Anterior Cervical and Upper Thoracic Column Reconstruction Using an Expandable Poly-Ether-Ether-Ketone Vertebral Body Replacement: A Retrospective Single Center Cohort Analysis
Abstract
:1. Introduction
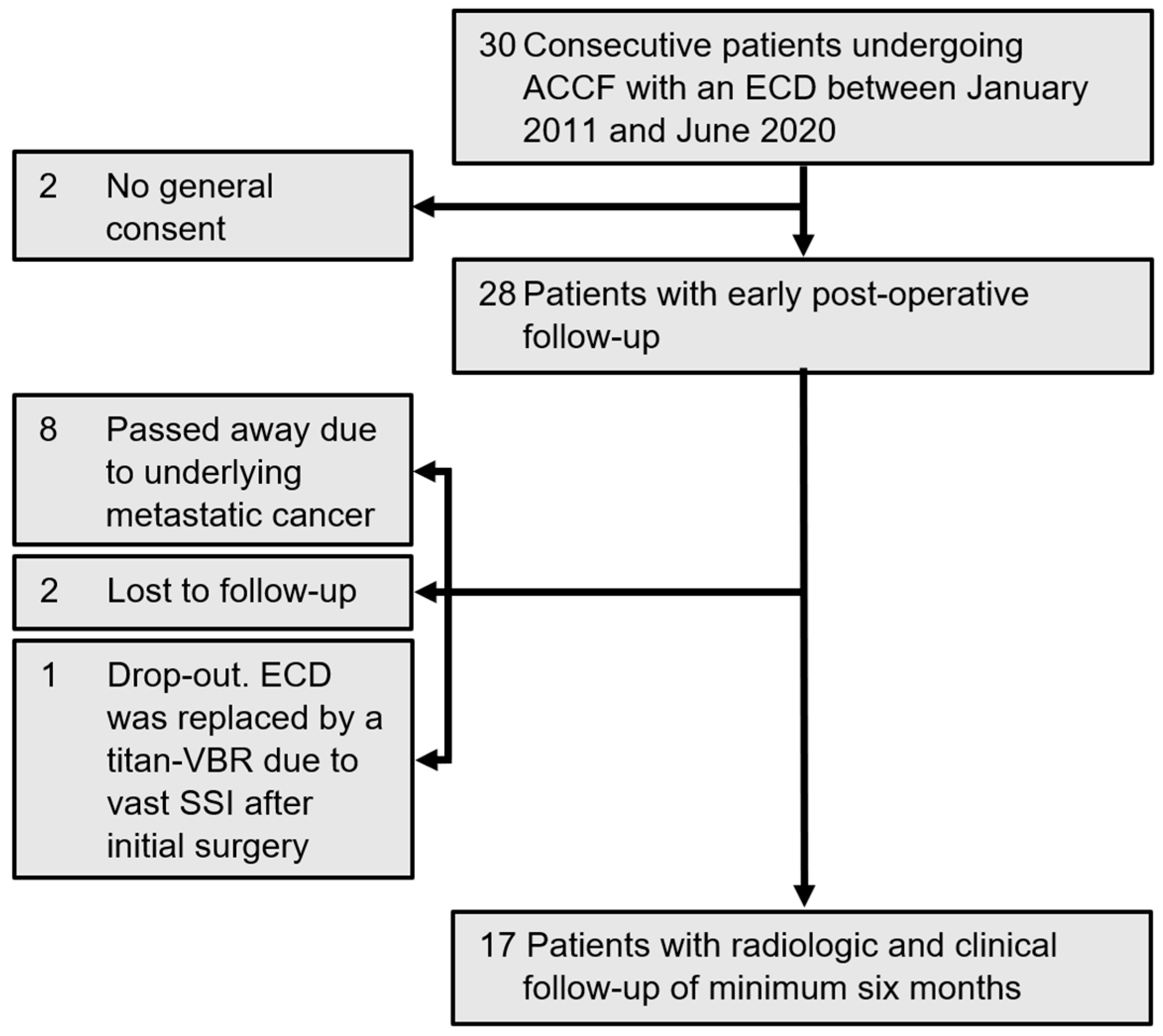
2. Materials and Methods
2.1. Study Population
2.2. Surgical Technique
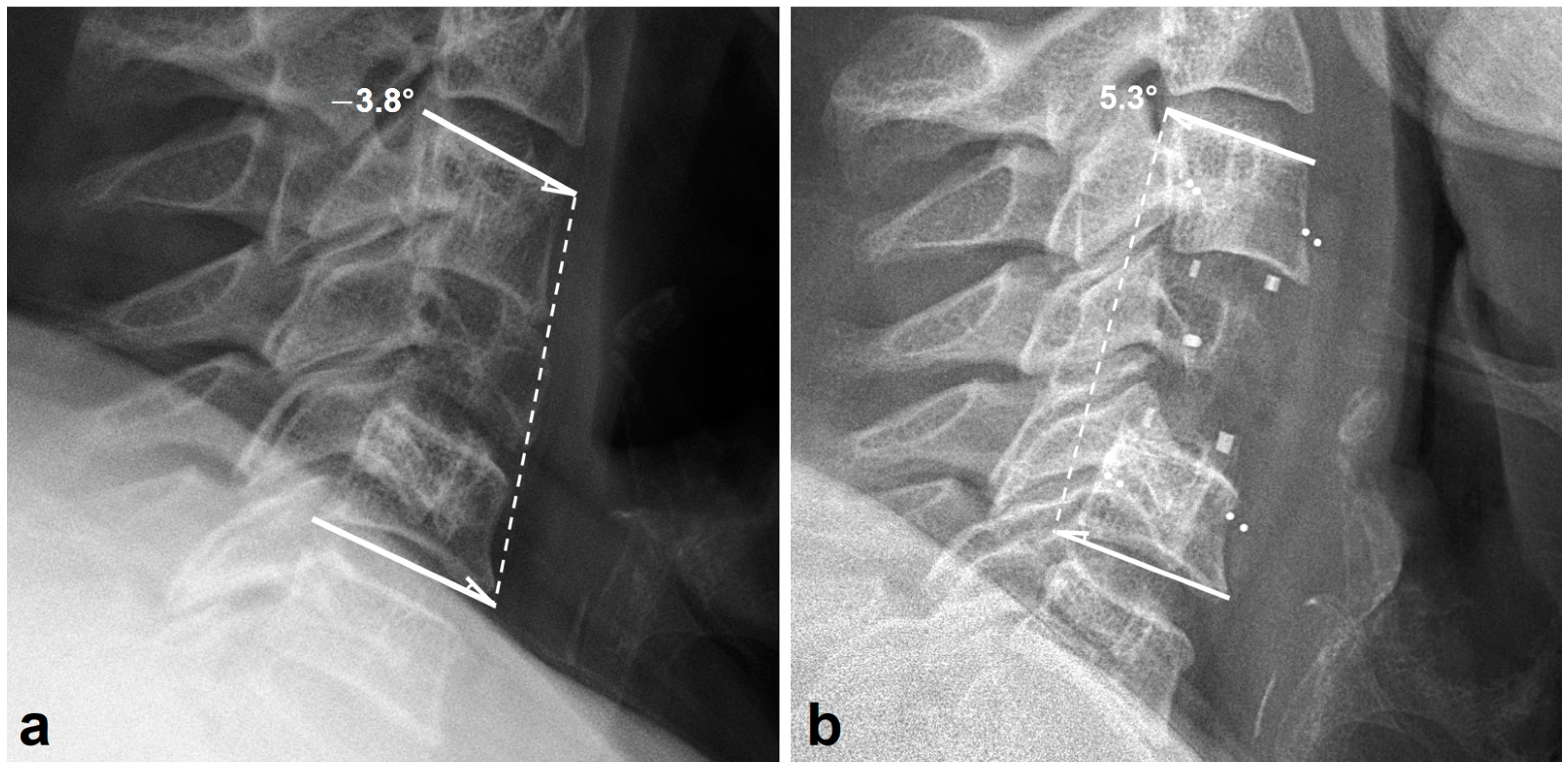
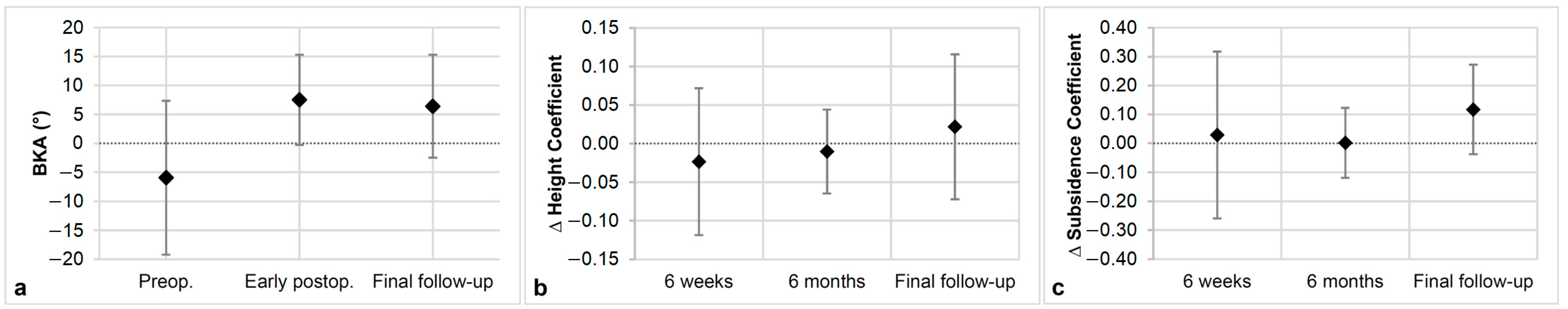
2.3. Radiologic Outcome
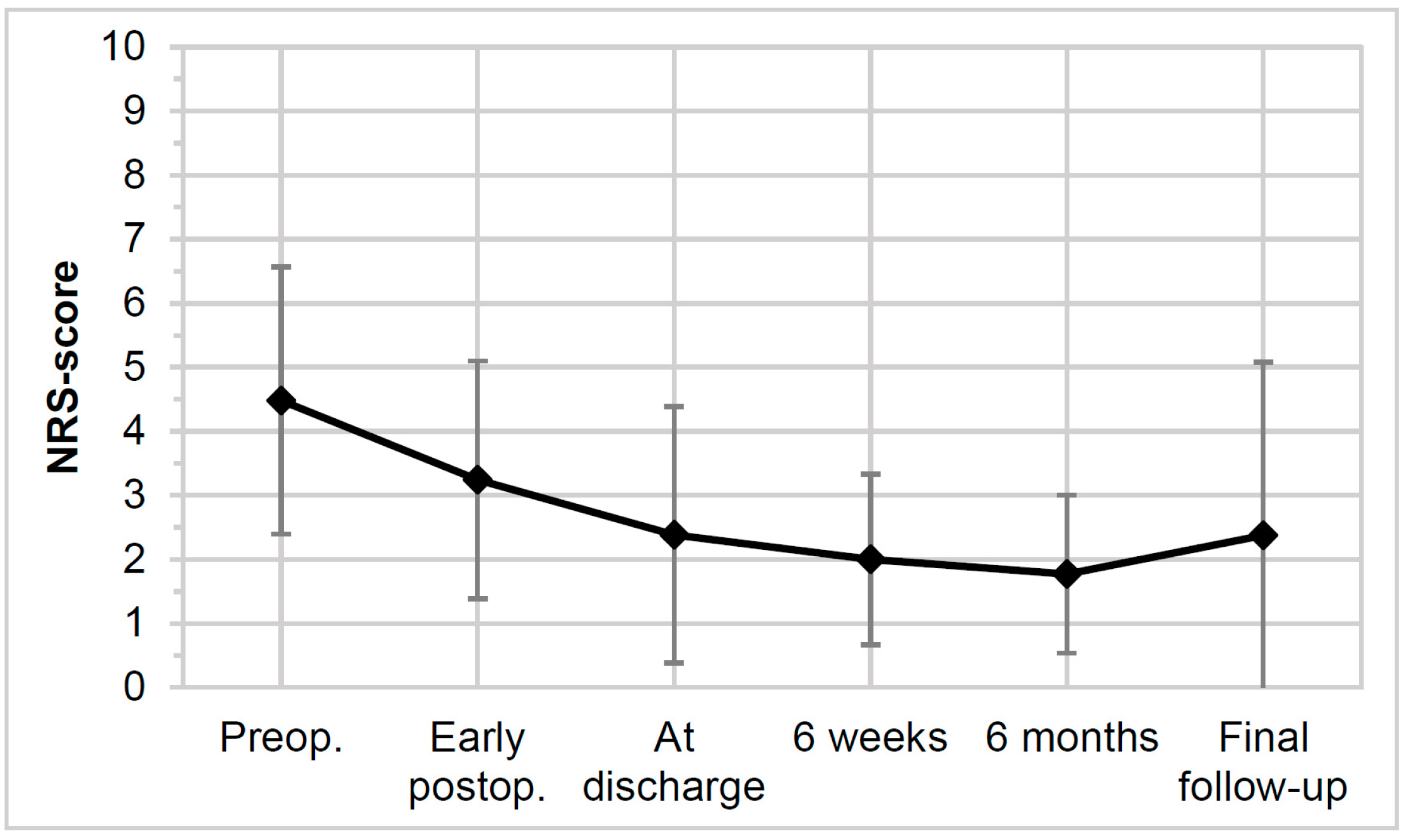
2.4. Clinical Outcome
2.5. Complication Assessment
2.6. Statistical Analysis
3. Results
3.1. Surgical Details
3.2. Radiologic Outcomes
3.3. Clinical Outcomes
3.4. Complications
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denis, F. The Three Column Spine and Its Significance in the Classification of Acute Thoracolumbar Spinal Injuries. Spine 1983, 8, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.W.; Robinson, R.A. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J. Bone Jt. Surg. Am. 1958, 40, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fei, Q.; Li, J.; Su, N.; Wang, B.; Li, D.; Meng, H.; Wang, Q.; Lin, J.; Ma, Z. Comparison between anterior cervical discectomy with fusion and anterior cervical corpectomy with fusion for the treatment of cervical spondylotic myelopathy: A meta-analysis. Ther. Clin. Risk Manag. 2015, 11, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Mauer, U.M.; Mathieu, R. Implantatassoziierte Komplikationen sowie klinischer und radiologischer Verlauf nach anteriorer zervikaler Korpektomie und Cage-Fusion—Retrospektiver Vergleich von PEEK-gegen Titan-Cages. Z. Orthop. Unfall. 2017, 155, 201–208. [Google Scholar] [CrossRef]
- Lu, D.C.; Wang, V.; Chou, D. The use of allograft or autograft and expandable titanium cages for the treatment of vertebral osteomyelitis. Neurosurgery 2009, 64, 122–129, discussion 129–130. [Google Scholar] [CrossRef] [PubMed]
- Elder, B.D.; Lo, S.-F.; Kosztowski, T.A.; Goodwin, C.R.; Lina, I.A.; Locke, J.E.; Witham, T.F. A systematic review of the use of expandable cages in the cervical spine. Neurosurg. Rev. 2016, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dorai, Z.; Morgan, H.; Coimbra, C. Titanium cage reconstruction after cervical corpectomy. J. Neurosurg. Spine 2003, 99, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Sénégas, J. Mechanical supplementation by non-rigid fixation in degenerative intervertebral lumbar segments: The Wallis system. Eur. Spine J. 2002, 11 (Suppl. S2), S164–S169. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.; Macnair, R.; MacDonald, C.; Wykman, A.; Goldie, I.; Grant, M. In vitro biocompatibility testing of polymers for orthopaedic implants using cultured fibroblasts and osteoblasts. Biomaterials 1995, 16, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Wenz, L.M.; Merritt, K.; Brown, S.A.; Moet, A.; Steffee, A.D. In vitro biocompatibility of polyetheretherketone and polysulfone composites. J. Biomed. Mater. Res. 1990, 24, 207–215. [Google Scholar] [CrossRef]
- Brandao, R.C.S.; Martins, W.d.S.; Arantes, A.; Gusmão, S.S.; Perrin, G.; Barrey, C. Titanium versus polyetheretherketone implants for vertebral body replacement in the treatment of 77 thoracolumbar spinal fractures. Surg. Neurol. Int. 2017, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Vadapalli, S.; Sairyo, K.; Goel, V.K.; Robon, M.; Biyani, A.; Khandha, A.; Ebraheim, N.A. Biomechanical Rationale for Using Polyetheretherketone (PEEK) Spacers for Lumbar Interbody Fusion—A Finite Element Study. Spine 2006, 31, E992–E998. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.H.; Gasbarrini, A.M.; Lui, D.F.; Reynolds, J.; Capua, J.; Boriani, S.M. Integrated Custom Composite Polyetheretherketone/Carbon fiber (PEEK/CF) Vertebral Body Replacement (VBR) in the Treatment of Bone Tumors of the Spine. Spine 2022, 47, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Hee, H.T.; Kundnani, V. Rationale for use of polyetheretherketone polymer interbody cage device in cervical spine surgery. Spine J. 2010, 10, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Raslan, F.; Koehler, S.; Berg, F.; Rueckriegel, S.; Ernestus, R.-I.; Meinhardt, M.; Westermaier, T. Vertebral body replacement with PEEK-cages after anterior corpectomy in multilevel cervical spinal stenosis: A clinical and radiological evaluation. Arch. Orthop. Trauma Surg. 2014, 134, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Synthes GmbH. ECD—Expandable Corpectomy Device: Surgical Technique. 2017. Available online: http://synthes.vo.llnwd.net/o16/LLNWMB8/INT%20Mobile/Synthes%20International/Product%20Support%20Material/legacy_Synthes_PDF/DSEM-SPN-1215-0389-1_LR.pdf (accessed on 15 October 2021).
- Das, A.K.; Purohit, D.K.; Gupta, A.; Kataria, R. Comparison of Radiological and Clinical Outcomes between Expandable and Non-expandable Cages Following Cervical Corpectomy: A Systematic Review and Meta-Analysis. Asian Spine J. 2023, 17, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Brenke, C.; Fischer, S.; Carolus, A.; Schmieder, K.; Ening, G. Complications associated with cervical vertebral body replacement with expandable titanium cages. J. Clin. Neurosci. 2016, 32, 35–40. [Google Scholar] [CrossRef]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef] [PubMed]
- Deml, M.C.; Sepulveda, C.A.M.; Albers, C.E.; Hoppe, S.; Bigdon, S.F.; Häckel, S.; Milavec, H.; Benneker, L.M. Anterior column reconstruction of the thoracolumbar spine with a new modular PEEK vertebral body replacement device: Retrospective clinical and radiologic cohort analysis of 48 cases with 1.7-years follow-up. Eur. Spine J. 2020, 29, 3194–3202. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Harrison, D.D.; Cailliet, R.; Troyanovich, S.J.; Janik, T.J.; Holland, B. Cobb method or harrison posterior tangent method: Which to choose for lateral cervical radiographic analysis. Spine 2000, 25, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Schnake, K.J.; Stavridis, S.I.; Kandziora, F. Five-year clinical and radiological results of combined anteroposterior stabilization of thoracolumbar fractures. J. Neurosurg. Spine 2014, 20, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Eck, K.R.; Lenke, L.G.; Bridwell, K.H.; Gilula, L.A.; Lashgari, C.J.; Riew, K.D. Radiographic Assessment of Anterior Titanium Mesh Cages. J. Spinal Disord. 2000, 13, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Odom, G.L.; Finney, W.; Woodhall, B. Cervical Disk Lesions. J. Am. Med. Assoc. 1958, 166, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kirshblum, S.; Snider, B.; Rupp, R.; Read, M.S. Updates of the International Standards for Neurologic Classification of Spinal Cord Injury: 2015 and 2019. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Karnofsky, D.A.; Abelmann, W.H.; Craver, L.F.; Burchenal, J.H. The use of the nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma. Cancer 1948, 1, 634–656. [Google Scholar] [CrossRef]
- Guo, G.-M.; Li, J.; Diao, Q.-X.; Zhu, T.-H.; Song, Z.-X.; Guo, Y.-Y.; Gao, Y.-Z. Cervical lordosis in asymptomatic individuals: A meta-analysis. J. Orthop. Surg. Res. 2018, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, X.; Jiang, D.; Luo, X.; Tang, K.; Zhao, Z.; Zhong, W.; Lei, T.; Quan, Z. Long-term results of anterior cervical corpectomy and fusion with nano-hydroxyapatite/polyamide 66 strut for cervical spondylotic myelopathy. Sci. Rep. 2016, 6, 26751. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Yu, S.; Yan, N.; Wang, J.; Hou, F.; Hou, T.; Cai, W. Risk factors for subsidence of titanium mesh cage following single-level anterior cervical corpectomy and fusion. BMC Musculoskelet. Disord. 2020, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Tatter, C.; Persson, O.; Burström, G.; Edström, E.; Elmi-Terander, A. Anterior Cervical Corpectomy and Fusion for Degenerative and Traumatic Spine Disorders, Single-Center Experience of a Case Series of 119 Patients. Oper. Neurosurg. 2020, 20, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Luo, D.; Ye, X.; Luo, X.; Yan, L.; Qian, H. Anatomy-related risk factors for the subsidence of titanium mesh cage in cervical reconstruction after one-level corpectomy. Int. J. Clin. Exp. Med. 2015, 8, 7405–7411. [Google Scholar] [PubMed]
- Hasegawa, K.; Abe, M.; Washio, T.; Hara, T. An experimental study on the interface strength between titanium mesh cage and vertebra in reference to vertebral bone mineral density. Spine 2001, 26, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Fehlings, M.G.; Tator, C.H.; Lozano, A.; Fleming, J.R.; Gentili, F.; Bernstein, M.; Wallace, M.C.; Tasker, R.R. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J. Neurosurg. 1997, 86, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Rieger, A.; Holz, C.; Marx, T.; Sanchin, L.; Menzel, M. Vertebral autograft used as bone transplant for anterior cervical corpectomy: Technical note. Neurosurgery 2003, 52, 449–454, discussion 453–454. [Google Scholar] [CrossRef] [PubMed]
- Rothrock, R.J.; Barzilai, O.; Reiner, A.S.; Lis, E.; Schmitt, A.M.; Higginson, D.S.; Yamada, Y.; Bilsky, M.H.; Laufer, I. Survival Trends After Surgery for Spinal Metastatic Tumors: 20-Year Cancer Center Experience. Neurosurgery 2020, 88, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Nater, A.; Tetreault, L.; Kopjar, B.; Arnold, P.; Dekutoski, M.; Finkelstein, J.; Fisher, C.; France, J.; Gokaslan, Z.; et al. Survival and Clinical Outcomes in Surgically Treated Patients With Metastatic Epidural Spinal Cord Compression: Results of the Prospective Multicenter AOSpine Study. J. Clin. Oncol. 2016, 34, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Krätzig, T.; Mende, K.C.; Mohme, M.; Kniep, H.; Dreimann, M.; Stangenberg, M.; Westphal, M.; Gauer, T.; Eicker, S.O. Carbon fiber–reinforced PEEK versus titanium implants: An in vitro comparison of susceptibility artifacts in CT and MR imaging. Neurosurg. Rev. 2021, 44, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Kandziora, F.; Pflugmacher, R.; Schaefer, J.; Scholz, M.; Ludwig, K.; Schleicher, P.; Haas, N.P. Biomechanical comparison of expandable cages for vertebral body replacement in the cervical spine. J. Neurosurg. Spine 2003, 99, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tian, X.-M.; Liu, S.-K.; Wang, H.; Zhang, Y.-Z.; Ding, W.-Y. Prevalence of complications after surgery in treatment for cervical compressive myelopathy. Medicine 2017, 96, e6421. [Google Scholar] [CrossRef] [PubMed]
- Fogel, G.R.; Toohey, J.S.; Neidre, A.; Brantigan, J.W. Fusion assessment of posterior lumbar interbody fusion using radiolucent cages: X-ray films and helical computed tomography scans compared with surgical exploration of fusion. Spine J. 2008, 8, 570–577. [Google Scholar] [CrossRef] [PubMed]





| Parameter | n | % of n = 28 | |
|---|---|---|---|
| Population | Female | 18 | 64.3 |
| Male | 10 | 35.7 | |
| Age | 60.7 ± 13 [20–84] * | ||
| BMI | 26.3 ± 5.4 [17.1–40.3] ** | ||
| Indication for surgery | Neoplasm | 19 | 67.9 |
| Degenerative | 5 | 17.9 | |
| Traumatic | 4 | 14.3 | |
| Presenting symptoms | Neck pain | 28 | 100 |
| Cervical radiculopathy | 18 | 64.3 | |
| Somatosensory disorder | 11 | 39.3 | |
| Reduced motor function | 11 | 39.3 | |
| Myelopathy | 5 | 17.9 | |
| Radiculopathy and myelopathy | 4 | 14.3 | |
| Radiculopathy with somatosensory disorder and motor deficits | 9 | 32.1 |
| Parameter | n, or Unit (x) | % of n = 28 | |
|---|---|---|---|
| General surgery information | Mean duration, SD, [range] (min) | 136 ± 54 [50–270] | - |
| Mean blood loss, SD, [range] (mL) | 451 ± 420 [40–1700] | - | |
| Single-stage procedures | 16 | 57.1 | |
| Two-stage procedures | 11 | 39.3 | |
| Three-stage procedures | 1 | 3.6 | |
| Number of vertebral bodies replaced | One | 20 | 71.4 |
| Two adjacent | 7 | 25.0 | |
| Three adjacent | 1 | 3.1 | |
| Anterior fusion osteosynthesis | Two segments | 14 | 50 |
| Three segments | 8 | 28.6 | |
| Four segments | 5 | 17.9 | |
| Six segments | 1 | 3.6 | |
| Posterior spondylodesis at initial surgery | Number of patients | 12 | 42.9 |
| Two segments | 1 | 3.6 | |
| Three segments | 1 | 3.6 | |
| Four and more segments | 10 | 35.7 |
| Preop. | Early Postop. | 6 Months | Final Follow-Up | ||
|---|---|---|---|---|---|
| ASIA Impairment Scale (in %) | Grade E | 59.3 | 71.4 | 78.6 | 93.8 |
| Grade D | 29.6 | 21.4 | 21.4 | 6.3 | |
| Grade C | 7.4 | 3.6 | 0 | 0 | |
| Grade B | 0 | 0 | 0 | 0 | |
| Grade A | 3.7 | 3.6 | 0 | 0 | |
| Odom’s criteria (in %) | Excellent | - | 10.7 | 28.6 | 50.0 |
| Good | - | 57.1 | 50.0 | 37.5 | |
| Fair | - | 25.0 | 21.4 | 12.5 | |
| Poor | - | 7.1 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štefanides, M.; Oswald, K.A.C.; Luyet, A.K.; Albers, C.E.; Benneker, L.M.; Deml, M.C. Anterior Cervical and Upper Thoracic Column Reconstruction Using an Expandable Poly-Ether-Ether-Ketone Vertebral Body Replacement: A Retrospective Single Center Cohort Analysis. Surg. Tech. Dev. 2024, 13, 107-121. https://doi.org/10.3390/std13020008
Štefanides M, Oswald KAC, Luyet AK, Albers CE, Benneker LM, Deml MC. Anterior Cervical and Upper Thoracic Column Reconstruction Using an Expandable Poly-Ether-Ether-Ketone Vertebral Body Replacement: A Retrospective Single Center Cohort Analysis. Surgical Techniques Development. 2024; 13(2):107-121. https://doi.org/10.3390/std13020008
Chicago/Turabian StyleŠtefanides, Martin, Katharina A. C. Oswald, Anaïs K. Luyet, Christoph E. Albers, Lorin M. Benneker, and Moritz C. Deml. 2024. "Anterior Cervical and Upper Thoracic Column Reconstruction Using an Expandable Poly-Ether-Ether-Ketone Vertebral Body Replacement: A Retrospective Single Center Cohort Analysis" Surgical Techniques Development 13, no. 2: 107-121. https://doi.org/10.3390/std13020008





