and pancreas should be avoided, and a drain should be left postoperatively after this procedure [55]. The space between the anterior part of the pancreatic head and the posterior part of the antro-pyloric region of the stomach should be exposed to remove potential hidden tumor deposits.
6.11. Lymphadenectomy
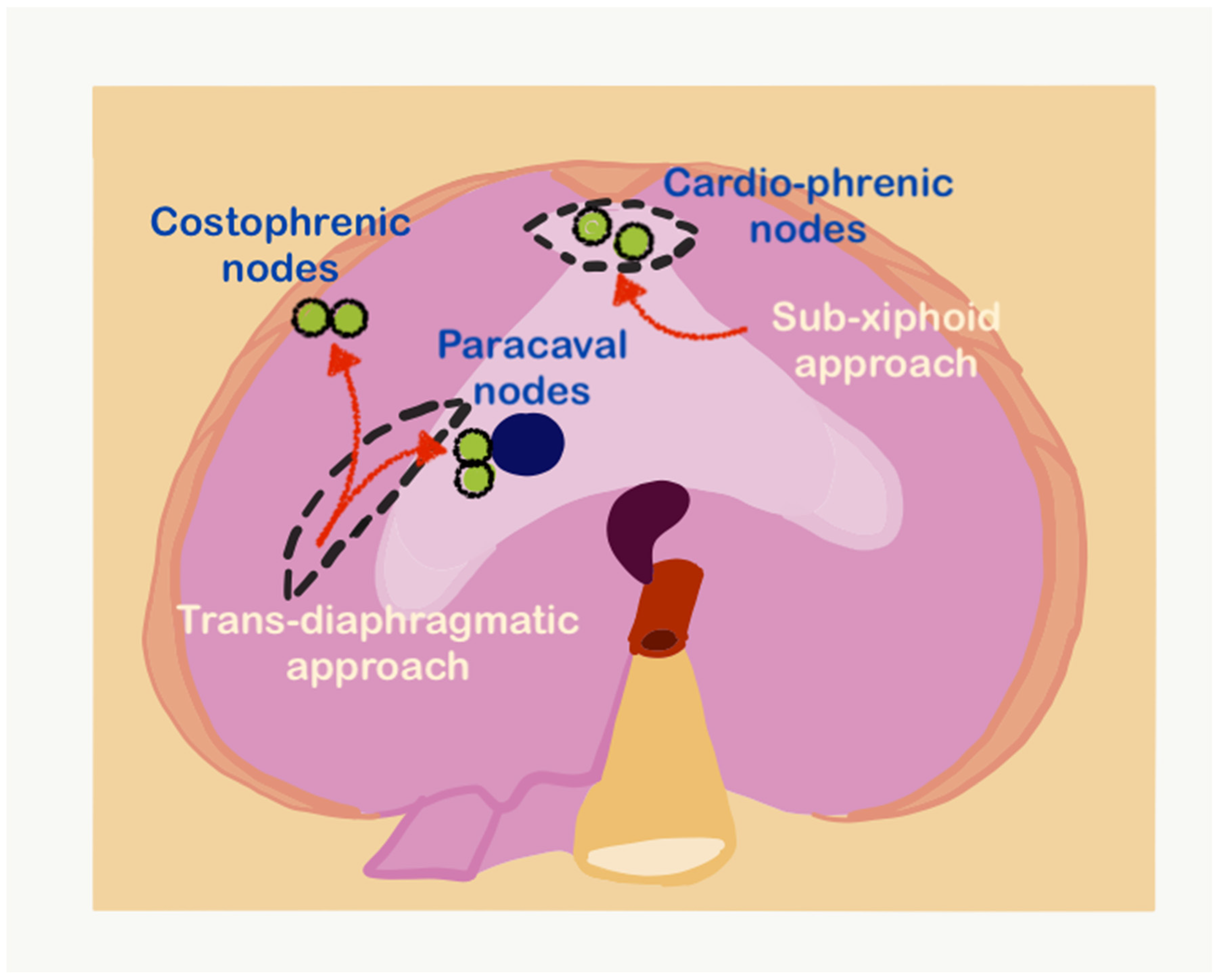
Upper-abdominal lymphadenectomy is performed if the lymph nodes are enlarged or suspicious on imaging or during surgery. The suprarenal para-aortic lymph nodes, left gastric nodes, and periportal and common bile duct nodes are dissected for other primary tumors. The technique of resection of celiac axis nodes for ovarian cancer has been described for ovarian cancer [71]. Periportal nodes may not be visible on preoperative imaging. If there is disease on the porta hepatis, the peritoneum is stripped and the region inspected for presence of enlarged nodes. The node along the lower half of the common bile duct on the right side, along the portal vein on the left side and the hepatic artery are are the nodes that are often enlarged. When there is disease on the porta, we resect these nodes if enlarged. If there is no disease, we leave them behind even if they are enlarged but no not seem grossly involved. Only the enlarged nodes are removed and all the fatty tissue is not removed. In case of future recurrence, this could prevent the disease from causing biliary obstruction. The variations in the anatomy of the hepatic artery such as a replaced right hepatic artery and of the biliary tree should be kept in mind. Greater and lesser omental nodes are usually found during pathological evaluation if these structures have been removed. Though the prognostic significance of these nodes is not known, documentation is essential for future research. The nodes that require particular mention as the supradiaphragmatic nodes. Cardiophrenic angle lymph nodes close to the xiphoid process can be removed by the sub-xiphoid approach (Figure 28) [55]. This approach utilizes the upper portion of the laparotomy incision and leaves the diaphragm intact, entering the pleural space anterior to the diaphragm and pericardium. The dissection plane is just under the xiphoid and allows direct access to the cardiophrenic lymph nodes. The other lymph nodes such as the costophrenic angle nodes and paracaval lymph nodes can be resected transdiaphragmatically or through video-assisted thoracoscopic surgery (VATS) [72].
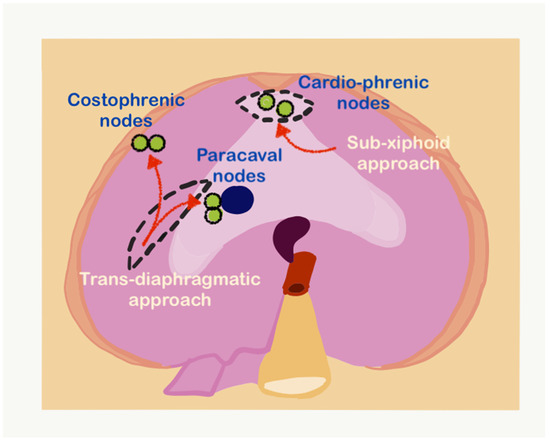
Figure 28.
The sub-xiphoid and transdiaphragmatic approaches for resecting supradiaphragmatic lymph nodes (adapted from [71] with permission).
In the transdiaphragmatic approach, the right diaphragm is incised lateral to the IVC, and the nodes in the costophrenic angle are identified and resected (Figure 28) [73]. The same approach can be used to resect the paracaval nodes lying in the posterior mediastinum. VATS has the advantage of allowing inspection of the pleural cavity as well for the presence of disease [72,74].
6.12. Topical Hemostatic Agents
Topical hemostatic agents such as Surgicel ®, TachoSil ®, Abgel ® to name a few can be used to control bleeding from the liver surface following hepatic resection or glissonectomy [75]. These agents can also be used to control bleeding from any rents in the spleen caused during dissection if preservation of the spleen is planned. For other regions these agents could be used if the need arises, for, e.g., in cases when anticoagulants cannot be stopped during surgery to reduce the transfusion rate [76]. However, it must be pointed out that meticulous coagulation of small bleeders using a bipolar cautery is an effective way of minimizing blood loss. This should be done simultaneously as the dissection proceeds. When there is uncontrolled bleeding, it is best to pack the area with surgical mops which could be soaked in topical hemostatic agents and reevaluate the situation after stabilizing the patient hemodynamically. Anesthesia management is crucial regarding the choice of fluids and blood components that are used during these procedures [77]. The recently published guidelines for perioperative care and ERAS for patients undergoing CRS have outlined the current standards for anesthesiology management and could be referred to [78].
6.13. Role of Minimally Invasive Surgery
Open surgery is considered to be the gold standard for CRS for advanced ovarian cancer. There are several reports suggesting that minimally invasive CRS could be performed for selected patients undergoing interval CRS resulting in similar oncological outcomes and a shorter hospital stay [79].
We do not advocate minimally invasive surgery (MIS) for upper-abdominal CRS in ovarian cancer outside clinical trials for the following reasons
- Even when there is limited disease, it is always accompanied by disease at other sites making the procedure technically challenging and time consuming if the peritonectomies are performed as described in the manuscript. We support our stand with the patient selection criteria for the LANCE trial in which patients with diaphragm, liver and splenic involvement are excluded from the trial [80].
- Though some papers show that for limited disease, MIS produces a survival compared to open surgery, the comparison is made between patients with limited disease undergoing MIS and the whole cohort of ovarian cancer patients undergoing open surgery which in our opinion is not a valid comparison [81]. Moreover, a study from the PSOGI registry showed a higher incidence of recurrence following MIS CRS for high-grade malignancies such as colorectal and ovarian cancer [82].
- For recurrent ovarian cancer, there could be two scenarios-either the diaphragm was previously stripped and there is a recurrence in the same region; in this scenario minimally invasive surgery is dangerous as there could be dense adhesions and without the tactile sensation that is available in open surgery, adhesionolysis should not be performed. In the second scenario, if the diaphragm was not stripped, we would perform a complete stripping in that region as described above.
Though there are many studies showing the safety and feasibility of diaphragm surgery in both the first-line and recurrent setting, only limited resection of the peritoneum or diaphragm is performed in all these studies [79,83,84,85,86,87].
- 4.
- There is a risk of missing small tumor nodules and some regions such as region 2 which is described above cannot be explored properly on MIS.
7. Morbidity of Upper-Abdominal Cytoreduction and Its Prevention
Upper-abdominal surgery, especially diaphragmatic surgery, carries its specific morbidity. In the surgical complexity score by Aletti et al., procedures such as diaphragmatic stripping, splenectomy, and liver resections are allotted a higher score [88]. The long incision coupled with the stripping of the diaphragm can compromise the respiratory movements in the postoperative period, leading to basal atelectasis and pneumonia. Pleural effusions are common, and most surgeons prefer to put in a chest tube if the diaphragm has been stripped [89,90]. There are several reports in which show no added benefit of placing chest tubes routinely [91]. However, these reports include most patients with focal stripping or resection and not complete stripping with or without full-thickness resection as we have described above. Not placing chest tubes could have adverse consequences in some patients [92]. Postoperative biliary fistulas could result from biliary injury or rarely following glissonectomy or segmental liver resection. Postoperative pancreatic fistulas could follow pancreatic resection or anterior capsular resection [93]. CRS-related complications such as fluid-electrolyte disturbances are more likely in patients undergoing upper-abdominal cytoreduction.
Prehabilitation, that is, optimizing the functional capacity to enable the patient to withstand surgery, is essential for patients undergoing upper-abdominal cytoreduction [94]. Specific measures include smoking cessation, physical exercise, breathing exercises, incentive spirometry, and nutritional status optimization. Enhanced recovery after surgery (ERAS) guidelines have been laid down by different societies and working groups of clinicians that include these prehabilitation measures and other perioperative practices [78,95,96]. Several studies have validated ERAS protocols and demonstrated the benefit of some of the practices such as prehabilitation [97,98]. However, validation of other practices such as avoiding drains and chest tubes and early removal of nasogastric tubes for patients who have extensive upper-abdominal surgery including stripping of the entire diaphragm with or without resection remains to be demonstrated. We recommend a more conservative approach for these practices following extensive upper-abdominal surgery.
The reported major morbidity in patients undergoing upper-abdominal procedures ranges from 8 to 30% [99,100]. Harter et al. reported a 30-day mortality of 2.3% in 396 patients undergoing extensive upper-abdominal cytoreduction. The major complications were essentially a result of bowel resections and anastomoses [13]. The complication rate was 12% in 115 patients treated at the Memorial Sloan Kettering center during their initial experience with extensive upper-abdominal cytoreduction [4]. In another study of 144 patients with advanced and recurrent EOC, all of whom had upper-abdominal cytoreduction, significant complications were seen in 31.9%; and 3.4% of patients died within 90 days of surgery [101]. Respiratory complications seen in 7.6% were the only complications attributed to upper-abdominal cytoreduction. The authors used non-invasive ventilation in the postoperative period and the other measures described above to reduce the incidence of respiratory complications. In another study of 34 patients, the authors reported complications in 56% and postoperative mortality in 6% of patients [102]. This was, however, their initial experience, and the complications were graded according to the old Clavien–Dindo classification. Though the exact incidence has not been mentioned, pulmonary complications were the most common complications seen in this study [102].
8. Conclusions
Upper-abdominal cytoreduction leads to a higher rate of complete gross resection and has an OS benefit in advanced EOC. Its benefit in patients with the recurrent disease has not been established. The role of upper-abdominal lymphadenectomy needs further study to determine its use in patients undergoing CRS. More than half the patients undergoing CRS for advanced EOC require upper-abdominal cytoreduction, and surgeons treating ovarian cancer should be well versed in these procedures or seek the help of surgical teams proficient in dealing with the upper-abdominal disease to obtain a complete cytoreduction. A thorough exploration of the upper abdomen should be performed in all patients with disease beyond the pelvis. The spaces and ligaments around the liver should be examined thoroughly to avoid missing disease in any region. The morbidity of upper-abdominal cytoreduction is acceptable. Prehabilitation and optimal perioperative management can reduce the incidence and severity of complications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/std12010001/s1, Figure S1. Widespread cancer distribution which is seen in epithelial ovarian cancer. Figure S2. The supradiaphragmatic nodes that are addressed during upper-abdominal cytoreduction: cardiophrenic angle nodes (red circle), paracaval nodes (yellow circle) and costophrenic nodes (blue circle).
Author Contributions
Conceptualization, A.B.; methodology, all authors; software, A.B. and G.B.; validation, all authors; formal analysis, A.B.; investigation, all authors; resources, all authors; data curation, A.B. and G.B.; visualization, G.B. and A.B.; supervision, O.G. and A.B.; project administration, A.B.; writing (original draft preparation), G.B. and A.B.; prepared the images, S.M.; Writing (review and editing), all authors. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no funding for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Doubeni, C.A.; Doubeni, A.R.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician 2016, 93, 937–944. [Google Scholar] [PubMed]
- Healy, J.C.; Reznek, R.H. The peritoneum, mesenteries and omenta: Normal anatomy and pathological processes. Eur. Radiol. 1998, 8, 886–900. [Google Scholar] [CrossRef] [PubMed]
- Pathiraja, P.N.; Garruto-Campanile, R.; Tozzi, R. Diaphragmatic peritonectomy versus full thickness diaphragmatic resection and pleurectomy during cytoreduction in patients with ovarian cancer. Int. J. Surg. Oncol. 2013, 2013, 876150. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.L.; Abu-Rustum, N.R.; Sonoda, Y.; Levine, D.A.; Poynor, E.A.; Aghajanian, C.; Jarnagin, W.R.; Chi, D.S.; Barakat, R.R.; DeMatteo, R.P.; et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol. Oncol. 2006, 103, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Eng, O.S.; Raoof, M.; Blakely, A.M.; Yu, X.; Lee, S.J.; Han, E.S.; Wakabayashi, M.T.; Yuh, B.; Lee, B.; Dellinger, T.H. A collaborative surgical approach to upper and lower abdominal cytoreductive surgery in ovarian cancer. J. Surg. Oncol. 2018, 118, 121–126. [Google Scholar] [CrossRef]
- Meyers, M.A. Distribution of intra-abdominal malignant seeding: Dependency on dynamics of flow of ascitic fluid. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1973, 119, 198–206. [Google Scholar] [CrossRef]
- Pannu, H.K.; Bristow, R.E.; Montz, F.J.; Fishman, E.K. Multidetector CT of peritoneal carcinomatosis from ovarian cancer. Radiographics 2003, 23, 687–701. [Google Scholar] [CrossRef]
- Martín-Cameán, M.; Delgado-Sánchez, E.; Piñera, A.; Diestro, M.D.; De Santiago, J.; Zapardiel, I. The role of surgery in advanced epithelial ovarian cancer. Ecancermedicalscience 2016, 10, 666. [Google Scholar] [CrossRef]
- Bianchi, F.; Camargo, A.; Habich, D.; Castano, R. The fundamental role of the exploration of the upper abdomen in ovarian cancer surgery. Obstet. Gynecol. Int. J. 2021, 12, 337–342. [Google Scholar] [CrossRef]
- Bhatt, A.; Parikh, L.; Mishra, S.; Glehen, O. Epithelial Serous Ovarian Cancer: Patterns of Peritoneal Dissemination and their Clinical Implications. In Pathology of Peritoneal Metastases; Glehen, O., Bhatt, A., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Chi, D.S.; Eisenhauer, E.L.; Zivanovic, O.; Sonoda, Y.; Abu-Rustum, N.R.; Levine, D.A.; Guile, M.W.; Bristow, R.E.; Aghajanian, C.; Barakat, R.R. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol. Oncol. 2009, 114, 26–31. [Google Scholar] [CrossRef]
- Chang, S.J.; Bristow, R.E.; Chi, D.S.; Cilby, W.A. Role of aggressive surgical cytoreduction in advanced ovarian cancer. J. Gynecol. Oncol. 2015, 26, 336–342. [Google Scholar] [CrossRef]
- Harter, P.; Muallem, Z.M.; Buhrmann, C.; Lorenz, D.; Kaub, C.; Hils, R.; Kommoss, S.; Heitz, F.; Traut, A.; du Bois, A. Impact of a structured quality management program on surgical outcome in primary advanced ovarian cancer. Gynecol. Oncol. 2011, 121, 615–619. [Google Scholar] [CrossRef]
- Ren, Y.; Jiang, R.; Yin, S.; You, C.; Liu, D.; Cheng, X.; Tang, J.; Zang, R. Radical surgery versus standard surgery for primary cytoreduction of bulky stage IIIC and IV ovarian cancer: An observational study. BMC Cancer 2015, 15, 583. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Gostout, B.S.; Jones, M.B.; Stanhope, C.R.; Wilson, T.O.; Podratz, K.C.; Cilby, W.A. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006, 107, 77–85. [Google Scholar] [CrossRef]
- Colombo, P.E.; Mourregot, A.; Fabbro, M.; Gutowski, M.; Saint-Aubert, B.; Quenet, F.; Gourgou, S.; Rouanet, P. Aggressive surgical strategies in advanced ovarian cancer: A monocentric study of 203 stage IIIC and IV patients. Eur. J. Surg. Oncol. 2009, 35, 135–143. [Google Scholar] [CrossRef]
- Chang, S.J.; Bristow, R.E.; Ryu, H.S. Impact of complete cytoreduction leaving no gross residual disease associated with radical cytoreductive surgical procedures on survival in advanced ovarian cancer. Ann. Surg. Oncol. 2012, 19, 4059–4067. [Google Scholar] [CrossRef]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.W.; Park, S.Y.; Kim, B.G.; Nam, J.H.; et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef]
- Shi, T.; Zhu, J.; Feng, Y.; Tu, D.; Zhang, Y.; Zhang, P.; Jia, H.; Huang, X.; Cai, Y.; Yin, S.; et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 439–449. [Google Scholar] [CrossRef]
- Zang, R.Y.; Harter, P.; Chi, D.S.; Sehouli, J.; Jiang, R.; Tropé, C.G.; Ayhan, A.; Cormio, G.; Xing, Y.; Wollschlaeger, K.M.; et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br. J. Cancer 2011, 105, 890–896. [Google Scholar] [CrossRef]
- Classe, J.M.; Glehen, O.; Decullier, E.; Bereder, J.M.; Msika, S.; Lorimier, G.; Abboud, K.; Meeus, P.; Ferron, G.; Quenet, F.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for First Relapse of Ovarian Cancer. Anticancer Res. 2015, 35, 4997–5005. [Google Scholar] [PubMed]
- Bhatt, A.; Bakrin, N.; Gertych, W.; Kammar, P.; Parikh, L.; Sheth, S.; Shaikh, S.; Devouassoux-Shisheboran, M.; Glehen, O. Extent and distribution of peritoneal disease in patients undergoing cytoreductive surgery for first platinum sensitive recurrence in ovarian cancer and its potential therapeutic implications. Eur. J. Surg. Oncol. 2020, 46, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Bacalbasa, N.; Dima, S.; Balescu, I.; David, L.; Brasoveanu, V.; Popescu, I. Results of Primary Cytoreductive Surgery in Advanced-stage Epithelial Ovarian Cancer: A Single-center Experience. Anticancer Res. 2015, 35, 4099–4104. [Google Scholar] [PubMed]
- Berek, J.S.; Kehoe, S.T.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet. 2018, 143, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Kawamura, T.; Bandou, E.; Tsukiyama, G.; Endou, Y.; Miura, M. The natural history of free cancer cells in the peritoneal cavity. In Advances in Peritoneal Surface Oncology; Gonzalez-Moreno, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 11–23. [Google Scholar]
- Kusamura, S.; Baratti, D.; Zaffaroni, N.; Villa, R.; Laterza, B.; Balestra, M.R.; Deraco, M. Pathophysiology and biology of peritoneal carcinomatosis. World J. Gastrointest. Oncol. 2010, 2, 12–18. [Google Scholar] [CrossRef]
- Sehouli, J.; Senyuva, F.; Fotopoulou, C.; Neumann, U.; Denkert, C.; Werner, L.; Gülten, O.O. Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. J. Surg. Oncol. 2009, 99, 424–427. [Google Scholar] [CrossRef]
- Bhatt, A.; Kammar, P.; Rousset, P.; Sinukumar, S.; Mehta, S.; Parkih, L.; Goswami, G.; Sheikh, S.; Kepenekian, V.; Bakrin, N.; et al. Greater-omentum lesion-score (GOLS) as a predictor of residual disease in different regions of the peritoneal cavity in patients undergoing interval cytoreductive surgery for advanced ovarian cancer and its potential clinical utility. Eur. J. Surg. Oncol. 2021, 47, 2925–2932. [Google Scholar] [CrossRef]
- Bhatt, A.; Yonemura, Y.; Mehta, S.; Benzerdjeb, N.; Kammar, P.; Parikh, L.; Shah, M.Y.; Shaikh, S.; Prabhu, A.; Mishra, S.; et al. Target region resection in patients undergoing cytoreductive surgery for peritoneal metastases-is it necessary in absence of visible disease? Eur. J. Surg. Oncol. 2020, 46, 582–589. [Google Scholar] [CrossRef]
- Bhatt, A.; Yonemura, Y.; Benzerdjeb, N.; Mehta, S.; Mishra, S.; Parikh, L.; Kammar, P.; Shah, M.Y.; Prabhu, A.; Shaikh, S.; et al. Pathological assessment of cytoreductive surgery specimens and its unexplored prognostic potential—A prospective multi-centric study. Eur. J. Surg. Oncol. 2019, 45, 2398. [Google Scholar] [CrossRef]
- Silverman, P.M. The subperitoneal space: Mechanisms of tumour spread in the peritoneal cavity, mesentery, and omentum. Cancer Imaging. 2003, 4, 25–29. [Google Scholar] [CrossRef][Green Version]
- Fritz, D.L.; Waag, D.M. Transdiaphragmatic lymphatic transport of intraperitoneally administered marker in hamsters. Lab. Anim. Sci. 1999, 49, 522–529. [Google Scholar] [PubMed]
- Abernethy, N.J.; Chin, W.; Hay, J.B.; Rodela, H.; Oreopoulos, D.; Johnston, M.G. Lymphatic drainage of the peritoneal cavity in sheep. Am. J. Physiol. 1991, 260, F353–F358. [Google Scholar] [CrossRef] [PubMed]
- Parungo, C.P.; Soybel, D.I.; Colson, Y.L.; Kim, S.W.; Ohnishi, S.; DeGrand, A.M.; Laurence, R.G.; Soltesz, E.G.; Chen, F.Y.; Cohn, L.H.; et al. Lymphatic drainage of the peritoneal space: A pattern dependent on bowel lymphatics. Ann. Surg. Oncol. 2007, 14, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Luna-Abanto, J.; García Ruiz, L.; Laura Martinez, J.; Álvarez Larraondo, M.; Villoslada Terrones, V. Liver Resection as Part of Cytoreductive Surgery for Ovarian Cancer. J. Gynecol. Surg. 2020, 36, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.J.; Long, K.C.; Feffer, J.B.; Leitao, M.M., Jr.; Abu-Rustum, N.R.; Barakat, R.R.; Chi, D.S.; Gardner, G.J. Parenchymal splenic metastasis is an independent negative predictor of overall survival in advanced ovarian, fallopian tube, and primary peritoneal cancer. Gynecol. Oncol. 2013, 128, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Madambath, J.G.; Somani, P.; Pathak, A.; Rameshbabu, C.S.; Bansal, R.; Ramasamy, K.; Patil, A. Endoscopic ultrasound of peritoneal spaces. Endosc. Ultrasound. 2017, 6, 90–102. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In Peritoneal Carcinomatosis: Principles of Management; Sugarbaker, P.H., Ed.; Kluwer: Boston, MA, USA, 1996; pp. 359–374. [Google Scholar]
- Meyers, M.A.; Charnsangavej, C.; Oliphant, M. Meyers’ Dynamic Radiology of the Abdomen, 6th ed.; Springer: New York, NY, USA, 2011; pp. 23–34. [Google Scholar]
- Kim, S.; Kim, T.U.; Lee, J.W.; Lee, T.H.; Lee, S.H.; Jeon, T.Y.; Kim, K.H. The perihepatic space: Comprehensive anatomy and CT features of pathologic conditions. Radiographics 2007, 27, 129–143. [Google Scholar] [CrossRef]
- Villeneuve, L.; Thivolet, A.; Bakrin, N.; Mohamed, F.; Isaac, S.; Valette, P.J.; Glehen, O.; Rousset, P.; BIG-RENAPE and RENAPE Working Groups. A new internet tool to report peritoneal malignancy extent. PeRitOneal MalIgnancy Stage Evaluation (PROMISE) application. Eur. J. Surg. Oncol. 2016, 42, 877–882. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef]
- Bhatt, A.; Mehta, S. Cytoreductive Surgery for Peritoneal Metastases: Principles and Techniques. In Management of Peritoneal Metastases-Cytoreductive Surgery, HIPEC and Beyond; Bhatt, A., Ed.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Lee, I.O.; Lee, J.Y.; Kim, H.J.; Nam, E.J.; Kim, S.; Kim, S.W.; Lee, C.Y.; Kang, W.J.; Kim, Y.T. Prognostic significance of supradiaphragmatic lymph node metastasis detected by 18F-FDG PET/CT in advanced epithelial ovarian cancer. BMC Cancer 2018, 18, 1165. [Google Scholar] [CrossRef]
- Buza, N. Staging. PathologyOutlines.com website. Available online: https://www.pathologyoutlines.com/topic/ovarytumorstage.html (accessed on 24 December 2021).
- Suppiah, S. The Past, Present and Future of Diagnostic Imaging in Ovarian Cancer. In Ovarian Cancer–From Pathogenesis to Treatment, Omer Devaja and Andreas Papadopoulos; IntechOpen: London, UK, 24 October; Available online: https://www.intechopen.com/chapters/59552 (accessed on 29 September 2022). [CrossRef]
- Verri, D. Pre-operative evaluation in advanced ovarian cancer: Is ultrasound ready to replace computed tomography? Int. J. Gynecol. Cancer 2019, 29, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Goswami, G.; Kammar, P.; Mangal, R.; Shaikh, S.; Patel, M.D.; Bhatt, A. Accuracy of CT Scan in Predicting the Surgical PCI in Patients Undergoing Cytoreductive Surgery with/without HIPEC-a Prospective Single Institution Study. Indian J. Surg. Oncol. 2019, 10, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, M.A.; Khader, L.; Cirigliano, A.; Cioffi Squitieri, N.; Guerrini, S.; Forzoni, B.; Marrelli, D.; Roviello, F.; Mazzei, F.G.; Volterrani, L. Accuracy of MDCT in the preoperative definition of Peritoneal Cancer Index (PCI) in patients with advanced ovarian cancer who underwent peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Abdom. Imaging. 2013, 38, 1422–1430. [Google Scholar] [CrossRef]
- Low, R.N.; Barone, R.M.; Lucero, J. Comparison of MRI and CT for predicating the peritoneal cancer index (PCI) in patients being considered for cytoreductive surgical procedures. Ann. Surg. Oncol. 2015, 22, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Dohan, A.; Hoeffel, C.; Soyer, P.; Jannot, A.S.; Valette, P.J.; Thivolet, A.; Passot, G.; Glehen, O.; Rousset, P. Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br. J. Surg. 2017, 104, 1244–1249. [Google Scholar] [CrossRef]
- Low, R.N.; Barone, R.M.; Rousset, P. Peritoneal MRI in patients undergoing cytoreductive surgery and HIPEC: History, clinical applications, and implementation. Eur. J. Surg. Oncol. 2021, 47, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Llueca, A.; Serra, A.; Rivadulla, I.; Gomez, L.; Escrig, J.; MUAPOS working group (Multidisciplinary Unit of Abdominal Pelvic Oncology Surgery). Prediction of suboptimal cytoreductive surgery in patients with advanced ovarian cancer based on preoperative and intraoperative determination of the peritoneal carcinomatosis index. World J. Surg. Oncol. 2018, 16, 37. [Google Scholar] [CrossRef]
- Mercier, F.; Mohamed, F.; Cazauran, J.B.; Kepenekian, V.; Vaudoyer, D.; Cotte, E.; Glehen, O.; Passot, G. An update of peritonectomy procedures used in cytoreductive surgery for peritoneal malignancy. Int. J. Hyperthermia. 2019, 36, 744–752. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Dagbert, F.; Passot, G.; Glehen, O.; Bakrin, N. Glisson capsulectomy for extensive superficial liver involvement in peritoneal carcinomatosis (with video). J. Visc. Surg. 2015, 152, 332–333. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Dissection by electrocautery with a ball tip. J. Surg. Oncol. 1994, 56, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Boerner, T.; Piso, P. Cytoreductive Surgery for Peritoneal Carcinomatosis from Gastric Cancer: Technical Details. J. Clin. Med. 2021, 10, 5263. [Google Scholar] [CrossRef] [PubMed]
- Vizzielli, G.; Conte, C.; Romano, M.; Fagotti, A.; Costantini, B.; Lodoli, C.; Gueli Alletti, S.; Gaballah, K.; Pacelli, F.; Ercoli, A.; et al. Clinical Impact of a Surgical Energy Device in Advanced Ovarian Cancer Surgery Including Bowel Resection. In Vivo 2018, 32, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Seror, J.; Bats, A.S.; Habchi, H.; Lécuru, F. Optimal surgical cytoreduction of the upper abdomen and the diaphragm for advanced ovarian cancer using PlasmaJet™ energy. Gynecol. Oncol. 2016, 140, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Fournier, I.; Christodoulou, M.; Seidler, S.; Besse, V.; Mathey, M.P.; Nef, J.; Hurni, Y. Stapled diaphragm resection: A new approach to diaphragmatic cytoreductive surgery for advanced-stage ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 279, 88–93. [Google Scholar] [CrossRef]
- Carboni, F.; Federici, O.; Zazza, S.; Sperduti, I.; Valle, M. Feasibility of diaphragmatic interventions in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: A 20-year experience. Eur. J. Surg. Oncol. 2021, 47, 143–148. [Google Scholar] [CrossRef]
- Zapardiel, I.; Peiretti, M.; Zanagnolo, V.; Biffi, R.; Bocciolone, L.; Landoni, F.; Aletti, G.; Colombo, N.; Maggioni, A. Diaphragmatic surgery during primary cytoreduction for advanced ovarian cancer: Peritoneal stripping versus diaphragmatic resection. Int. J. Gynecol. Cancer. 2011, 21, 1698–1703. [Google Scholar] [CrossRef]
- Fanfani, F.; Fagotti, A.; Gallotta, V.; Ercoli, A.; Pacelli, F.; Costantini, B.; Vizzielli, G.; Margariti, P.A.; Garganese, G.; Scambia, G. Upper abdominal surgery in advanced and recurrent ovarian cancer: Role of diaphragmatic surgery. Gynecol. Oncol. 2010, 116, 497–501. [Google Scholar] [CrossRef]
- Passot, G.; Kim, B.J.; Vaudoyer, D.; Kepenekian, V.; Bonnefoy, I.; Bakrin, N.; Cotte, E.; Glehen, O. Digital glissonectomy: A safe perihepatic peritonectomy. Ann. Surg. Oncol. 2016, 23, 3978–3985. [Google Scholar] [CrossRef]
- Han, S.S.; Sugarbaker, P.H. Kocher maneuver to facilitate cytoreduction within the foramen of Winslow. J. Surg. Oncol. 2017, 115, 788–790. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Cytoreductive Surgery and Perioperative Chemotherapy: Textbook and Video Atlas; Cine-Med Publishing: Woodbury, CT, USA, 2013. [Google Scholar]
- Yan, T.D.; Bijelic, L.; Sugarbaker, P.H. Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann. Surg. Oncol. 2007, 14, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Pont hepatique (hepatic bridge), an important anatomic structure in cytoreductive surgery. J. Surg. Oncol. 2010, 101, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Ferrandina, G.; Vizzielli, G.; Conte, C.; Lucidi, A.; Costantini, B.; De Rose, A.M.; Di Giorgio, A.; Zannoni, G.F.; Fagotti, A.; et al. Hepatoceliac Lymph Node Involvement in Advanced Ovarian Cancer Patients: Prognostic Role and Clinical Considerations. Ann. Surg. Oncol. 2017, 24, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, C.; Angeles, M.A.; Leray, H.; Tanguy Le Gac, Y.; Ferron, G.; Martinez, A. Transdiaphragmatic and transxiphoid cardiophrenic lymph node resection step-by-step in advanced ovarian cancer. Int. J. Gynecol. Cancer. 2020, 30, 1646–1647. [Google Scholar] [CrossRef] [PubMed]
- Cowan, R.A.; Tseng, J.; Murthy, V.; Srivastava, R.; Long Roche, K.C.; Zivanovic, O.; Gardner, G.J.; Chi, D.S.; Park, B.J.; Sonoda, Y. Feasibility, safety and clinical outcomes of cardiophrenic lymph node resection in advanced ovarian cancer. Gynecol. Oncol. 2017, 147, 262–266. [Google Scholar] [CrossRef]
- Lim, M.C.; Lee, H.S.; Jung, D.C.; Choi, J.Y.; Seo, S.S.; Park, S.Y. Pathological diagnosis and cytoreduction of cardiophrenic lymph node and pleural metastasis in ovarian cancer patients using video-assisted thoracic surgery. Ann. Surg. Oncol. 2009, 16, 1990–1996. [Google Scholar] [CrossRef]
- Emilia, M.; Luca, S.; Francesca, B.; Luca, B.; Paolo, S.; Giuseppe, F.; Gianbattista, B.; Carmela, M.; Luigi, M.; Mauro, L. Topical hemostatic agents in surgical practice. Transfus. Apher. Sci. 2011, 45, 305–311. [Google Scholar] [CrossRef]
- Watrowski, R.; Jäger, C.; Forster, J. Improvement of Perioperative Outcomes in Major Gynecological and Gynecologic-Oncological Surgery with Hemostatic Gelatin-Thrombin Matrix. In Vivo 2017, 31, 251–258. [Google Scholar] [CrossRef][Green Version]
- Sargant, N.; Roy, A.; Simpson, S.; Chandrakumaran, K.; Alves, S.; Coakes, J.; Bell, J.; Knight, J.; Wilson, P.; Mohamed, F.; et al. A protocol for management of blood loss in surgical treatment of peritoneal malignancy by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Transfus. Med. 2016, 26, 118–122. [Google Scholar] [CrossRef]
- Hübner, M.; Kusamura, S.; Villeneuve, L.; Al-Niaimi, A.; Alyami, M.; Balonov, K.; Bell, J.; Bristow, R.; Guiral, D.C.; Fagotti, A.; et al. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced recovery after surgery (ERAS®) Society Recommendations—Part I: Preoperative and intraoperative management. Eur. J. Surg. Oncol. 2020, 46, 2292–2310. [Google Scholar] [CrossRef]
- Pereira, A.; Magrina, J.F.; Magtibay, P.M.; Neto, J.S.; Siufi, D.F.S.; Chang, Y.H.; Perez-Medina, T. Does MIS Play a Role in the Treatment of Advanced Ovarian Cancer? Cancers 2022, 14, 3579. [Google Scholar] [CrossRef] [PubMed]
- Nitecki, R.; Rauh-Hain, J.A.; Melamed, A.; Scambia, G.; Pareja, R.; Coleman, R.L.; Ramirez, P.T.; Fagotti, A. Laparoscopic cytoreduction After Neoadjuvant ChEmotherapy (LANCE). Int. J. Gynecol. Cancer 2020, 30, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Gueli Alletti, S.; Corrado, G.; Cola, E.; Vizza, E.; Vieira, M.; Andrade, C.E.; Tsunoda, A.; Favero, G.; Zapardiel, I.; et al. The International Mission study: Minimally invasive surgery in ovarian neoplasms after neoadjuvant chemotherapy. Int. J. Gynecol. Cancer 2019, 29, 5–9. [Google Scholar] [CrossRef]
- Arjona-Sanchez, A.; Esquivel, J.; Glehen, O.; Passot, G.; Turaga, K.K.; Labow, D.; Rufian-Peña, S.; Morales, R.; van der Speeten, K. A minimally invasive approach for peritonectomy procedures and hyperthermic intraperitoneal chemotherapy (HIPEC) in limited peritoneal carcinomatosis: The American Society of Peritoneal Surface Malignancies (ASPSM) multi-institution analysis. Surg. Endosc. 2019, 33, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Magrina, J.F.; Guardiola, T.C.; Magtibay PM 3rd Kosiorek, H.E.; Magtibay, P.M. Minimally Invasive Surgery for Resection of Diaphragm Metastases in Ovarian Cancer. J. Minim Invasive Gynecol. 2019, 26, 1268–1272. [Google Scholar] [CrossRef]
- Magrina, J.F.; Magtibay, P.M. Robotic Resection of Diaphragm Metastases in Ovarian Cancer: Technical Aspects. J. Minim Invasive Gynecol. 2020, 27, 1417–1422. [Google Scholar] [CrossRef]
- Uccella, S.; Franchi, M.P.; Cianci, S.; Zorzato, P.C.; Bertoli, F.; Alletti, S.G.; Ghezzi, F.; Scambia, G. Laparotomy vs. minimally invasive surgery for ovarian cancer recurrence: A systematic review. Gland Surg. 2020, 9, 1130–1139. [Google Scholar] [CrossRef]
- Gueli Alletti, S.; Capozzi, V.A.; Rosati, A.; De Blasis, I.; Cianci, S.; Vizzielli, G.; Uccella, S.; Gallotta, V.; Fanfani, F.; Fagotti, A.; et al. Laparoscopy vs. laparotomy for advanced ovarian cancer: A systematic review of the literature. Minerva Med. 2019, 110, 341–357. [Google Scholar] [CrossRef]
- Loverro, M.; Ergasti, R.; Conte, C.; Gallitelli, V.; Nachira, D.; Scaglione, G.; Fagotti, A.; Scambia, G.; Gallotta, V. Minimally Invasive Secondary Cytoreductive Surgery for Superficial Celiac and Cardio-Phrenic Isolated Nodal Recurrence of Ovarian Cancer. Ann. Surg. Oncol. 2022, 29, 2603–2604. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am. J. Obstet. Gynecol. 2007, 197, 676.e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, W.; Gao, C.; Zhou, Y.; Xie, Y. Postoperative pulmonary complications and outcomes in cytoreductive surgery for ovarian cancer: A propensity-matched analysis. BMC Anesthesiol. 2022, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.C.; Loewen, R.T.; Aletti, G.; Feitoza, S.S.; Cliby, W. Assessment of outcomes and morbidity following diaphragmatic peritonectomy for women with ovarian carcinoma. Gynecol. Oncol. 2008, 109, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Ditto, A.; Martinelli, F.; Lorusso, D.; Chiappa, V.; Donfrancesco, C.; Di Donato, V.; Indini, A.; Aletti, G.; Raspagliesi, F. Surgical Techniques for Diaphragmatic Resection During Cytoreduction in Advanced or Recurrent Ovarian Carcinoma: A Systematic Review and Meta-analysis. Int. J. Gynecol. Cancer. 2016, 26, 371–380. [Google Scholar] [CrossRef]
- Sinukumar, S.; Naik, S.; Khurjekar, D.; Munde, Y.; Bhosale, S. Pancreaticopleural Fistula After Cytoreductive Surgery and HIPEC for Pseudomyxoma Peritonei-a Rare Presentation and Rare Complication. Indian J. Surg. Oncol. 2020, 11, 174–177. [Google Scholar] [CrossRef]
- Bhatt, A.; Mehta, A.M. Management of Complications of CRS and HIPEC. In Management of Peritoneal Metastases-Cytoreductive Surgery, HIPEC and Beyond; Bhatt, A., Ed.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Cortés-Guiral, D.; Mohamed, F.; Glehen, O.; Passot, G. Prehabilitation of patients undergoing cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal malignancy. Eur. J. Surg. Oncol. 2021, 47, 60–64. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Planchamp, F.; Aytulu, T.; Chiva, L.; Cina, A.; Ergönül, Ö.; Fagotti, A.; Suria, S.; Knapp, P.; Morice, P.; et al. European Society of Gynaecological Oncology guidelines for the peri-operative management of advanced ovarian cancer patients undergoing debulking surgery. Int. J. Gynecol. Cancer. 2021, 31, 1199–1206. [Google Scholar] [CrossRef]
- Hubner, M.; Kusamura, S.; Villeneuve, L.; Al-Niaimi, A.; Alyami, M.; Balonov, K.; Bell, J.; Altman, A.; Fagotti, A.; Mack, L.; et al. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced Recovery After Surgery (ERAS) Society Recommendations—Part II: Postoperative management and special considerations. Eur. J. Surg. Oncol. 2020, 46, 2311–2323. [Google Scholar] [CrossRef]
- Dhanis, J.; Keidan, N.; Blake, D.; Rundle, S.; Strijker, D.; van Ham, M.; Pijnenborg, J.M.A.; Smits, A. Prehabilitation to Improve Outcomes of Patients with Gynaecological Cancer: A New Window of Opportunity? Cancers 2022, 14, 3448. [Google Scholar] [CrossRef]
- Diaz-Feijoo, B.; Agusti-Garcia, N.; Sebio, R.; López-Hernández, A.; Sisó, M.; Glickman, A.; Carreras-Dieguez, N.; Fuste, P.; Marina, T.; Martínez-Egea, J.; et al. Feasibility of a Multimodal Prehabilitation Programme in Patients Undergoing Cytoreductive Surgery for Advanced Ovarian Cancer: A Pilot Study. Cancers 2022, 14, 1635. [Google Scholar] [CrossRef]
- Mehta, S.S.; Bhatt, A.; Glehen, O. Cytoreductive Surgery and Peritonectomy Procedures. Indian J. Surg. Oncol. 2016, 7, 139–151. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Taskiran, C. Principles of safe and efficacious upper abdominal surgery. Gynecol. Pelvic Med. 2021, 4, 35. [Google Scholar] [CrossRef]
- Bhatt, A.; Kammar, P.; Sinukumar, S.; Goswami, G.; Mishra, B.; Bhavsar, M.; Shaikh, S.; Bhosale, S.; Aggarwal, D.; Bhorkar, N.; et al. Perioperative outcomes in patients treated with total parietal peritonectomy and multi-visceral resections with or without HIPEC at different time points in the history of advanced ovarian cancer. Eur. J. Gynaecol. Oncol. 2021, 42, 711–720. [Google Scholar]
- Llueca, J.; Herraiz, J.; Catala, C.; Serra, A.; Rivadulla, I.; Escrig, J.; MUAPOS Working Group. Effectiveness and Safety of Cytoreduction Surgery in Advanced Ovarian Cancer: Initial Experience at a University General Hospital. J. Clin. Gynecol. Obstet. 2015, 4, 251–257. Available online: https://www.jcgo.org/index.php/jcgo/article/view/345/191 (accessed on 24 December 2021). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).