Abstract
Background: The arthroscopically assisted stabilization of AC joint dislocations with a suture button system is an established procedure that is widely and successfully used in everyday practice. The main advantages of this one-step method are the minimally invasive procedure and the anatomical reconstruction of the ruptured coracoclavicular ligaments with a permanent implant. With this technical note study, for the first time, the new method of navigated suture button implantation in everyday clinical practice is described with the future goal of further reducing invasiveness and increasing precision. Materials and Methods: The surgical technique is explained using precise descriptions and illustrations, photos, X-rays, and 3D reconstructions based on clinical cases. The step-by-step system setup and patient positioning, AC joint reduction and retention, 3D scan and drill tunnel planning, stab incision and Kirschner wire navigation, and cannulated drilling and implant positioning, as well as closure and documentation are described in detail. Results: The standard coracoclavicular stabilization of AC joint dislocations with the 3D C-arm navigated suture button method is described in detail. Furthermore, the feasibility of an additive horizontal acromioclavicular suture cerclage, the implantation of an additional coracoclavicular suture button system, and the single-stage cannulated screw fixation of non-displaced fractures is demonstrated. Conclusion: The navigated suture button method aims to be simple, safe, minimally invasive, and precise. Prospective clinical studies with a long follow-up should be carried out to determine the clinical and radiological outcome in comparison with current methods.
1. Introduction
With an incidence of 3–4 cases per 100,000 inhabitants per year and a share of 4–12% of all shoulder injuries, acromioclavicular (AC) joint separations occur frequently. These are usually sports injuries in young men after a fall on the outstretched arm or a direct impact trauma to the shoulder [1]. In 1963, Tossy published the first useful and practical classification of typical vertical AC joint instability in three degrees of severity, which was supplemented by Rockwood in 1984 with three further grades [2,3].
There is a general consensus for the conservative treatment of type 1 and 2 injuries and surgical treatment of type 4 to 6 injuries. For type 3 injuries, there is just a relative indication for surgery [4], 3a (horizontally stable) more conservative, and 3b (horizontally unstable) more surgical [5,6]. A wide variety of different surgical treatment options are known [4]. However, two well-established procedures are currently used most frequently and widely: conventional open reduction and hook plate (HP) fixation and arthroscopic closed reduction and minimally invasive implantation of a suture button system (ASB) [7,8]. The HP is used more in trauma surgery [9], the ASB more in orthopedics and specialized centers [10]. Another option for surgical stabilization, which is sometimes used for acute as well as chronic dislocations, is the Weaver–Dunn procedure, in which the ruptured coracoclavicular ligaments are replaced by the transposition of the coracoacromial ligament from the anterior acromion tip to the laterally resected clavicle [11,12,13,14].
The advantages of the ASB include slightly better clinical outcomes with a higher patient satisfaction and quality of life, an earlier return to sport and return to athletic level, a lower rate of horizontal instability (depending on the study considered), no need for the secondary removal of osteosynthesis material (lower revision rate), and no direct rotator cuff damage or osteolysis/fracture of the acromion [6,15,16,17].
Risks and complications such as the loss of reduction (26.8%), implant failure (25%), and fractures of the clavicle and coracoid process (5.3%), as well as infections (3.8%) are mentioned as general disadvantages of ASB [18]. Not mentioned are movement restrictions (frozen shoulder) after shoulder arthroscopy in general and tunnel widening due to the bone contact of the implant during mobilization [18].
Therefore, the ASB has been modified several times since it was first described in 2001 [13,19]. To increase the stability of the reconstruction, the human anatomy of the coracoclavicular (CC) ligaments, consisting of the trapezoideal ligament and the conoidal ligament, was imitated by implanting two separate suture button systems (double-button technique (DSB)) [20]. However, several studies reported complications with iatrogenic fractures and, again, residual horizontal instability [21,22,23]. Later, an additive horizontal AC cerclage was recommended as a supplement to the conventional ASB technique [24,25,26]. The benefit of this horizontal suture cerclage is supported by biomechanical and clinical studies [27,28,29,30], but there is also some controversy in the literature [31]. One study even examined the double-strengthening of the conventional ASB method using an additional suture button system CC and an additive horizontal cerclage AC [32].
In order to improve the intraoperative visualization of the bony structures of the shoulder for everyday surgery, three-dimensional (3D) digital volume tomography (DVT (-is another name for cone beam computed tomography (CBCT)) imaging with a mobile C-arm image intensifier was successfully investigated [33,34,35,36,37]. For example, Theopold et al. were able to demonstrate the superiority of intraoperative 3D imaging in several studies with regard to detecting the position of Kirschner wires and screws in the proximal humerus and glenoid [36,37,38]. Böhringer et al. confirmed the ability to accurately assess the fracture reduction, plate and screw position, and cement flow in proximal humerus fractures by using 3D DVT in everyday clinical practice [33]. In a further clinical study, Hepp et al. were able to demonstrate the diagnostic superiority of multiplanar imaging over X-ray imaging in two planes with regard to screw perforation in the plate osteosynthesis of proximal humerus fractures [34].
The clinical feasibility of intraoperative 3D imaging on the shoulder can make image-based computer navigation possible with the option of navigated drill tunnel creation and suture button system implantation (navigated suture button (NSB) method). A few in vitro studies on cadaver shoulders and synthetic shoulder models have already been published [39,40,41], but the method has not yet been demonstrated clinically. However, in the future, it may be possible to achieve a further reduction in invasiveness while, at the same time, increasing the precision of implant placement for the best possible anatomical reconstruction.
However, this study should first describe the clinical procedure of the method step by step. Approval for the study was obtained from the local ethics committee with the application number 88/23.
2. Surgical Technique
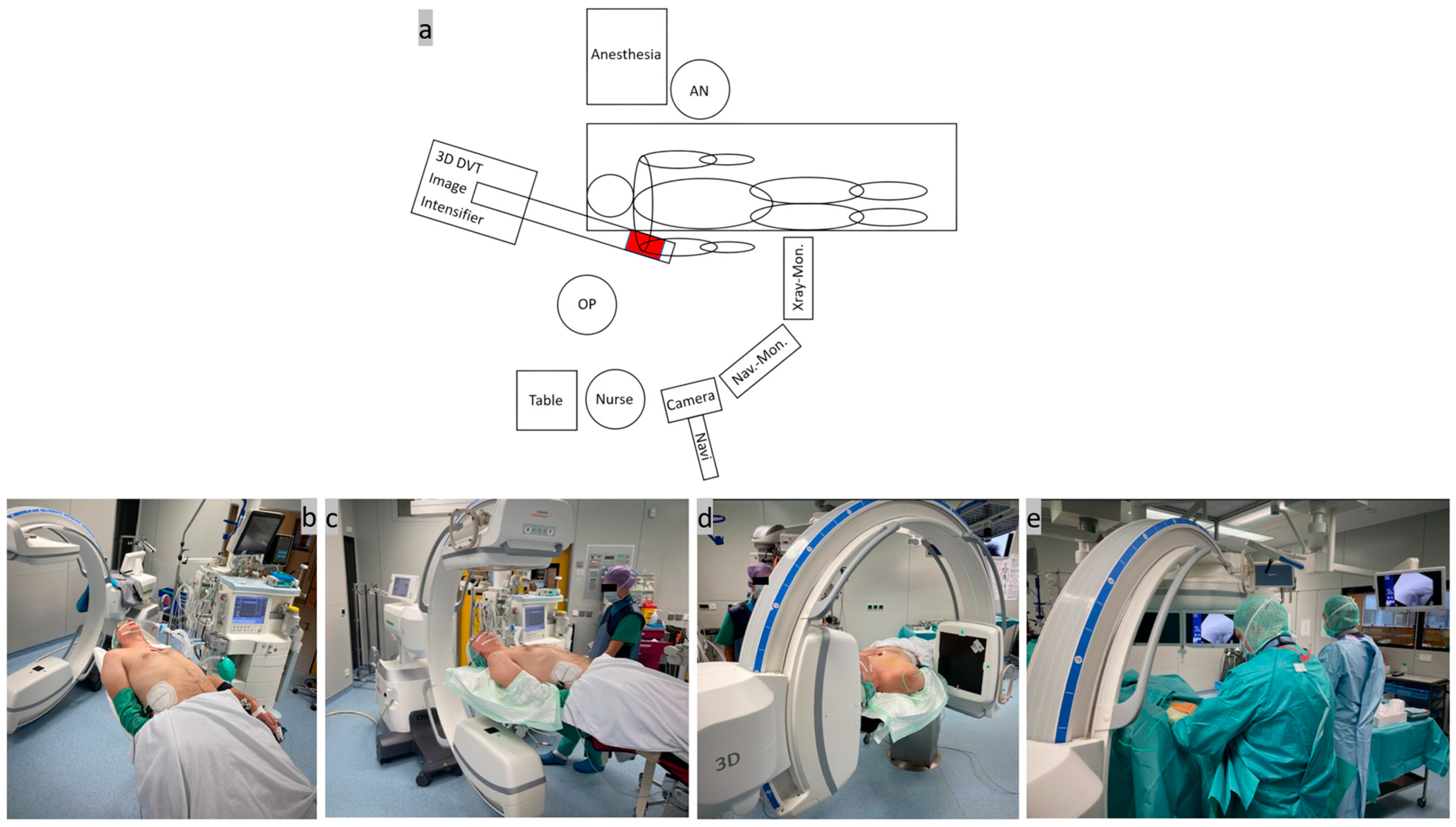
Step one, patient positioning and system setup (Figure 1): A rigid, simple, full-carbonate table was used to position the patient in a beach-chair-like position in the operating room. The patient was positioned on the slightly inclined table up to the edge of the head and torso on the side of the shoulder to be operated on. The shoulder in question should protrude over the edge of the table. A firm foam cushion was used under the seat and a narrow ring under the head to fix the position. The arm was placed on the abdomen and secured with a sling. Two leather straps were used to secure the thorax and legs and a bandage was used to slightly tilt the head to one side during the DVT scan. To protect the lenses, the patient was wearing lead goggles, and an X-ray-proof lead mat was placed under the thorax, abdomen, and gonads. As usual, the mobile isocentric C-arm image intensifier (Siemens® Cios-Spin, Siemens Healthineers AG, Forchheim, Germany) was placed at the patient’s head end in a slightly oblique, parasagittal position in order to exclusively image the shoulder area in question. The exact position of the image intensifier was determined before the start of the operation by aligning the laser cross on the AC joint in two planes with the C-arm freely rotating through 220°. The X-ray machine has a 30 × 30 cm flat detector plate and a motorized rotation range of 195° in which up to 400 individual images are calculated into a 3D data set [42]. The camera of the image-based navigation system ((Brainlab “Buzz”, consisting of hardware (instruments, references, cameras, and screens) and software (Backbone 1.6.2.54, Backbone Viewer 1.6.2.578, Brainlab Buzz 1.0.0.12, and Brainlab Nodemaster 1.6.0.48)) was movably installed on the ceiling on the same side as the shoulder and the C-arm. After careful skin disinfection of the entire shoulder girdle, the operating area was covered with sterile drapes and 2 g of cefazolin® (Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany) was given as an intravenous single-shot antibiotic. An interdisciplinary team time-out was carried out as is standard before the start of the operation.
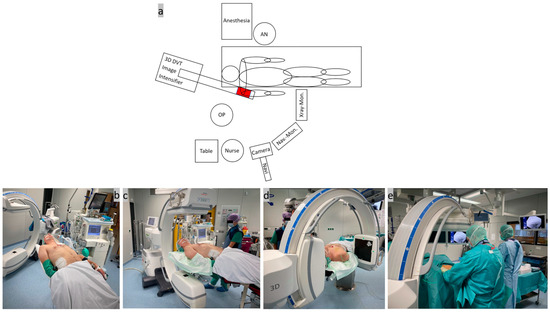
Figure 1.
(a) Sketch of the system arrangement in the operating room with the equipment positioned around the patient; (b) positioning of the patient on the outer edge of the carbon table in a slight beach chair position; (c,d) adjustment of the mobile C-arm image intensifier by X-ray images in 2 planes; and (e) surgical site after covering with sterile drapes.
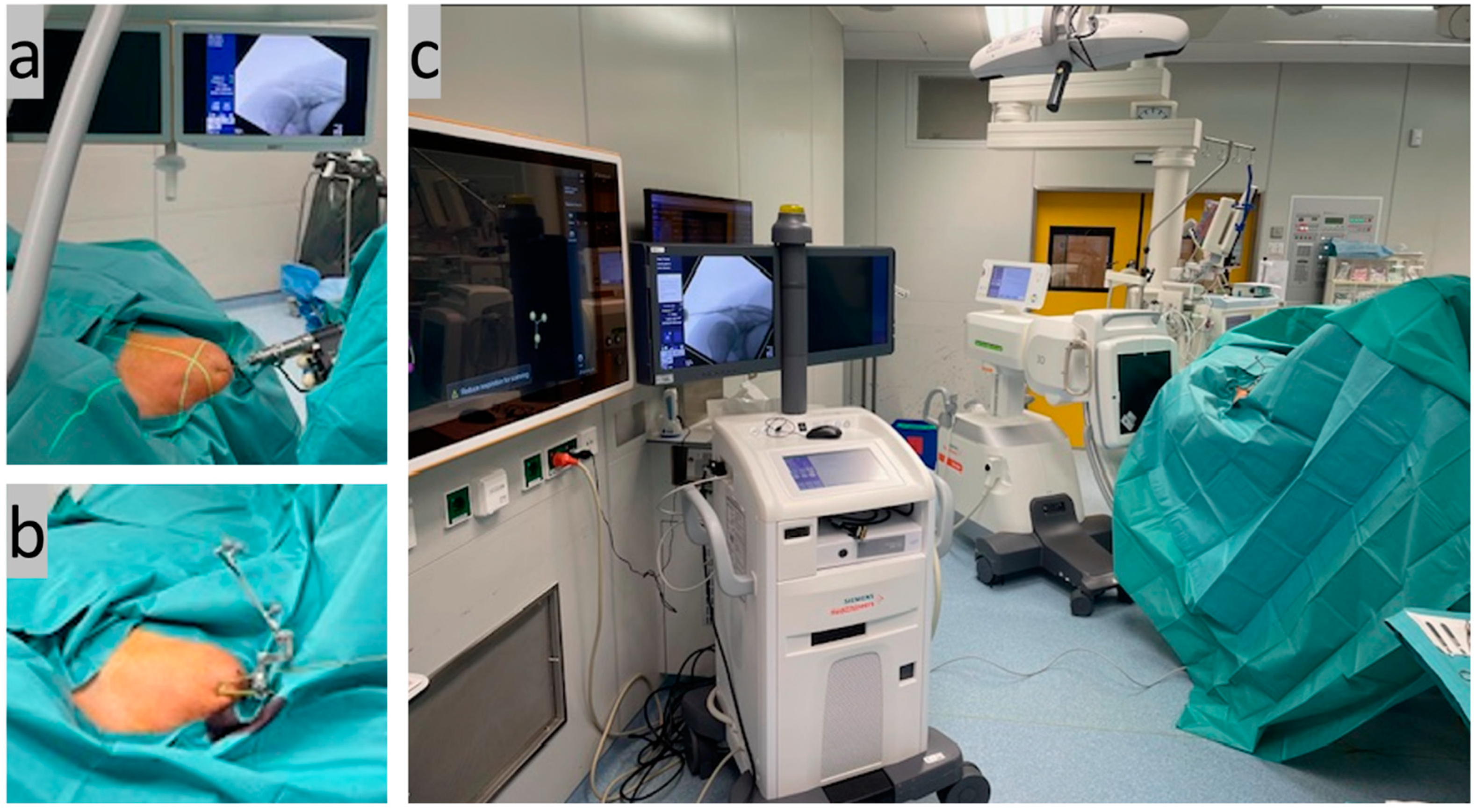
Step two, AC joint reduction and retention (Figure 2): Closed reduction and retention of the AC joint (temporary transfixation) was then performed percutaneously through the acromion using a 2.5 mm Kirschner wire (K-wire) under two-dimensional (2D) X-ray control. The wire was inserted horizontally from the anterior lateral acromion edge through the acromion and AC joint into the lateral clavicle, crossing three stable corticalices. A 3 mm-diameter threaded wire was then inserted into the acromion dorsally parallel to the first to securely attach the navigation reference laterally. The navigation camera was then adjusted so that both references (both the one installed on the C-arm and the one attached to the acromion) could be captured together. The first DVT was then performed and the 3D data set was created. This resulted in an average radiation exposure from 0.45 mSv for tissue weighting factor ‘skin and bones’ (0.01), up to 5.38 mSv for tissue weighting factor ‘other tissues and thoracic organs’ (0.12) (scan data: 110 kV, 0.31 mAs, 15 pulse frequency, 0.139 dose step, 33.1 scene length, 448.55 DAP, 28.244 air kerma ref, large focus).
Figure 2.
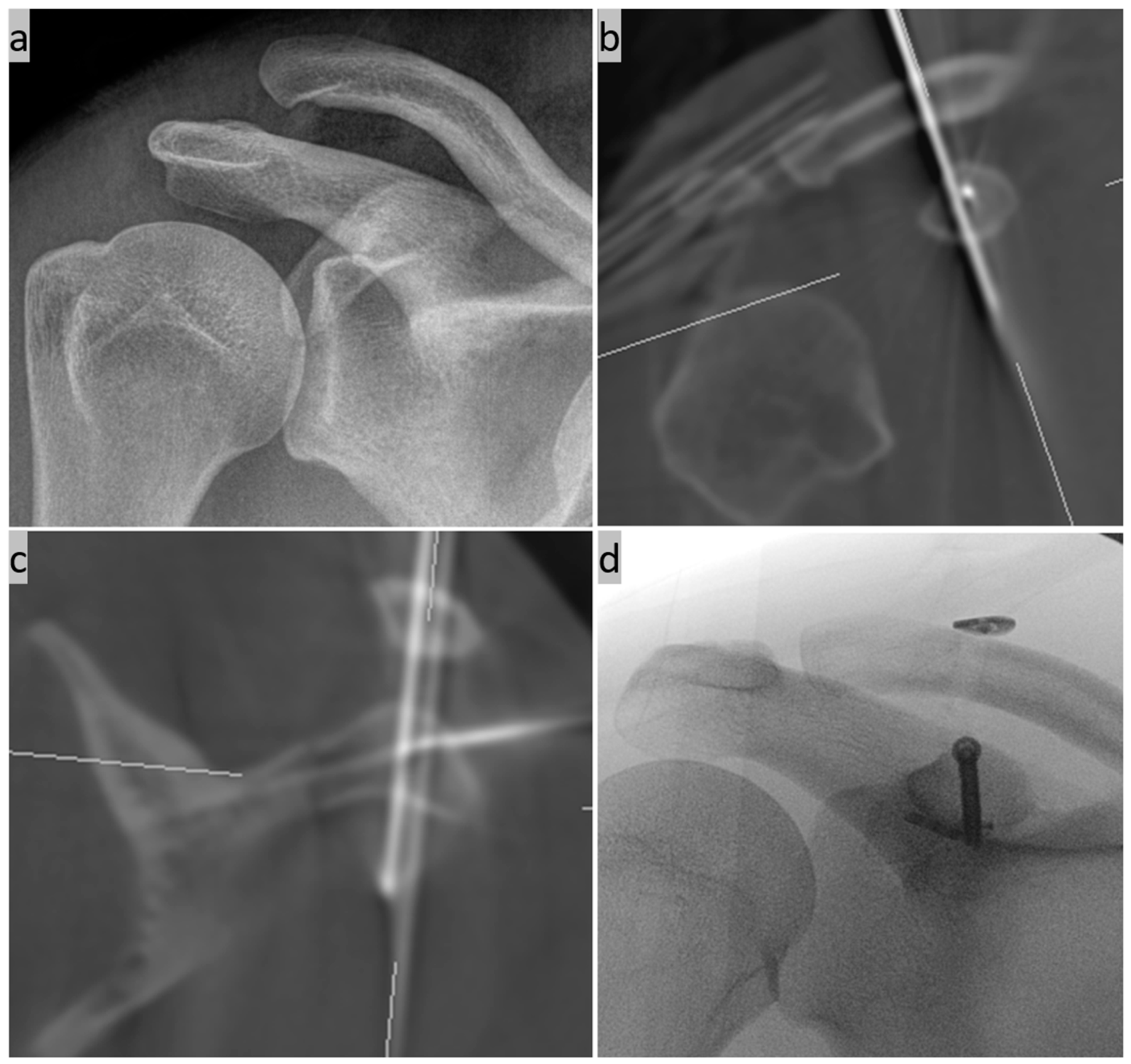
(a) Closed AC joint reduction with percutaneous K-wire transfixation under X-ray control; (b) mounted navigation reference on the lateral acromion edge fixed via a second K-wire; AND (c) start of the first 3D scan.
Step three, 3D scan and drill tunnel planning (Figure 3): The anatomical AC joint position was checked exactly using the multiplanar layers of the 3D data set. The CC drill tunnel was then precisely planned on the navigation monitor. Care was taken to ensure that the single tunnel would run as close as possible to the anatomy along the original ligaments between the clavicle and coracoid. The center of the drill hole was planned to be 25–35 mm from the lateral clavicular edge [43].

Figure 3.
(a) 3D model reconstruction of the repositioned and retained AC joint; (b–d) CC drill tunnel planning: (b) alignment of the pointer over the lateral clavicle and (c) on the X-ray image; and (d) drill tunnel planning on the navigation monitor in the three DVT slices coronal, sagittal, and axial.
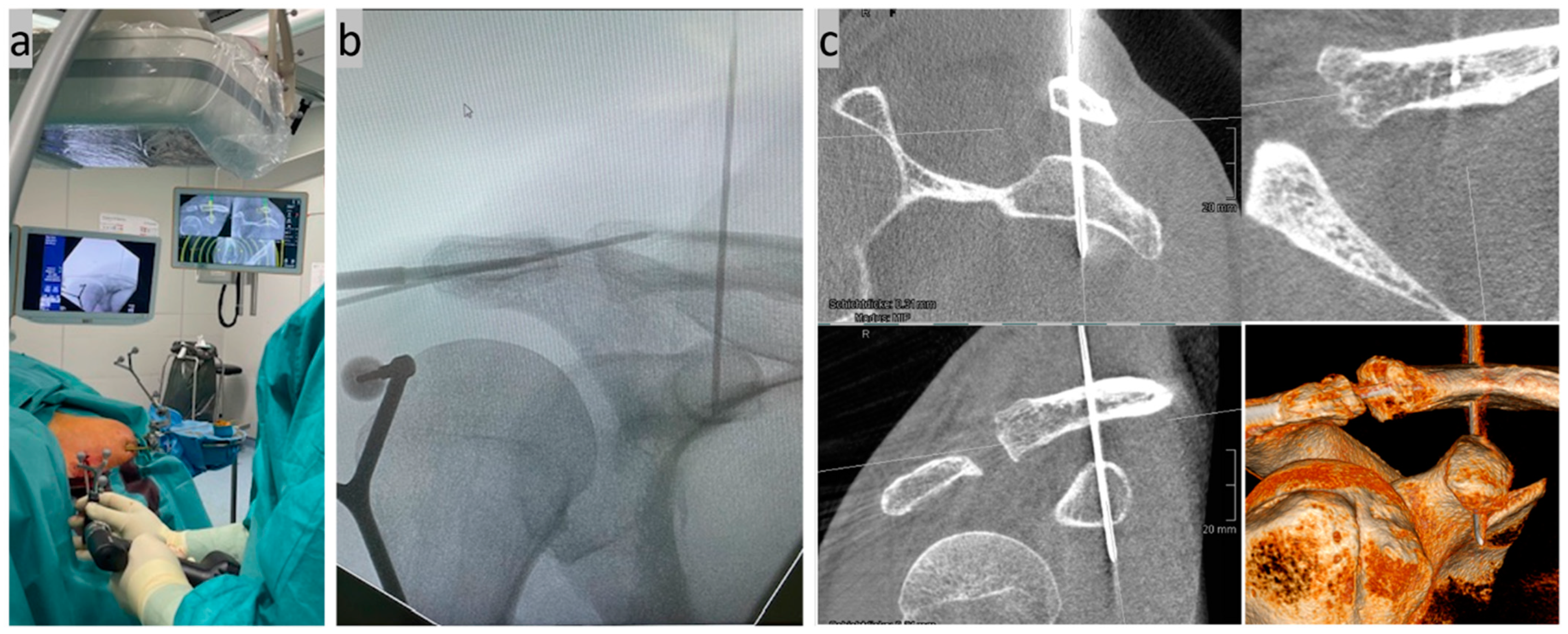
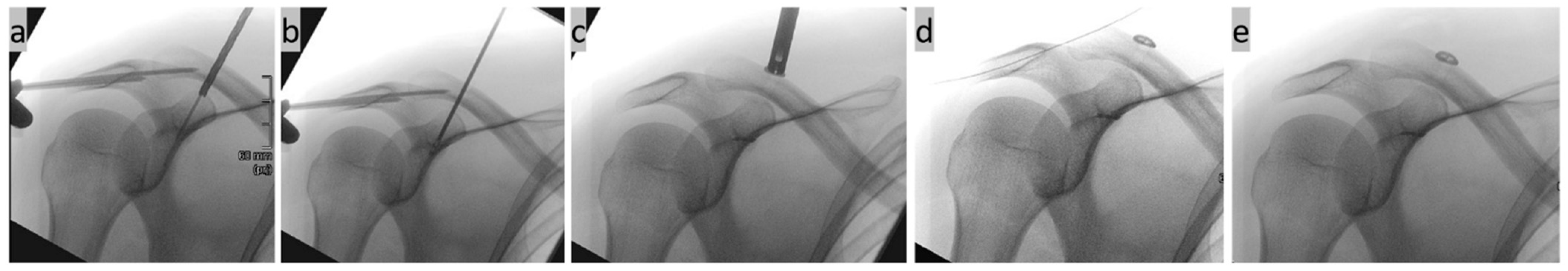
Step four, stab incision and K-wire navigation (Figure 4): In accordance with the drill tunnel planning on the monitor, a 1.8 mm K-wire was inserted through a stab incision above the clavicle using a navigated Yamshidi needle. The four corticalices of the two small tubular bones (clavicle and coracoid) were noticeably penetrated. A second 3D DVT scan was then carried out to check the exact position.
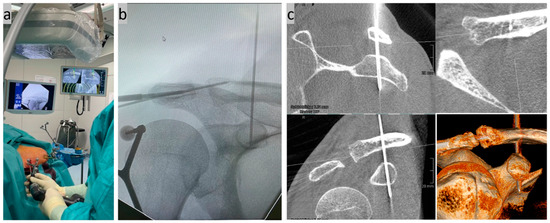
Figure 4.
(a) Minimally invasive insertion of a K-wire in CC direction using a navigated Jamshidi needle under real-time control on the monitor; (b) control of the wire position on a conventional X-ray image; and (c) exact position control using a second DVT scan in the sagittal, axial, and coronal slices as well as a 3D reconstruction.
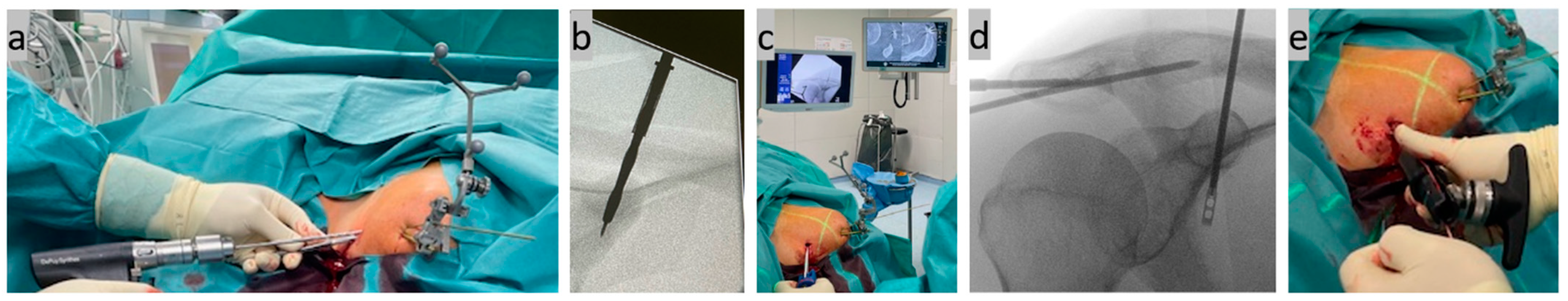
Step five, cannulated drilling and implant positioning (Figure 5): The inserted 1.8 mm K-wire was overdrilled to 4 mm with a cannulated drill, with the four corticalices again being noticeable. The implant (Arthrex AC TightRope® suture button system, Arthrex GmbH, Munich, Germany) was then inserted through the drill tunnel and anchored below the coracoid arch by flipping the titanium button. Finally, the AC joint K-wire transfixation was removed, the suture button system was tightened with a tensioning device to 80 Newton meters, and the suture ends were knotted to double-secure the knotless system.
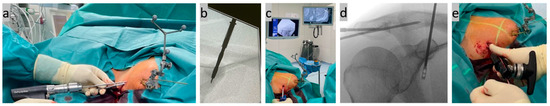
Figure 5.
(a,b) Cannulated overdrilling of the inserted K-wire; (c,d) subsequent insertion of the implant through the CC drill tunnel; and (e) tightening of the implant system with a tensioning device after anchoring the lower titanium button.
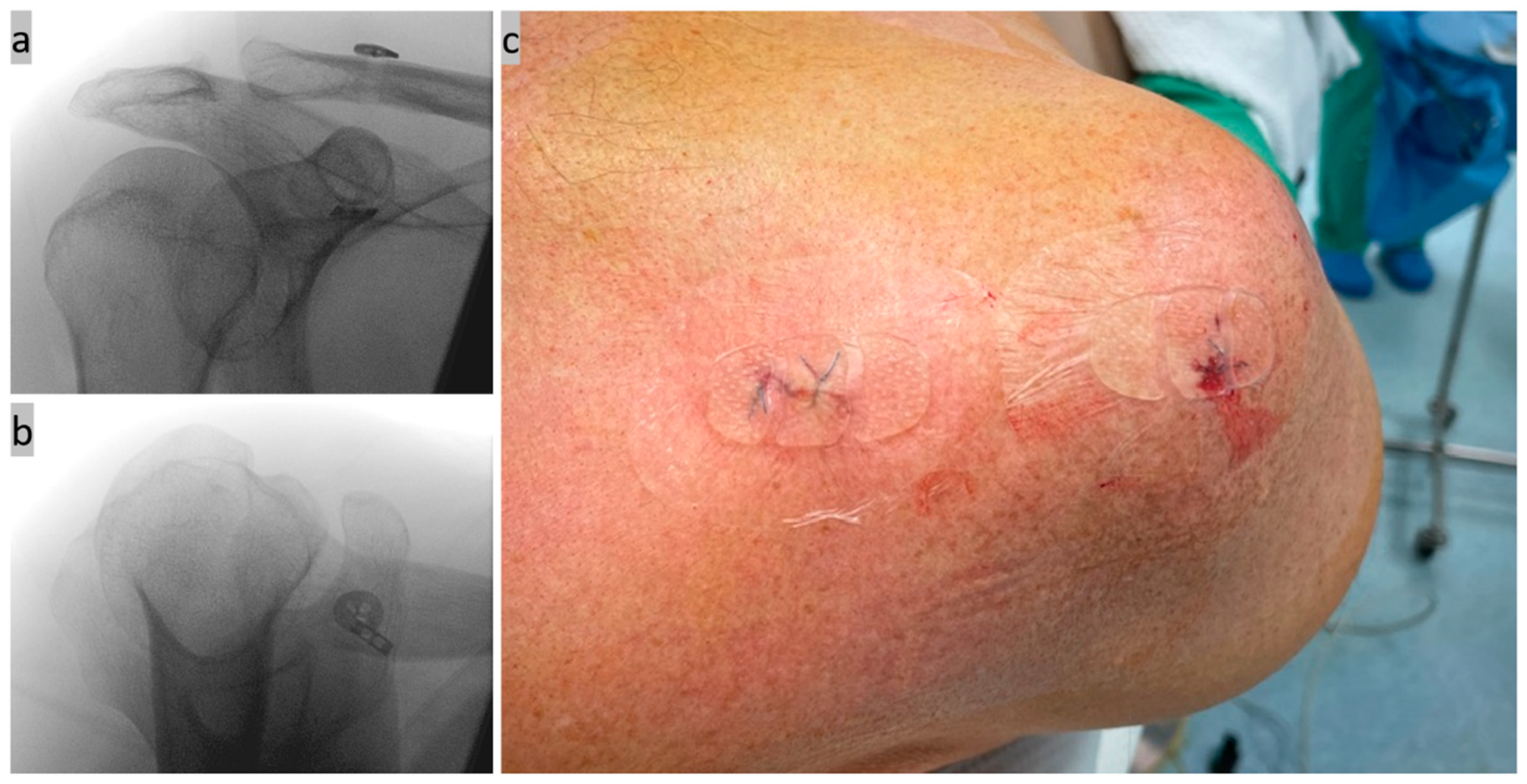
Step six, final check, documentation, and wound closure (Figure 6): Finally, the anatomical AC joint position and correct implant position were checked and documented using X-ray control in two planes (a.p. and axial). A slight overcorrection was also tolerated [44]. The wound was closed over the implant above the lateral clavicle and the removed reference on the lateral acromion edge using non-absorbable sutures and sterile plasters. The average operating time was initially around 50 min.
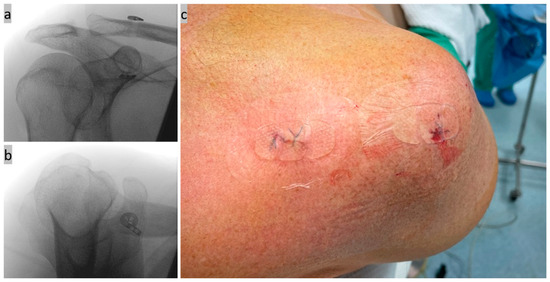
Figure 6.
(a,b) Final X-ray control in 2 planes; and (c) wound closure picture of the stab incisions.
3. Possible Applications and Extensions of the New Method
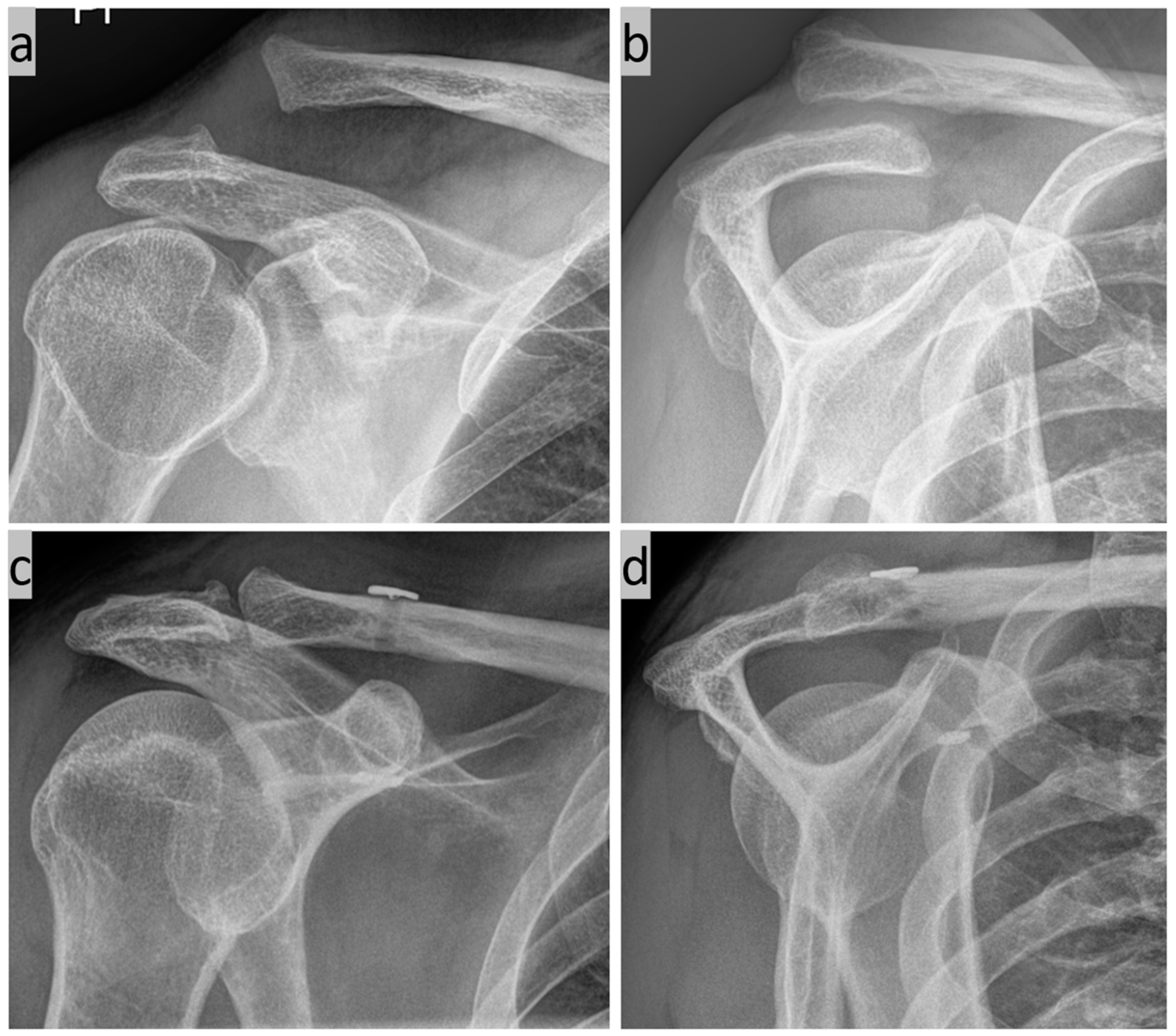
Various possible applications for the method are listed below. First, we have the simple 3D C-arm navigated AC joint stabilization for a Rockwood 3b injury (Figure 7).
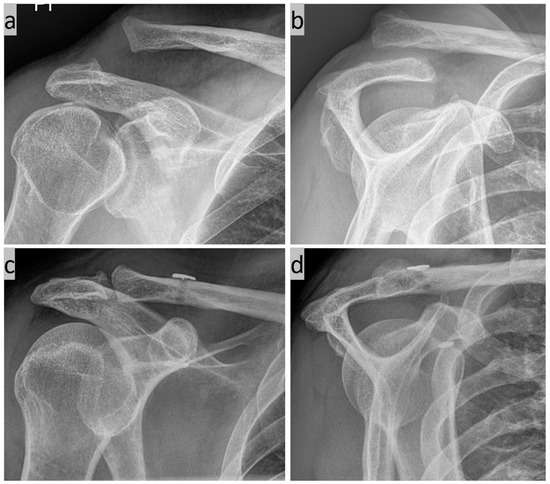
Figure 7.
(a) Zanca view and (b) Y-view of a Tossy 3b injury to the right shoulder; and (c,d) postoperative follow-up after 3 months.
In addition to CC stabilization, it is also possible to apply a horizontal AC suture cerclage (Figure 8). A drill tunnel was created in the central extension of the lateral clavicle from the lateral acromion edge to the upper edge of the AC joint. One suture end of the knotted CC implant was passed through the drill channel and the second through the soft tissue directly above the acromion to the lateral side. There, the two sutures were knotted together again under the transverse compression of the AC joint for horizontal AC cerclage.
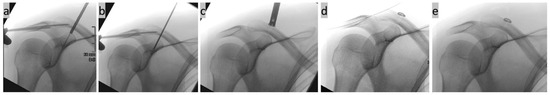
Figure 8.
(a–e) Intraoperative PA (posterior anterior) view X-ray images of a right shoulder; (a) cannulated overdrilling of a CC K-wire implanted using navigation; (b) insertion of the implant through the drill tunnel; (c) tightening of the CC Suture Button System; (d) nithinol wire is inserted through a horizontally drilled tunnel through the acromion; and (e) final check after tightening the horizontal AC cerclage.
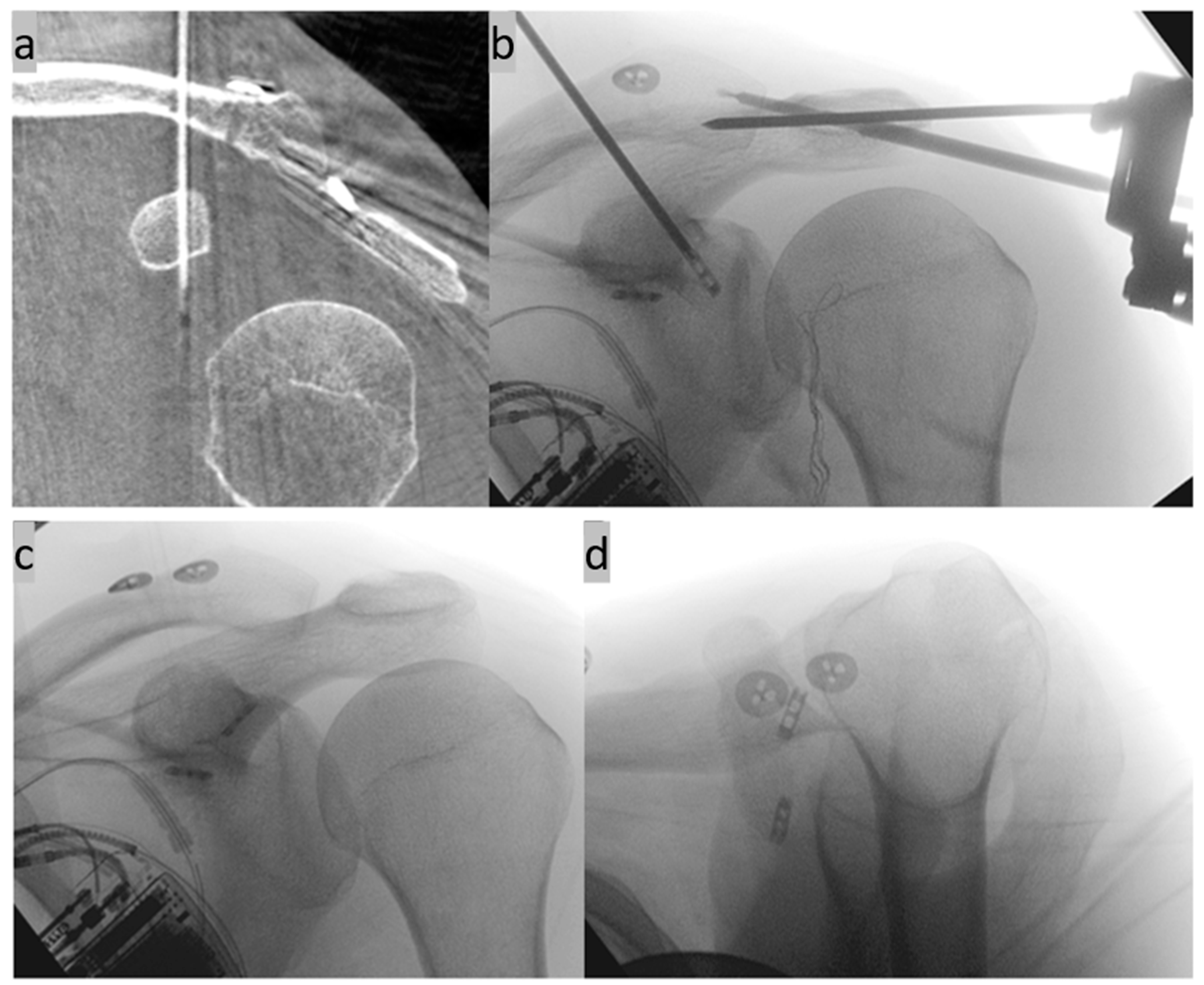
Another option for additive AC joint stabilization is the implantation of a second CC implant in a V-shaped configuration (Figure 9). The anatomical course of the original ligaments can be reproduced exactly here [43].
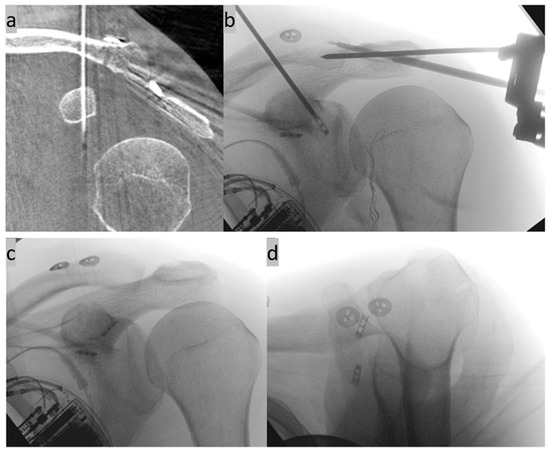
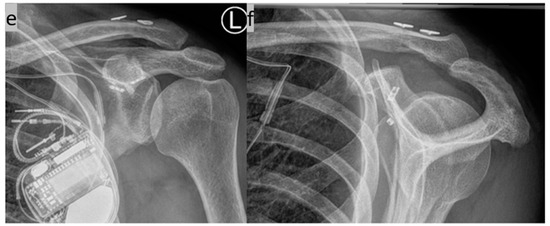
Figure 9.
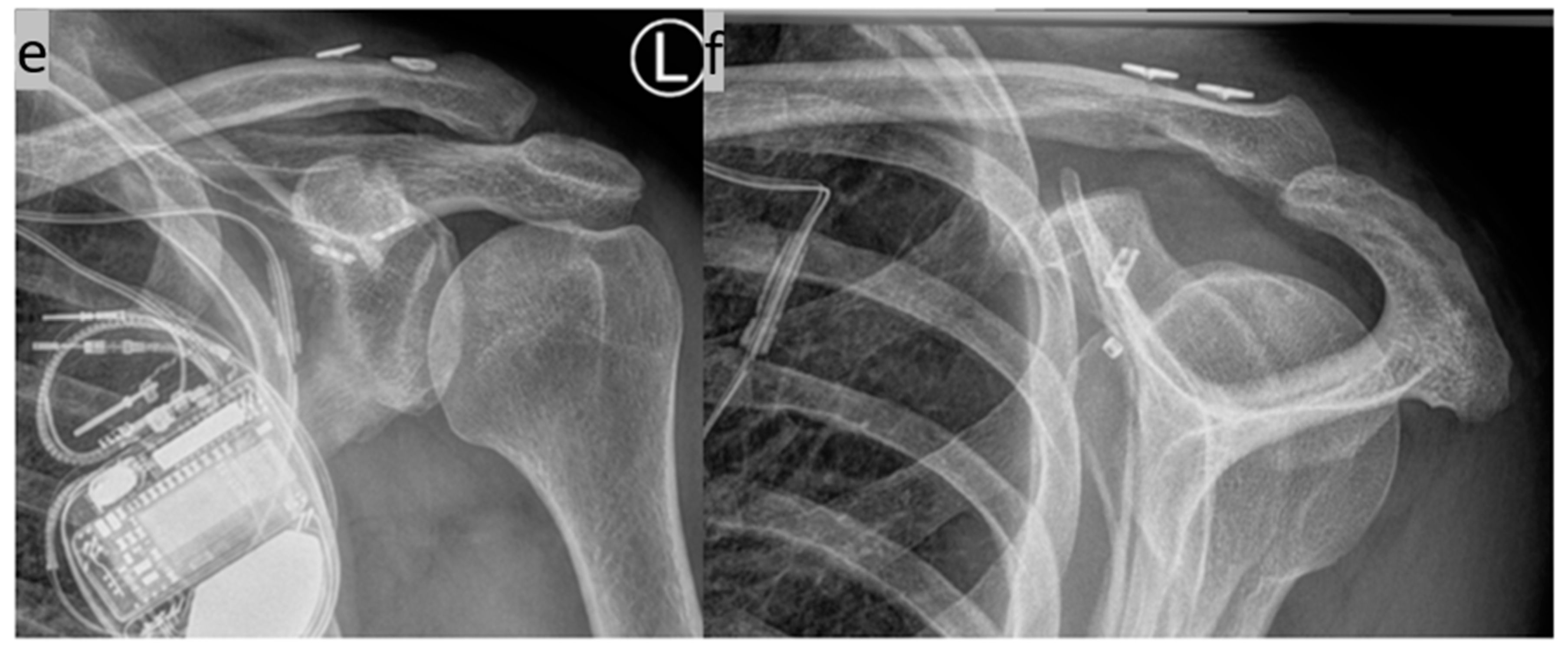
(a–d) Implantation of two suture button systems in a V-shaped arrangement for CC AC joint stabilization. The anatomical course of the original ligaments is mimicked (anterior trapezoid ligament and posterior conoid ligament). (e,f) X-ray control three months postoperatively in two planes.
Furthermore, non-displaced fractures (e.g., of the coracoid base) can be both recognized and screwed in a minimally invasive manner using a stab incision and a navigated K-wire (Figure 10).
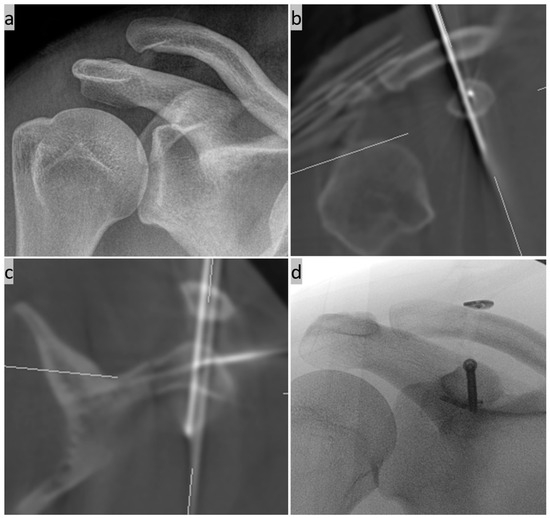
Figure 10.
(a) Preoperative X-ray image of a supposed simple AC joint dislocation; (b,c) intraoperative 3D imaging with an additional non-displaced fracture of the coracoid base and an already inserted additional K-wire for one-stage cannulated screw osteosynthesis; and (d) final X-ray control with the implanted CC Suture Button System and the 3.5 mm cannulated titanium screw osteosynthesis of the coracoid.
4. Risks and Limitations
The general risks of the method are the usual surgical complications such as injuries to nerves, vessels, muscles, tendons, ligaments, and organs, with numbness, paralysis, bleeding, hematoma, and dysfunction, as well as infection, impaired wound healing, pain, restriction of movement, implant failure, iatrogenic tissue damage/fracture, persistence/recurrence of symptoms, and possible follow-up surgery. However, the NSB method aims to further reduce surgical invasiveness while, at the same time, increasing accuracy in order to avoid these risks and complications.
The special risks of the suture button method, in general, are primarily implant failure due to elongation and malpositioning with persistent instability, loss of reduction, and widening of the drill tunnel, as well as implant migration/irritation and wound healing problems [1,18]. Here, too, increasing the implantation accuracy aims to reduce the risks.
The specific disadvantages and risks of 3D-image-based computer navigation are the increased effort required in the operating room, the necessary equipment (such as a full-carbon table, a 3D DVT C-arm image intensifier, and an image-based computer navigation system), and the increased radiation exposure for the patient due to the intraoperative DVT scan. However, with the NSB method, only the shoulder area in question is imaged. Nevertheless, the known radiation protection measures must be exhausted. Among other things, in order to protect the sensitive organs in the best possible way, lead mats and protective goggles as well as gonad protection are used as standard.
Possible technical limitations of the new method could be patient-specific body characteristics. For patients with narrow shoulders, a short neck, and a thick head, positioning for exposure of the relevant shoulder area is certainly not easy. Furthermore, the method is currently not recommended for children of growing age due to the possible increased radiation exposure and the remaining implant. The method should also not be performed on chronically unstable AC joint dislocations due to a reduced/lack of biological ligament healing potential.
In order to minimize the surgical risk and possible complications as best as possible, a very careful indication and precise planning of the surgical procedure is necessary [1].
5. Rehabilitation
The shoulder is protected in a Gilchrist bandage until the skin sutures are removed two weeks postoperatively. The patient is then allowed to move the shoulder freely below the horizontal plane without weight-bearing until the end of the sixth postoperative week. A clinical radiologic follow-up is then performed to allow pain-adapted successive movement and subsequent weight-bearing. Three months postoperatively, a further clinical radiological follow-up is performed for complete release.
6. Discussion
ASB techniques for AC joint reconstruction have risen in importance in recent years [9,44]. Several advantages of the method over the conventional open treatment are mentioned in the literature. These include the minimally invasive procedure with less tissue damage and, therefore, less pain postoperatively, faster convalescence, a lower risk of infection, and a good cosmetic result. The use of the suture button system also enables the anatomical reconstruction of the original ligaments and, as a one-step procedure, eliminates the need for further surgery to remove the implant, although this remains possible in the future at the patient’s request [6,15,16,17,44].
By using intraoperative 3D imaging with image-guided computer navigation to assess the AC joint position and drill tunnel placement, the NSB method should be even less invasive and more accurate [39,40,41,45].
Several studies on the ASB method point to the advantage of detecting intra-articular comorbidities and the possibility of treating them in one go. However, it is not yet clear to what extent these findings are of traumatic or degenerative origin, whether they are symptomatic pathologies or incidental findings, and whether immediate treatment (usually only debridement) is really necessary. Thereby, the general risks of shoulder arthroscopy, such as postoperative shoulder stiffness, are barely mentioned in these studies [46,47].
Various extensions of the ASB method include the additive horizontal AC suture cerclage and the double CC suture button system implantation in a V-shaped arrangement [24,27,29,30,31]. With the navigated technique, both extensions can be performed as well as the navigated screw fixation of fractures that are not significantly displaced.
7. Conclusions
The NSB method aims to be simple, minimally invasive, safe, and precise. In order to validate this and compare it with established methods, prospective clinical trials with a large number of patients and a long follow-up period should be carried out. The clinical and radiological outcome between the open, arthroscopic, and navigated methods should be investigated using standardized examinations with the evaluation of radiological measurement data and clinical questionnaires on complaints and the function of the affected extremity. A comprehensive long-term comparison with the hook plate and the conservative approach would certainly be just as interesting, but no less challenging.
In order to achieve the best possible postoperative outcome, however, very careful patient selection and good surgical planning, including the choice of a suitable additive stabilization procedure, should be ensured.
Author Contributions
Conceptualization, A.B. and K.S.; methodology, A.B. and K.S.; validation, A.B., C.P., A.E., F.G. and K.S.; formal analysis, A.B. and K.S.; investigation, A.B., C.P., A.E., F.G. and K.S.; data curation, A.B., A.E. and C.P.; writing-original draft preparation, A.B.; writing-review and editing, A.B. and K.S.; visualization, A.B.; supervision, F.G.; project administration, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
No author is affiliated to any of the supporting companies, or received or will receive any form of payment related to this study.
Institutional Review Board Statement
This technical note study involving human participants was in accordance with the ethical standards of the Institutional and National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Ethics Committee of Ulm University approved the application with the number 88/23—FSt/Sta on 20 April 2023.
Informed Consent Statement
Informed consent was obtained in each case by each patient consenting to study participation with detailed written information. Consent to the publication of the anonymized study data was also obtained in writing from each patient.
Data Availability Statement
All authors decided that the data and material will not be deposited in a public repository, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Martetschläger, F.; Kraus, N.; Scheibel, M.; Streich, J.; Venjakob, A.; Maier, D. The Diagnosis and Treatment of Acute Dislocation of the Acromioclavicular Joint. Dtsch. Arztebl. Int. 2019, 116, 89–95. [Google Scholar] [CrossRef]
- Gorbaty, J.D.; Hsu, J.E.; Gee, A.O. Classifications in Brief: Rockwood Classification of Acromioclavicular Joint Separations. Clin. Orthop. Relat. Res. 2017, 475, 283–287. [Google Scholar] [CrossRef]
- Tossy, J.D.; Mead, N.C.; Sigmond, H.M. Acromioclavicular separations: Useful and practical classification for treatment. Clin. Orthop. Relat. Res. 1963, 28, 111–119. [Google Scholar] [CrossRef]
- Berthold, D.P.; Muench, L.N.; Dyrna, F.; Mazzocca, A.D.; Garvin, P.; Voss, A.; Scheiderer, B.; Siebenlist, S.; Imhoff, A.B.; Beitzel, K. Current concepts in acromioclavicular joint (AC) instability—A proposed treatment algorithm for acute and chronic AC-joint surgery. BMC Musculoskelet. Disord. 2022, 23, 1078. [Google Scholar] [CrossRef]
- Beitzel, K.; Mazzocca, A.D.; Bak, K.; Itoi, E.; Kibler, W.B.; Mirzayan, R.; Imhoff, A.B.; Calvo, E.; Arce, G.; Shea, K.; et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the Rockwood classification for acromioclavicular joint injuries. Arthroscopy 2014, 30, 271–278. [Google Scholar] [CrossRef]
- Rosso, C.; Martetschläger, F.; Saccomanno, M.F.; Voss, A.; Lacheta, L.; ESA DELPHI Consensus Panel; Beitzel, K.; Milano, G. High degree of consensus achieved regarding diagnosis and treatment of acromioclavicular joint instability among ESA-ESSKA members. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 2325–2332. [Google Scholar] [CrossRef]
- Arirachakaran, A.; Boonard, M.; Piyapittayanun, P.; Phiphobmongkol, V.; Chaijenkij, K.; Kongtharvonskul, J. Comparison of surgical outcomes between fixation with hook plate and loop suspensory fixation for acute unstable acromioclavicular joint dislocation: A systematic review and meta-analysis. Eur. J. Orthop. Surg. Traumatol. 2016, 26, 565–574. [Google Scholar] [CrossRef]
- Lloyd, A.J.; Hurley, E.T.; Davey, M.S.; Pauzenberger, L.; Mullet, H. Arthroscopic Suture-Button versus Hook-Plate Fixation for Acromioclavicular Joint Injuries-A Systematic Review of Comparative Studies. Arthrosc. Sports Med. Rehabil. 2020, 2, e671–e676. [Google Scholar] [CrossRef]
- Balke, M.; Schneider, M.M.; Akoto, R.; Bäthis, H.; Bouillon, B.; Banerjee, M. Acute acromioclavicular joint injuries. Changes in diagnosis and therapy over the last 10 years. Unfallchirurg 2015, 118, 851–857. [Google Scholar] [CrossRef]
- Allemann, F.; Halvachizadeh, S.; Waldburger, M.; Schaefer, F.; Pothmann, C.; Pape, H.C.; Rauer, T. Different treatment strategies for acromioclavicular dislocation injuries: A nationwide survey on open/minimally invasive and arthroscopic concepts. Eur. J. Med. Res. 2019, 24, 18. [Google Scholar] [CrossRef]
- Beitzel, K.; Obopilwe, E.; Chowaniec, D.M.; Niver, G.E.; Nowak, M.D.; Hanypsiak, B.T.; Guerra, J.J.; Arciero, R.A.; Mazzocca, A.D. Biomechanical comparison of arthroscopic repairs for acromioclavicular joint instability: Suture button systems without biological augmentation. Am. J. Sports Med. 2011, 39, 2218–2225. [Google Scholar] [CrossRef]
- Deshmukh, A.V.; Wilson, D.R.; Zilberfarb, J.L.; Perlmutter, G.S. Stability of acromioclavicular joint reconstruction: Biomechanical testing of various surgical techniques in a cadaveric model. Am. J. Sports Med. 2004, 32, 1492–1498. [Google Scholar] [CrossRef]
- Gowd, A.K.; Liu, J.N.; Cabarcas, B.C.; Cvetanovich, G.L.; Garcia, G.H.; Manderle, B.J.; Verma, N.N. Current Concepts in the Operative Management of Acromioclavicular Dislocations: A Systematic Review and Meta-analysis of Operative Techniques. Am. J. Sports Med. 2019, 47, 2745–2758. [Google Scholar] [CrossRef]
- McKee, M.D. Operative Fixation of Chronic Acromioclavicular Joint Dislocation with Hook Plate and Modified Ligament Transfer. J. Orthop. Trauma. 2016, 30 (Suppl. S2), S7–S8. [Google Scholar] [CrossRef]
- Moatshe, G.; Kruckeberg, B.M.; Chahla, J.; Godin, J.A.; Cinque, M.E.; Provencher, M.T.; LaPrade, R.F. Acromioclavicular and Coracoclavicular Ligament Reconstruction for Acromioclavicular Joint Instability: A Systematic Review of Clinical and Radiographic Outcomes. Arthroscopy 2018, 34, 1979–1995.e8. [Google Scholar] [CrossRef]
- Müller, D.; Reinig, Y.; Hoffmann, R.; Blank, M.; Welsch, F.; Schweigkofler, U.; Stein, T. Return to sport after acute acromioclavicular stabilization: A randomized control of double-suture-button system versus clavicular hook plate compared to uninjured shoulder sport athletes. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3832–3847. [Google Scholar] [CrossRef]
- Stein, T.; Müller, D.; Blank, M.; Reinig, Y.; Saier, T.; Hoffmann, R.; Welsch, F.; Schweigkofler, U. Stabilization of Acute High-Grade Acromioclavicular Joint Separation: A Prospective Assessment of the Clavicular Hook Plate Versus the Double Double-Button Suture Procedure. Am. J. Sports Med. 2018, 46, 2725–2734. [Google Scholar] [CrossRef]
- Woodmass, J.M.; Esposito, J.G.; Ono, Y.; Nelson, A.A.; Boorman, R.S.; Thornton, G.M.; Lo, I.K. Complications following arthroscopic fixation of acromioclavicular separations: A systematic review of the literature. Open Access J. Sports Med. 2015, 6, 97–107. [Google Scholar] [CrossRef]
- Wolf, E.M.; Pennington, W.T. Arthroscopic reconstruction for acromioclavicular joint dislocation. Arthroscopy 2001, 17, 558–563. [Google Scholar] [CrossRef]
- Scheibel, M.; Dröschel, S.; Gerhardt, C.; Kraus, N. Arthroscopically assisted stabilization of acute high-grade acromioclavicular joint separations. Am. J. Sports Med. 2011, 39, 1507–1516. [Google Scholar] [CrossRef]
- Jensen, G.; Katthagen, J.C.; Alvarado, L.E.; Lill, H.; Voigt, C. Has the arthroscopically assisted reduction of acute AC joint separations with the double tight-rope technique advantages over the clavicular hook plate fixation? Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 422–430. [Google Scholar] [CrossRef]
- Saier, T.; Plath, J.E.; Beitzel, K.; Minzlaff, P.; Feucht, J.M.; Reuter, S.; Martetschläger, F.; Imhoff, A.B.; Aboalata, M.; Braun, S. Return-to-activity after anatomical reconstruction of acute high-grade acromioclavicular separation. BMC Musculoskelet. Disord. 2016, 17, 145. [Google Scholar] [CrossRef]
- Venjakob, A.J.; Salzmann, G.M.; Gabel, F.; Buchmann, S.; Walz, L.; Spang, J.T.; Vogt, S.; Imhoff, A.B. Arthroscopically assisted 2-bundle anatomic reduction of acute acromioclavicular joint separations: 58-month findings. Am. J. Sports Med. 2013, 41, 615–621. [Google Scholar] [CrossRef]
- Braun, S.; Beitzel, K.; Buchmann, S.; Imhoff, A.B. Arthroscopically Assisted Treatment of Acute Dislocations of the Acromioclavicular Joint. Arthrosc. Tech. 2015, 4, e681–e685. [Google Scholar] [CrossRef]
- Saier, T.; Venjakob, A.J.; Minzlaff, P.; Föhr, P.; Lindell, F.; Imhoff, A.B.; Vogt, S.; Braun, S. Value of additional acromioclavicular cerclage for horizontal stability in complete acromioclavicular separation: A biomechanical study. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1498–1505. [Google Scholar] [CrossRef]
- Wellmann, M.; Smith, T.; Windhagen, H.; Siebert, C.H. Biomechanik aktueller Rekonstruktionstechniken bei Schultereckgelenksprengungen. Obere Extrem. 2011, 6, 85–89. [Google Scholar] [CrossRef]
- Dyrna, F.; Imhoff, F.B.; Haller, B.; Braun, S.; Obopilwe, E.; Apostolakos, J.M.; Morikawa, D.; Imhoff, A.B.; Mazzocca, A.D.; Beitzel, K. Primary Stability of an Acromioclavicular Joint Repair Is Affected by the Type of Additional Reconstruction of the Acromioclavicular Capsule. Am. J. Sports Med. 2018, 46, 3471–3479. [Google Scholar] [CrossRef]
- Hann, C.; Kraus, N.; Minkus, M.; Maziak, N.; Scheibel, M. Combined arthroscopically assisted coraco- and acromioclavicular stabilization of acute high-grade acromioclavicular joint separations. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 212–220. [Google Scholar] [CrossRef]
- Jensen, G.; Dey Hazra, R.-O.; Al-Ibadi, M.; Salmoukas, K.; Katthagen, J.C.; Lill, H.; Ellwein, A. Arthroscopically assisted single tunnel reconstruction for acute high-grade acromioclavicular joint dislocation with an additional acromioclavicular joint cerclage. Eur. J. Orthop. Surg. Traumatol. 2022, 33, 1185–1192. [Google Scholar] [CrossRef]
- Schär, M.O.; Jenni, S.; Fessel, G.; Snedeker, J.G.; Scheibel, M.; Zumstein, M.A. Biomechanical comparison of two biplanar and one monoplanar reconstruction techniques of the acromioclavicular joint. Arch. Orthop. Trauma. Surg. 2019, 139, 779–786. [Google Scholar] [CrossRef]
- Theopold, J.; Schöbel, T.; Fischer, J.-P.; Löffler, S.; Osterhoff, G.; Schleifenbaum, S.; Hepp, P. Acromioclavicular joint reconstruction: An additional acromioclavicular cerclage does not improve horizontal stability in double coraco-clavicular tunnel technique. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 3827–3834. [Google Scholar] [CrossRef]
- Minkus, M.; Kraus, N.; Hann, C.; Scheibel, M. Arthroscopic Reconstruction After Acute Acromioclavicular Separation Injuries. JBJS Essent. Surg. Tech. 2017, 7, e7. [Google Scholar] [CrossRef]
- Böhringer, A.; Cintean, R.; Eickhoff, A.; Gebhard, F.; Schütze, K. Intraoperative 3D imaging in plate osteosynthesis of proximal humerus fractures. Arch. Orthop. Trauma. Surg. 2023, 143, 4993–5000. [Google Scholar] [CrossRef]
- Hepp, P.; Theopold, J.; Jarvers, J.-S.; Marquaß, B.; von Dercks, N.; Josten, C. Multiplanar reconstruction with mobile 3D image intensifier. Surgical treatment of proximal humerus fractures. Unfallchirurg 2014, 117, 437–444. [Google Scholar] [CrossRef]
- Theopold, J.; Pieroh, P.; Henkelmann, R.; Osterhoff, G.; Hepp, P. Real-time intraoperative 3D image intensifier-based navigation in reversed shoulder arthroplasty-analyses of image quality. BMC Musculoskelet. Disord. 2019, 20, 262. [Google Scholar] [CrossRef]
- Theopold, J.; Weihs, K.; Feja, C.; Marquaß, B.; Josten, C.; Hepp, P. Detection of articular perforations of the proximal humerus fracture using a mobile 3D image intensifier—A cadaver study. BMC Med. Imaging 2017, 17, 47. [Google Scholar] [CrossRef]
- Theopold, J.; Weihs, K.; Marquaß, B.; Josten, C.; Hepp, P. Detection of primary screw perforation in locking plate osteosynthesis of proximal humerus fracture by intra-operative 3D fluoroscopy. Arch. Orthop. Trauma. Surg. 2017, 137, 1491–1498. [Google Scholar] [CrossRef]
- Theopold, J.; Pieroh, P.; Scharge, M.L.; Marquaß, B.; Hohmann, T.; Josten, C.; Hepp, P. Improved accuracy of K-wire positioning into the glenoid vault by intraoperative 3D image intensifier-based navigation for the glenoid component in shoulder arthroplasty. Orthop. Traumatol. Surg. Res. 2016, 102, 575–581. [Google Scholar] [CrossRef]
- Böhringer, A.; Gebhard, F.; Dehner, C.; Eickhoff, A.; Cintean, R.; Pankratz, C.; Schütze, K. 3D C-arm navigated acromioclavicular joint stabilization. Arch. Orthop. Trauma. Surg. 2023, 144, 601–610. [Google Scholar] [CrossRef]
- Stübig, T.; Jähnisch, T.; Petri, M.; Hawi, N.; Zeckey, C.; Krettek, C.; Citak, M.; Meller, R. Navigated versus conventional transfixation of AC joint injuries: Feasibility and accuracy. Comput. Aided Surg. 2013, 18, 68–75. [Google Scholar] [CrossRef]
- Stübig, T.; Jähnisch, T.; Reichelt, A.; Krettek, C.; Citak, M.; Meller, R. Navigated vs arthroscopic-guided drilling for reconstruction of acromioclavicular joint injuries: Accuracy and feasibility. Int. J. Med. Robot. 2013, 9, 359–364. [Google Scholar] [CrossRef]
- Sheth, N.M.; De Silva, T.; Uneri, A.; Ketcha, M.; Han, R.; Vijayan, R.; Osgood, G.M.; Siewerdsen, J.H. A mobile isocentric C-arm for intraoperative cone-beam CT: Technical assessment of dose and 3D imaging performance. Med. Phys. 2020, 47, 958–974. [Google Scholar] [CrossRef]
- Peebles, L.A.; Aman, Z.S.; Kraeutler, M.J.; Mulcahey, M.K. Qualitative and Quantitative Anatomic Descriptions of the Coracoclavicular and Acromioclavicular Ligaments: A Systematic Review. Arthrosc. Sports Med. Rehabil. 2022, 4, e1545–e1555. [Google Scholar] [CrossRef]
- Minkus, M.; Maziak, N.; Moroder, P.; Scheibel, M. Arthroscopic low-profile reconstruction for acute acromioclavicular joint instability. Obere Extrem. 2019, 14, 60–65. [Google Scholar] [CrossRef]
- Theopold, J.; Weihs, K.; Löffler, S.; Marquass, B.; von Dercks, N.; Josten, C.; Hepp, P. Image-free navigated coracoclavicular drilling for the repair of acromioclavicular joint dislocation: A cadaver study. Arch. Orthop. Trauma. Surg. 2015, 135, 1077–1082. [Google Scholar] [CrossRef]
- Brislin, K.J.; Field, L.D.; Savoie, F.H. Complications after arthroscopic rotator cuff repair. Arthroscopy 2007, 23, 124–128. [Google Scholar] [CrossRef]
- Weber, S.C.; Abrams, J.S.; Nottage, W.M. Complications associated with arthroscopic shoulder surgery. Arthroscopy 2002, 18, 88–95. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).