Abstract
(1) Background: The aim of this study was to investigate potential translational factors for optical 3D reconstruction in an in vivo setting using a newly developed computerized bowel length measurement system (BMS) as a real-time application. (2) Methods: The BMS was evaluated in an in vivo porcine experiment for the influence of light source power (Watt), laparoscope-to-bowel distance (cm), bowel rotation, image background, and surgical objects in the image. Endpoints were robustness, calculated as success rate (SR) in percent, and accuracy, defined as relative error (RE) in percent of BMS measurement result to ground truth. (3) Results: A total of 1992 bowel measurements were performed on n = 7 pigs using the BMS. Bowel measurements were robust and accurate regardless of light source power, at a laparoscope-to-bowel distance of 5 cm (SR 100%, RE 18 ± 38.5%), when the small bowel was aligned horizontally (SR 100%, RE 7.3 ± 36.2%) or in the image background (SR 100%, RE 15.2 ± 23.4%), and when no additional instruments were in the image. (4) Conclusions: Applications based on optical 3D reconstruction are feasible for intraoperative use and could enable quantitative laparoscopy.
1. Introduction
Laparoscopy has revolutionized surgery and has become the standard technique for many surgical procedures. While laparoscopy offers numerous benefits to the patient, such as less trauma, less pain, shorter hospital stay, and faster recovery [1,2,3], it comes at the cost of several technical drawbacks for the surgeon. The main drawbacks are a limited field of view, a limited range of motion, poor ergonomics, and a loss of depth perception [4]. To overcome the latter, 3D laparoscopy, i.e., stereoscopy, has been introduced. This has been shown to reduce procedure time and decrease performance errors when performing laparoscopic tasks in a simulated environment [5].
In the clinical setting multiple studies have been conducted by different surgical disciplines comparing 3D laparoscopy with conventional 2D laparoscopy. Although 3D laparoscopy did not always result in shorter operative times [6,7,8,9], it demonstrated high feasibility and facilitation of particularly complex procedures [7,10,11,12] with similar or lower complication rates and comparable clinical outcomes [13,14,15]. As 3D laparoscopy is increasingly used in clinical practice, researchers have suggested using 3D laparoscopy to improve the visualization of the surgical site through vision-based techniques such as optical 3D reconstruction [16,17]. Here, a real-time 3D model of the surgical site is created from stereoscopic images of the 3D laparoscope. This 3D model can be used, for example, to provide intraoperative information by augmenting the laparoscopic view with preoperative imaging [18]. To date, different techniques for optical 3D reconstruction have been validated for accuracy and robustness in an in vitro study [19] and for accuracy in an in vivo porcine study for open surgery [20]. As a real-time application for laparoscopy based on optical 3D reconstruction, a computer-assisted bowel length measurement system (BMS) has recently been developed and validated in a clinical feasibility study by our group [21]. The aim of this study was to investigate potential translational factors for optical 3D reconstruction in an in vivo setting with BMS as a real-time application.
2. Materials and Methods
2.1. Bowel Length Measurement System (BMS)
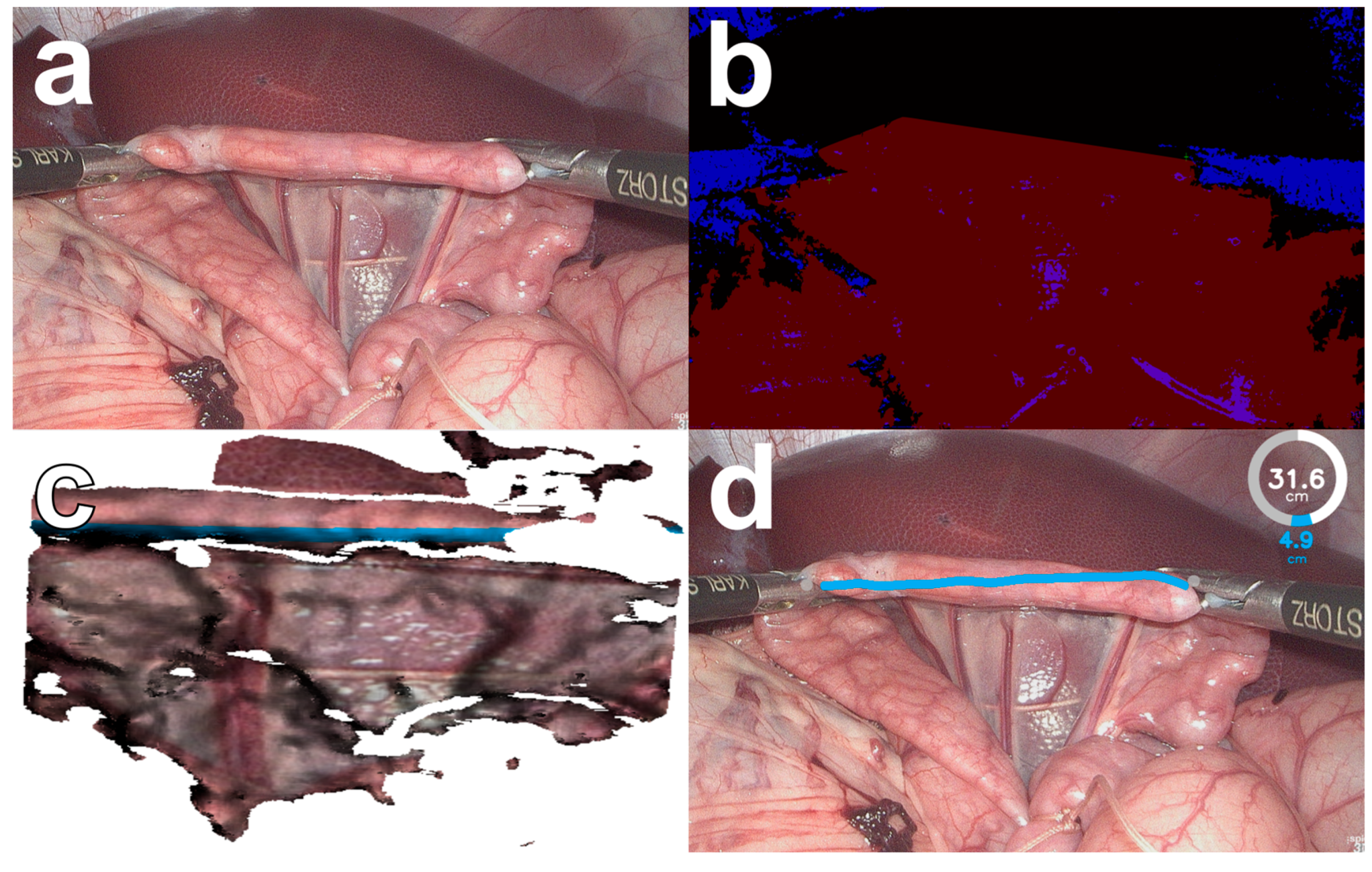
The BMS has recently been developed for computer-assisted laparoscopic bowel length measurement in close collaboration between computer scientists and surgeons for rapid clinical translation [22], leading to a successful first-in-human study [21]. In addition, the BMS has been compared with conventional methods of bowel measurement in a laboratory setting [23]. It is a software add-on for conventional 3D laparoscopes that requires a personal computer, a monitor, a USB foot switch, and standard laparoscopic instruments. When measuring the bowel with the BMS, the surgeon holds the bowel in the field of view of the 3D laparoscope and gives measurement commands by pressing the left pedal of a USB foot switch. Using image classification and optical 3D reconstruction, the BMS creates a virtual bowel model and measures the shortest distance along the virtual bowel surface between the two laparoscopic instruments (Figure 1). The measurement result is then displayed to the surgeon on a separate screen as augmented reality with a graphical user interface [24]. If the surgeon is not satisfied with a measurement, the last measurement can be deleted by pressing the right pedal of the USB foot switch.
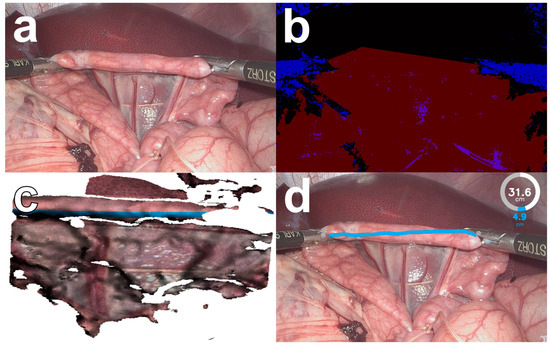
Figure 1.
The bowel length measurement system (BMS, version 2) in an in vivo porcine setting. Images from a 3D laparoscope (a) are pre-processed and segmented by the computer vision algorithm of the BMS (b) into instruments (blue) and bowel (red). A virtual bowel model is created and the shortest distance along the virtual bowel surface is measured (c). The calculated bowel length measurement is displayed to the surgeon in augmented reality (d).
2.2. Study Design
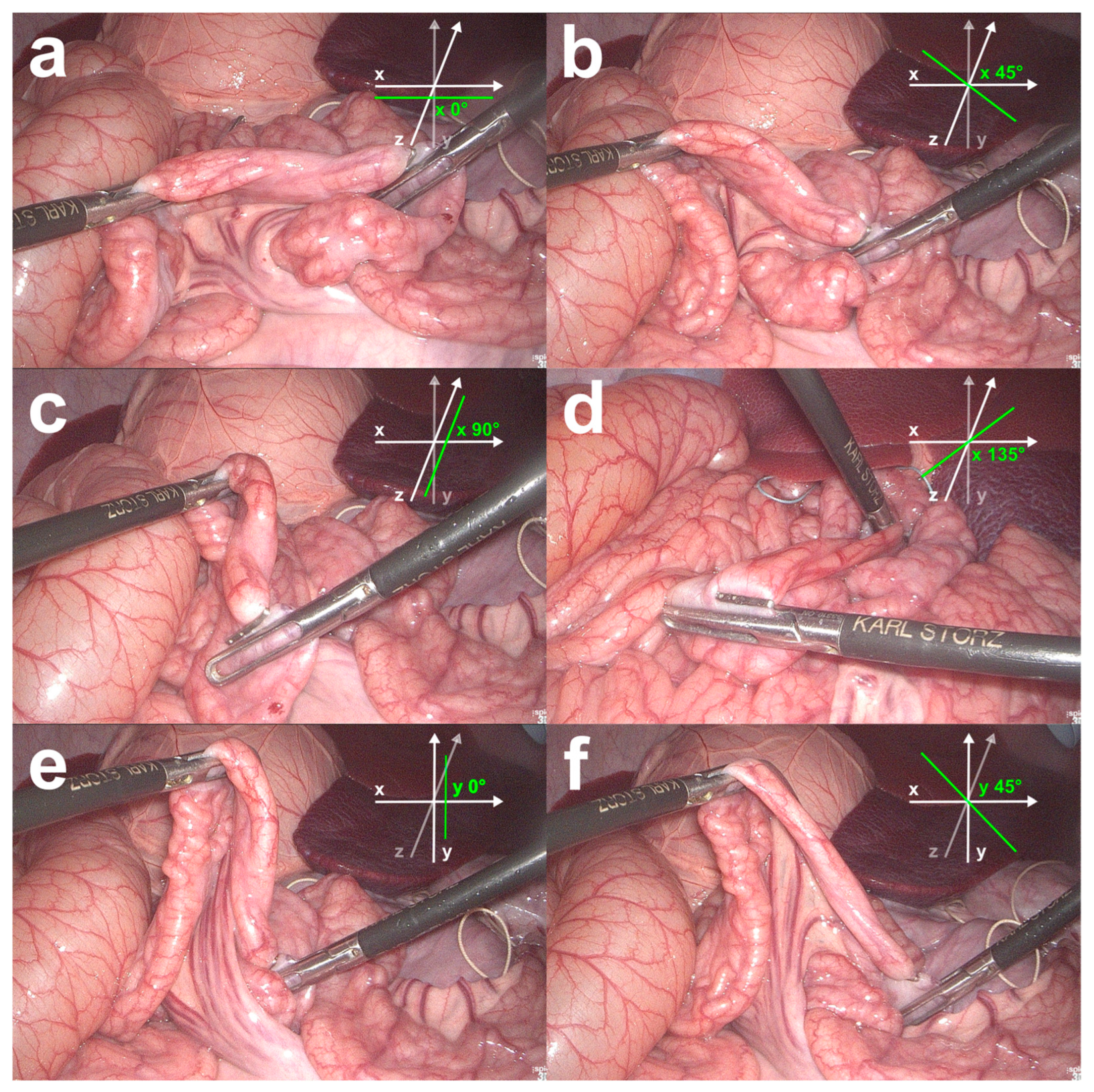
Five influencing factors, namely, (1) light source power in Watts, (2) laparoscope-to-bowel distance in cm, (3) bowel rotation in the image (Figure 2), (4) image background, and (5) surgical objects, were tested during bowel length measurement with the BMS. The influencing factors light source power, laparoscope-to-bowel distance, bowel rotation in the image, and image background were chosen because their influence on the optical surface reconstruction has been demonstrated in an in vitro setting [19]. Surgical objects in the image were chosen as an influencing factor due to experiences with the BMS in a clinical setting, where additional instruments in the image led to failed bowel length measurements [21].
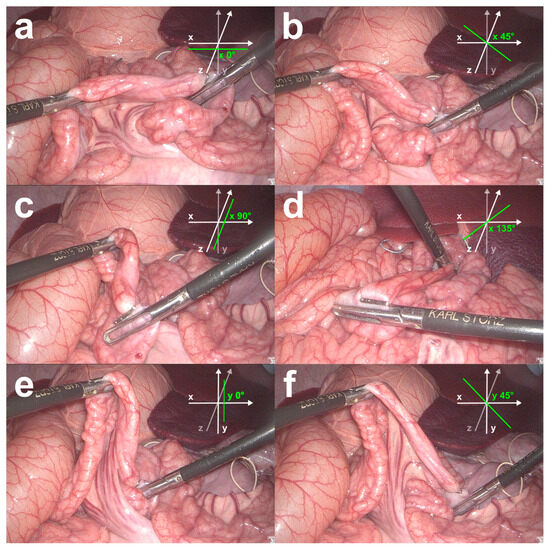
Figure 2.
Bowel rotations investigated in the in vivo validation study. The bowel was measured when aligned horizontally along the x-axis (a), and when rotated horizontally in the xz-plane and measured at 45° (b), 90° (c), and 135° (d) angles between the bowel- and the x-axis. The bowel was also measured along the y-axis (e) and when rotated vertically within the xy-plane and measured at a 45° angle between the bowel- and the y-axis (f).
Light source power was tested as a measure of light intensity in 30 Watt increments from 30 to 300 Watts as adjusted on a xenon light source (300 Watt Xenon SCB, Karl Storz GmbH & Co. KG, Tuttlingen, Germany). The distance to the bowel was measured in 2 cm increments from 3 cm to 11 cm distance. To determine the distance to the bowel, the position of the laparoscope shaft was marked with a tape at the level of the trocar entrance at 0 cm distance. The laparoscope was then retracted according to the increment that had to be measured. Bowel rotation in the image was tested in six different positions. The bowel was first measured horizontally in alignment with the x-axis (x 0°). Then, it was rotated horizontally in the xz-plane and measured at an angle of 45°, 90°, and 135° between the bowel- and the x-axis (x 45°, x 90°, and x 135°). In addition, the bowel was measured vertically along the y-axis (y 0°), rotated vertically within the xy-plane, and measured at a 45° angle between the bowel- and the y-axis (y 45°) (Figure 2). Factors involving the image background were tested with the liver, colon, and small bowel in the background. The influence of surgical objects was tested with five different objects in the image that are likely to be used during laparoscopic bowel length measurement. These included clips in the background of the image, Overholts instead of bowel graspers as laparoscopic instruments for bowel length measurement, an additional grasper in the background of the image or on the bowel, a trocar in the background of the image, and a compress in the image. In order to compare the measurement results for the influence factor surgical objects, a control group was subsequently formed that included the measurement results for the influence factor light source power at 120 Watts.
The default setting, when the influence factor was not tested, was a light source power of 120 Watts, a laparoscope-to-bowel distance of 5 cm, the bowel held at a 0° position to the x- and y-axes, the small bowel as the image background, and no additional surgical objects in the image. For each value of an influencing factor, two surgeons measured one bowel segment ten times with the BMS in each pig. This was repeated for n = 7 pigs as described in the following experimental setup.
2.3. Experimental Setup
The BMS was evaluated in a clinically realistic setting with a standardized setup and medical staff operating the equipment. The experiment was performed using a 3D TIPCAM®1 HD laparoscope (Karl Storz GmbH & Co. KG, Tuttlingen, Germany) with a 30° optical system and a chip-on-tip design with infinite focus. A universal calibration file was generated for the 3D laparoscope which was used in all pigs for the rectification and 3D triangulation of the acquired images. In total, 58 images of in vivo porcine bowel measurements were manually segmented for bowel, background, and laparoscopic instruments to train the image classification algorithm of the BMS (Figure 1b). The surgeons used passive polarized glasses to view the laparoscopic images on a 32″ medical 3D full HD LCD monitor (EJ-MDA32E-K, Panasonic Corporation, Kadoma, Japan). Laparoscopy was performed with a xenon light source (300 Watt Xenon SCB, Karl Storz GmbH & Co. KG, Tuttlingen, Germany). The surgeons used standard bowel graspers (Karl Storz GmbH & Co. KG, Tuttlingen, Germany) for bowel handling. The BMS required a double USB foot switch (USB Foot Switch 2 Double, Scythe Co. Ltd., Tokyo, Japan), a personal computer (Intel Core i7-2700K CPU, GeForce GTX 650Ti GPU and 16GB RAM) with a DVI video capture card (DVI2PCIe Duo capture card, Epiphan Systems Inc., Palo Alto, CA, USA), and a conventional LCD monitor.
This study was the second in vivo study to investigate the BMS because the intraoperative factors affecting the BMS were not known at the time of the first translational study, in which only the feasibility of the BMS was investigated [21]. The staff of the Biomedical Research Facility at the University of Heidelberg provided veterinary handling and care. All animals were treated in accordance with the National Research Council’s criteria for humane care as described in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health [25]. Animals were fasted for 12 h prior to surgery. A modified anesthesia protocol based on Clutton et al. was used [26]. The pigs were kept under general anesthesia with either through intravenous ketamine and midazolam or through inhaled isoflurane (2–3 Vol %) and a mixture of oxygen and nitrogen dioxide (40% oxygen). Ventilation was maintained manually or mechanically (frequency = 12–14/min; volume = 250–320 mL). Abdominal access was created by inserting five trocars in a standardized approach. The ligament of Treitz was identified and the starting point of the first measurement was marked on the small bowel with a suture. All bowel measurements with the BMS were performed under full stretch, as suggested by Tacchino et al. [27]. The start and end points of each measurement were marked with clips at the antimesenteric border of the bowel. For each value of the influence factors, a bowel segment was measured ten times with the BMS. The measured length and the number of successful measurements were recorded in a log file automatically generated by the BMS and stored on the computer. At the end of the experiment, laparotomy was performed to manually measure the ground truth. All distances were measured with umbilical tape along the antimesenteric border of the bowel using full extension [27]. All pigs were euthanized at the end of the procedure with an intravenous injection of a lethal dose of potassium chloride according to protocol.
2.4. Data Analysis
Statistical analysis was performed using SPSS software (v. 28.0.0.0 (190), IBM; Armonk, NY, USA) and Microsoft Excel 2010 (Microsoft Corporation; Redmond, WA, USA). The endpoints were robustness and accuracy. Robustness was calculated as the success rate in percent of the number of successful measurements divided by the number of measurements per bowel segment. Accuracy was defined as the relative error calculated as the percentage of the positive or negative difference between the length in cm measured with the BMS and the ground truth in cm divided by the ground truth in cm. All results were stated as mean values with standard deviation (SD). The results for robustness and accuracy within each value of an influence factor were analyzed using descriptive statistics and compared using nonparametric Friedman and Wilcoxon tests. Statistical significance was defined as p < 0.05.
3. Results
The BMS was evaluated in an in vivo porcine experiment (n = 7) with 275 measurements per pig for the influence of light source power in Watts, laparoscope-to-bowel distance in cm, bowel rotation, image background, and surgical objects in the image (Table 1). The in vivo bowel length measurement with the BMS on the porcine bowel showed a high success rate independent of the light source power. Bowel measurement was not possible with 0 Watt light source power. No statistically significant differences in success rates were found between each 30 Watt light source power increment. Bowel length measurements with the BMS and a laparoscope-to-bowel distance of 5 cm (100 ± 0%) and 7 cm (100 ± 0%) had a higher success rate than with 3 cm (91.7 ± 27.9, p = 0.046) and 9 cm (88.7 ± 31.9, p = 0.008). An increase in relative error was observed with increasing distance from laparoscope to bowel. No statistical analysis was performed for bowel length measurements with a distance of 11 cm from laparoscope to bowel, as this was only feasible in three pigs due to space limitations. Success rates for laparoscopic bowel measurements with the BMS were low when the bowel was measured vertically in the xy-plane (y 0°: 84.4 ± 36.6%; y 45°: 79.7 ± 40.6%) and higher for horizontal measurements in the xz-plane (x 0°: 100 ± 0%, p < 0.002; x 5°: 100 ± 0%, p < 0.034; x 90°: 96.7 ± 20.5%, p < 0.002; x 135°: 93.9 ± 24.2%, p < 0.021). Laparoscopic bowel length measurement with the BMS showed a negative relative error when the bowel was held horizontally and rotated at an angle of 90° (−24 ± 24.3%, p < 0.001) or 135° (−13.1 ± 22.8%, p < 0.001) between the bowel axis and the x-axis. Laparoscopic bowel length measurements with the BMS resulted in higher success rates when the small bowel (100 ± 0%) was in the image background instead of the liver (91.3 ± 28.4%, p = 0.014) (Table 1). With the colon in the image background, the BMS measured bowel lengths with the lowest relative error (10.3 ± 27.2%) compared to the liver (15.6 ± 34%, p = 0.008) or the small bowel (15.2 ± 23.4%, p = 0.008). The relative error was higher for measurements with Overholts (26.3 ± 18.2%) or a trocar (28.6 ± 40%) in the image compared to clips (8.8 ± 42.2%, p < 0.001) and an additional instrument in the background (1.1 ± 46.6, p < 0.001) or on the bowel surface (1.5 ± 35.2, p < 0.001).

Table 1.
Results of in vivo measurements with the bowel length measurement system (BMS).
4. Discussion
4.1. In Vivo Bowel Length Measurement
The BMS measured bowel length with adequate robustness and accuracy in an in vivo setting, independent of the light source power level. Measurements with a light source power of 30 Watts were more accurate than measurements at higher light source power levels; however, the surgical site was not adequately illuminated, and therefore low light source levels do not appear to be feasible for clinical application.
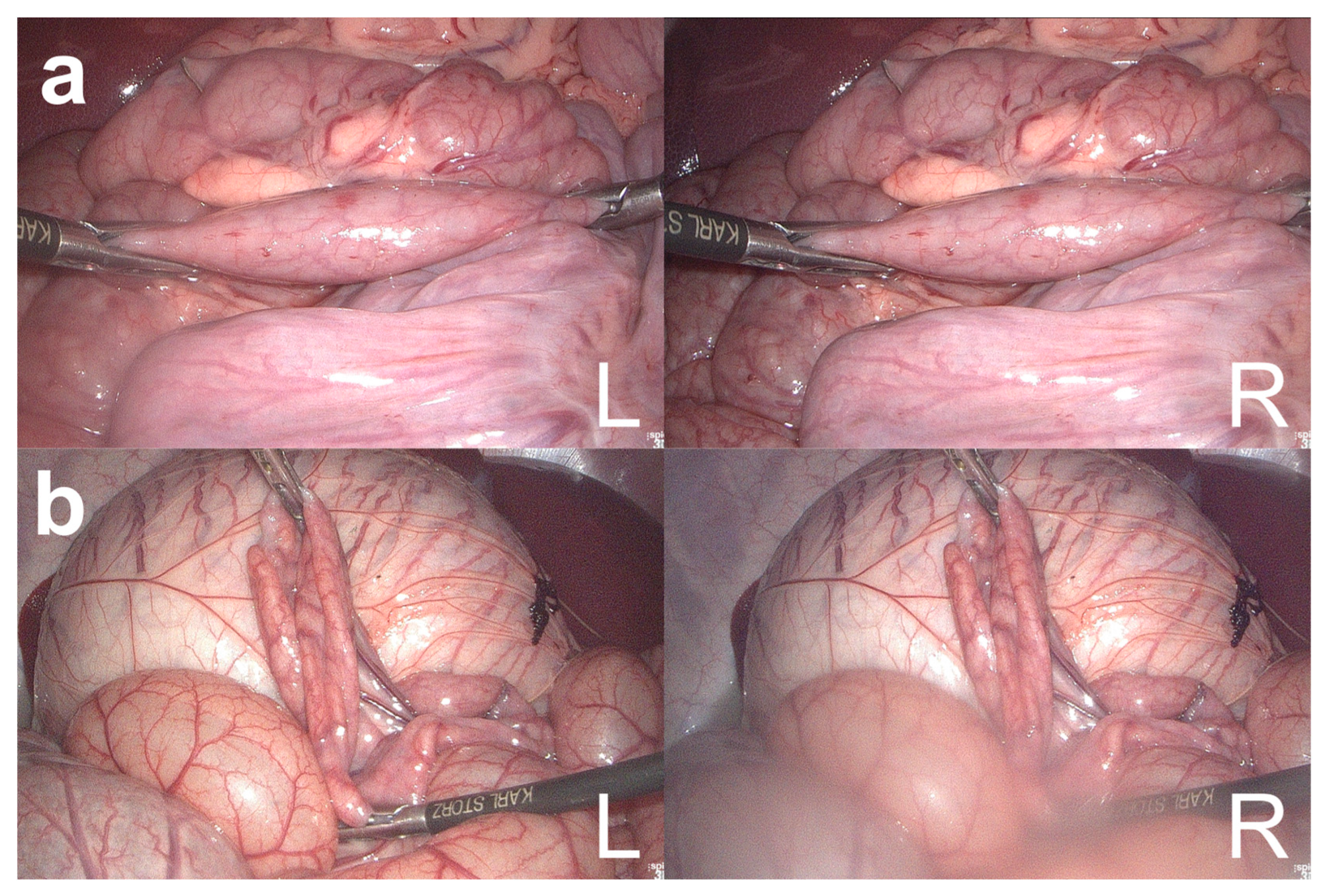
For smaller laparoscope-to-bowel distances, the BMS measured bowel lengths with higher success rates and accuracy. This is consistent with the previous work by Maier-Hein et al., who found a decrease in robustness and accuracy with increasing laparoscope-to-bowel distance [19]. Bowel length measurements at a distance of 3 cm were more accurate but less successful than measurements at a distance of 5 cm. A possible explanation could be that it is difficult to keep both laparoscopic instruments in the image at such a short distance. Therefore, the BMS cannot detect the instrument tips in the image and measure the bowel segment between them (Figure 3a). The robustness and accuracy of bowel length measurements with the BMS could also depend on the light source level and the laparoscope-to-bowel distance used during the creation of the calibration file. The calibration file is used to rectify all captured images from the 3D laparoscope prior to further image processing. In this study, the calibration file was created using images from the 3D laparoscope at a constant light source level and at different distances and angles. Bowel length measurements with the BMS had a higher success rate when the bowel was held horizontally in the xz-plane. It may have been easier to keep both instruments in the image with this bowel orientation than when the bowel was measured in a vertical orientation. The negative relative error for measurements with the bowel held horizontally in the xz-plane at a 90° and 135° to the x-axis could be due to the impaired 3D reconstruction of the bowel segment. Since less bowel surface is visible in this position, a shorter segment is considered in the 3D reconstruction and bowel length is therefore calculated on a shorter virtual bowel surface (Figure 2c,d). Regarding the image background, the BMS was able to measure bowel lengths with increased robustness and accuracy with the colon and small bowel as an image background. In addition, the BMS was able to discriminate well between metallic objects in the image, but had problems performing robust and accurate measurements with non-metallic surgical objects. Both findings can be explained by the fact that the image classification algorithm of the BMS was trained to detect and distinguish between two laparoscopic graspers, the small bowel, and the image background. Therefore, the image classification algorithm failed to discriminate in cases where the image background was unknown and the objects in the image were not visually similar to laparoscopic graspers, such as non-metallic surgical objects. Based on the results of the in vivo validation study, the optimal intraoperative conditions for bowel length measurements with the BMS can be defined:
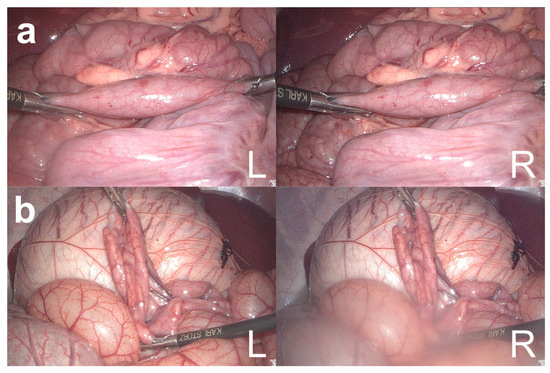
Figure 3.
Failed measurements of the bowel length measurement system (BMS). Failed bowel measurements by BMS because the right laparoscopic grasper could not be detected in the right image (a) and because of a blurred right laparoscope lens (b). L = left image; R = right image.
- Adequate illumination of the surgical site;
- Laparoscope-to-bowel distance of 5 cm;
- Colon or small bowel in the image background;
- Horizontal alignment of the bowel;
- Only two laparoscopic instruments in the image.
To date, translational factors and optimal intraoperative conditions for assistance system based on 3D laparoscopy have not been investigated. However, from our experience at the Heidelberg Center for Obesity and Diabetes, surgeons using the BMS found that the conditions under which the BMS functioned well were adequate for the clinical application of intraoperative laparoscopic bowel length measurement.
4.2. Limitations
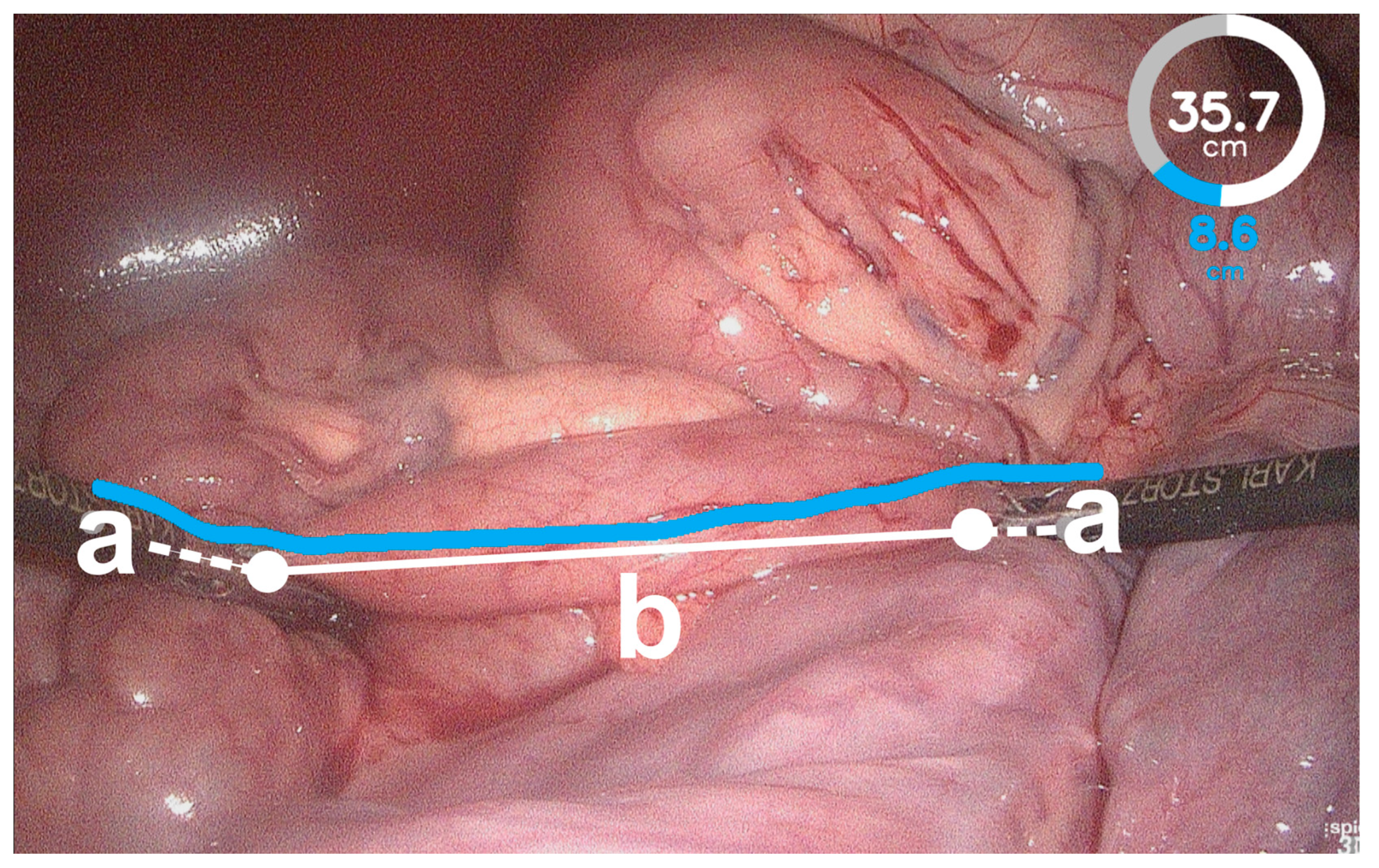
Overall, the BMS showed a tendency to overestimate bowel length. This may be due to impaired instrument detection. If the BMS does not detect the instrument tips on the bowel surface, but further up on the instrument shaft, the length of the instrument shaft is added to the bowel length measurement on the bowel surface, resulting in overestimated bowel lengths (Figure 4). Further training of the image classification algorithm of the BMS would be required to improve the instrument detection and recognition of the relevant bowel segments. The BMS was investigated in an exploratory approach, and therefore the influencing factors were evaluated to the maximum extent to reveal the limitations of the system. Inherently, this resulted in low success rates for some values and a smaller number of results for the accuracy of the BMS available for statistical analysis. Irrespective of the influence factor investigated, the results for bowel length measurement with the BMS varied between pigs. This can be explained by the use of a universal calibration file that is used to rectify all images acquired by the 3D laparoscope for further image processing. Although creating an individual calibration file for each pig would probably have resulted in better rectification of the acquired images, we decided to use a universal calibration file to simulate the conditions in a clinical setting without preoperative preparation.
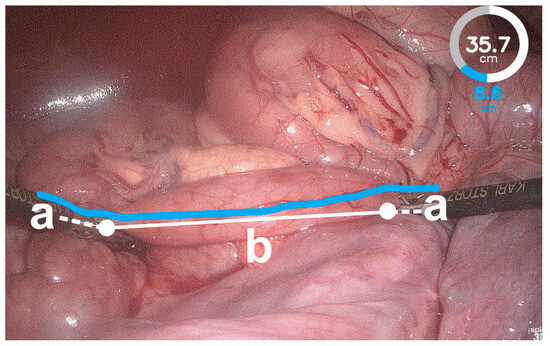
Figure 4.
Failed instrument detection of the bowel length measurement system (BMS). The BMS detected the instrument tips on the instrument shaft (a) and not on the bowel surface (white dots). Therefore, the bowel length measured by the BMS exceeded the actual bowel length (b) because the distances along the instrument shafts were added (white dashed lines).
4.3. Translational Factors
All bowel length measurements with the BMS were subject to conditions inherent to use in an in vivo setting. Conditions that affected all bowel length measurements, regardless of the influence factor studied, were the blurring of the laparoscope lens (Figure 3b), failed measurement when only one instrument was visible in the laparoscopic image (Figure 3a), surgeon shaking while guiding the 3D laparoscope, and organ movement. While the blurring of the lens and the visibility of instruments could be improved by increasing the power of the light source to warm the tip of the laparoscope [28] and optimizing port placement [29], organ movement is inevitable during surgery. Therefore, in our intention to reproduce a realistic clinical setting, we decided not to mount the laparoscope on a fixed holding arm or to stop ventilation during laparoscopic bowel length measurement. Since smoke and blood are not typically present during laparoscopic bowel length measurement, they were not investigated as influence factors. However, in a pilot study, we found that blood and smoke hamper optical 3D reconstruction in a manner similar to blurred laparoscope lenses, which is consistent with the findings of Maier-Hein et al. [19].
4.4. Quantitative Laparoscopy
The majority of clinical applications of real-time optical 3D reconstruction have focused on the mapping of patient anatomy with preoperative imaging via optical 3D reconstruction for the improved visualization of the surgical field [20,30,31,32,33]. Only a few groups have developed applications that use optical 3D reconstruction to add a quantitative function to laparoscopy. In 2002, Yao et al. published a study in which they measured the size of gastric lesions in 50 human patients using a 3D endoscope by manual triangulation compared to conventional radiographic measurements [34]. In 2014, Bernal et al. compared the conventional defect size measurement of ventral hernias by inserting needles or measuring tape with a new measurement using optical 3D reconstruction from images of a 2D laparoscope via a visual simultaneous localization and mapping (Visual SLAM) algorithm [35]. Both studies showed favorable results for the optical 3D reconstruction-based measurement method compared to conventional size measurement methods. However, in both studies, size measurements were performed retrospectively on recorded images, and the relevant corresponding image points had to be manually selected. While this approach yields higher accuracy, it does not consider the influence factors at hand during real-time intraoperative use and does not allow real-time computer-assisted surgery. Here, similar to our approach, the implementation of recent image processing technology and classification algorithms could help to overcome the technical limitations and enable real-time applications.
5. Conclusions
With the increasing use of 3D laparoscopy, applications based on optical 3D reconstruction will gain interest and enable quantitative laparoscopy. By identifying and investigating influence factors on real-time optical 3D reconstruction in an intraoperative setting, we hope to provide insights that will facilitate the clinical translation of future applications. These applications could add functional value to 3D laparoscopy, enabling safer and more accurate computer-assisted surgery.
Author Contributions
Conceptualization, B.F.B.M., H.G.K. and K.F.K.; methodology, B.F.B.M., H.G.K. and K.F.K.; software, S.B. and S.S.; validation, B.F.B.M. and S.B.; data curation, B.F.B.M., S.B., P.M., R.R., L.M.S. and K.F.K.; writing—original draft preparation, B.F.B.M.; writing—review and editing, S.S., H.G.K. and K.F.K.; funding acquisition, S.S. and H.G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Research Foundation (DFG) within the Transregional Collaborative Research Center 125 (SFB 125): “Cognition Guided Surgery” in project A01 by S.S. and H.G.K. B.F.B.M was supported by the MINT-Clinician Scientist program of the Medical Faculty Tübingen, funded by the DFG–493665037.
Institutional Review Board Statement
The study protocol for the in vivo porcine experiment was approved by the local ethics committee in Heidelberg and the regional committee in Karlsruhe, Germany (approval code is 35-9185.81/G-127/11, and approval date in day September 2011). All animals were treated in compliance with the National Research Council’s criteria for humane care as described in the Guide for the Care and Use of Laboratory Animals prepared by the National Institutes of Health.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Simon Mayer, School of Design of Pforzheim University, Germany, for the design of the graphical user interface of the Bowel Length Measurement System.
Conflicts of Interest
B.F.B.M., S.S. and H.G.K. worked with the company Karl Storz in the joint research project “InnOPlan”, funded by the German Federal Ministry of Economic Affairs and Energy (grant number BMWI 01MD15002E). S.B. and S.S. worked with device manufacturer Karl Storz GmbH & Co. KG in the joint research project “OP4.1”, funded by the German Federal Ministry of Economic Affairs and Energy (grant number BMWI 01MT17001C). P.M., R.R., L.M.S. and K.F.K. have no conflicts of interest or financial ties to disclose.
References
- Colon Cancer Laparoscopic or Open Resection Study Group; Buunen, M.; Veldkamp, R.; Hop, W.C.J.; Kuhry, E.; Jeekel, J.; Haglind, E.; Påhlman, L.; Cuesta, M.A.; Msika, S.; et al. Survival after Laparoscopic Surgery versus Open Surgery for Colon Cancer: Long-Term Outcome of a Randomised Clinical Trial. Lancet Oncol. 2009, 10, 44–52. [Google Scholar] [CrossRef]
- Kitano, S.; Shiraishi, N.; Fujii, K.; Yasuda, K.; Inomata, M.; Adachi, Y. A Randomized Controlled Trial Comparing Open vs Laparoscopy-Assisted Distal Gastrectomy for the Treatment of Early Gastric Cancer: An Interim Report. Surgery 2002, 131, S306–S311. [Google Scholar] [CrossRef]
- Keus, F.; de Jong, J.; Gooszen, H.G.; Laarhoven, C.J. Laparoscopic versus Open Cholecystectomy for Patients with Symptomatic Cholecystolithiasis. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; ISBN 1465-1858. [Google Scholar]
- Ballantyne, G.H.M. The Pitfalls of Laparoscopic Surgery: Challenges for Robotics and Telerobotic Surgery. Surg. Laparosc. Endosc. Percutaneous Tech. 2002, 12, 1–5. [Google Scholar] [CrossRef]
- Sørensen, S.M.D.; Savran, M.M.; Konge, L.; Bjerrum, F. Three-Dimensional versus Two-Dimensional Vision in Laparoscopy: A Systematic Review. Surg. Endosc. 2016, 30, 11–23. [Google Scholar] [CrossRef]
- Ajao, M.O.; Larsen, C.R.; Manoucheri, E.; Goggins, E.R.; Rask, M.T.; Cox, M.K.B.; Mushinski, A.; Gu, X.; Cohen, S.L.; Rudnicki, M.; et al. Two-Dimensional (2D) versus Three-Dimensional (3D) Laparoscopy for Vaginal Cuff Closure by Surgeons-in-Training: A Randomized Controlled Trial. Surg. Endosc. 2019, 34, 1237–1243. [Google Scholar] [CrossRef]
- Kinoshita, H.; Nakagawa, K.; Usui, Y.; Iwamura, M.; Ito, A.; Miyajima, A.; Hoshi, A.; Arai, Y.; Baba, S.; Matsuda, T. High-Definition Resolution Three-Dimensional Imaging Systems in Laparoscopic Radical Prostatectomy: Randomized Comparative Study with High-Definition Resolution Two-Dimensional Systems. Surg. Endosc. 2015, 29, 2203–2209. [Google Scholar] [CrossRef]
- Kanaji, S.; Suzuki, S.; Harada, H.; Nishi, M.; Yamamoto, M.; Matsuda, T.; Oshikiri, T.; Nakamura, T.; Fujino, Y.; Tominaga, M.; et al. Comparison of Two- and Three-Dimensional Display for Performance of Laparoscopic Total Gastrectomy for Gastric Cancer. Langenbecks Arch. Surg. 2017, 402, 493–500. [Google Scholar] [CrossRef]
- Bagan, P.; De Dominicis, F.; Hernigou, J.; Dakhil, B.; Zaimi, R.; Pricopi, C.; Le Pimpec Barthes, F.; Berna, P. Complete Thoracoscopic Lobectomy for Cancer: Comparative Study of Three-Dimensional High-Definition with Two-Dimensional High-Definition Video Systems. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 820–824. [Google Scholar] [CrossRef]
- Schwab, K.E.; Curtis, N.J.; Whyte, M.B.; Smith, R.V.; Rockall, T.A.; Ballard, K.; Jourdan, I.C. 3D Laparoscopy Does Not Reduce Operative Duration or Errors in Day-Case Laparoscopic Cholecystectomy: A Randomised Controlled Trial. Surg. Endosc. 2019, 34, 1745–1753. [Google Scholar] [CrossRef]
- Currò, G.; Malfa, G.L.; Caizzone, A.; Rampulla, V.; Navarra, G. Three-Dimensional (3D) Versus Two-Dimensional (2D) Laparoscopic Bariatric Surgery: A Single-Surgeon Prospective Randomized Comparative Study. Obes. Surg. 2015, 25, 2120–2124. [Google Scholar] [CrossRef]
- Kozlov, Y.; Kovalkov, K.; Nowogilov, V. 3D Laparoscopy in Neonates and Infants. J. Laparoendosc. Adv. Surg. Tech. 2016, 26, 1021–1027. [Google Scholar] [CrossRef]
- Lee, K.; Youn, S.I.; Won, Y.; Min, S.-H.; Park, Y.S.; Ahn, S.-H.; Park, D.J.; Kim, H.-H. Prospective Randomized Controlled Study for Comparison of 2-Dimensional versus 3-Dimensional Laparoscopic Distal Gastrectomy for Gastric Adenocarcinoma. Surg. Endosc. 2021, 35, 934–940. [Google Scholar] [CrossRef]
- Zhao, B.; Lv, W.; Mei, D.; Luo, R.; Bao, S.; Huang, B.; Lin, J. Comparison of Short-Term Surgical Outcome between 3D and 2D Laparoscopy Surgery for Gastrointestinal Cancer: A Systematic Review and Meta-Analysis. Langenbecks Arch. Surg. 2020, 405, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hermosa, J.I.; Ranea, A.; Delisau, O.; Planellas-Giné, P.; Cornejo, L.; Pujadas, M.; Codony, C.; Gironès, J.; Codina-Cazador, A. Three-Dimensional (3D) System versus Two-Dimensional (2D) System for Laparoscopic Resection of Adrenal Tumors: A Case-Control Study. Langenbecks Arch. Surg. 2020, 405, 1163–1173. [Google Scholar] [CrossRef]
- Stoyanov, D.; Mylonas, G.P.; Deligianni, F.; Darzi, A.; Yang, G.Z. Soft-Tissue Motion Tracking and Structure Estimation for Robotic Assisted MIS Procedures. Med. Image Comput. Comput. Assist. Interv. 2005, 8, 139–146. [Google Scholar]
- Lin, B.; Sun, Y.; Qian, X.; Goldgof, D.; Gitlin, R.; You, Y. Video-Based 3D Reconstruction, Laparoscope Localization and Deformation Recovery for Abdominal Minimally Invasive Surgery: A Survey. Int. J. Med. Robot. Comput. Assist. Surg. 2016, 12, 158–178. [Google Scholar] [CrossRef]
- Penza, V.; Ortiz, J.; Mattos, L.S.; Forgione, A.; Momi, E.D. Dense Soft Tissue 3D Reconstruction Refined with Super-Pixel Segmentation for Robotic Abdominal Surgery. Int. J. CARS 2016, 11, 197–206. [Google Scholar] [CrossRef]
- Maier-Hein, L.; Groch, A.; Bartoli, A.; Bodenstedt, S.; Boissonnat, G.; Chang, P.L.; Clancy, N.T.; Elson, D.S.; Haase, S.; Heim, E.; et al. Comparative Validation of Single-Shot Optical Techniques for Laparoscopic 3-D Surface Reconstruction. IEEE Trans. Med. Imaging 2014, 33, 1913–1930. [Google Scholar] [CrossRef]
- Kowalczuk, J.; Meyer, A.; Carlson, J.; Psota, E.T.; Buettner, S.; Pérez, L.C.; Farritor, S.M.; Oleynikov, D. Real-Time Three-Dimensional Soft Tissue Reconstruction for Laparoscopic Surgery. Surg. Endosc. 2012, 26, 3413–3417. [Google Scholar] [CrossRef]
- Wagner, M.; Mayer, B.F.B.; Bodenstedt, S.; Stemmer, K.; Fereydooni, A.; Speidel, S.; Dillmann, R.; Nickel, F.; Fischer, L.; Kenngott, H.G. Computer-Assisted 3D Bowel Length Measurement for Quantitative Laparoscopy. Surg. Endosc. 2018, 32, 4052–4061. [Google Scholar] [CrossRef]
- Marcus, H.J.M.; Payne, C.J.; Hughes-Hallett, A.M.; Gras, G.M.; Leibrandt, K.M.; Nandi, D.D.; Yang, G.-Z. Making the Leap: The Translation of Innovative Surgical Devices From the Laboratory to the Operating Room. Ann. Surg. 2016, 263, 1077–1078. [Google Scholar] [CrossRef]
- Wagner, M.; Mayer, B.F.B.; Bodenstedt, S.; Kowalewski, K.F.; Nickel, F.; Speidel, S.; Kenngott, H.G.; Müller-Stich, B.P. Comparison of Conventional Methods for Bowel Length Measurement in Laparoscopic Surgery to a Novel Computer-Assisted 3D Measurement System. Obes. Surg. 2021, 31, 4692–4700. [Google Scholar] [CrossRef]
- Cleary, K.; Peters, T.M. Image-Guided Interventions: Technology Review and Clinical Applications. Annu. Rev. Biomed. Eng. 2010, 12, 119–142. [Google Scholar] [CrossRef]
- National Research Council (US). Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Clutton, R.E.; Blissitt, K.J.; Bradley, A.A.; Camburn, M.A. Comparison of Three Injectable Anaesthetic Techniques in Pigs. Vet. Rec. 1997, 141, 140–146. [Google Scholar] [CrossRef]
- Tacchino, R.M. Bowel Length: Measurement, Predictors, and Impact on Bariatric and Metabolic Surgery. Surg. Obes. Relat. Dis. 2015, 11, 328–334. [Google Scholar] [CrossRef]
- Bessell, J.R.; Flemming, E.; Kunert, W.; Buess, G. Maintenance of Clear Vision during Laparoscopic Surgery. Minim. Invasive Ther. Allied Technol. 1996, 5, 450–455. [Google Scholar] [CrossRef]
- Kunert, W.; Storz, P.; Kirschniak, A. For 3D Laparoscopy: A Step toward Advanced Surgical Navigation: How to Get Maximum Benefit from 3D Vision. Surg. Endosc. 2013, 27, 696–699. [Google Scholar] [CrossRef]
- Stoyanov, D.; Scarzanella, M.V.; Pratt, P.; Yang, G.-Z. Real-Time Stereo Reconstruction in Robotically Assisted Minimally Invasive Surgery. Med. Image Comput. Comput. Assist. Interv. 2010, 13, 275–282. [Google Scholar]
- Maier-Hein, L.; Mountney, P.; Bartoli, A.; Elhawary, H.; Elson, D.; Groch, A.; Kolb, A.; Rodrigues, M.; Sorger, J.; Speidel, S.; et al. Optical Techniques for 3D Surface Reconstruction in Computer-Assisted Laparoscopic Surgery. Med. Image Anal. 2013, 17, 974–996. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Wang, Y.; Wang, H.; Wang, F.; Zeng, X.; Wang, Q.; Egger, J. Development of a Surgical Navigation System Based on Augmented Reality Using an Optical See-through Head-Mounted Display. J. Biomed. Inform. 2015, 55, 124–131. [Google Scholar] [CrossRef]
- Sui, C.; Wu, J.; Wang, Z.; Ma, G.; Liu, Y.-H. A Real-Time 3D Laparoscopic Imaging System: Design, Method, and Validation. IEEE Trans. Biomed. Eng. 2020, 67, 2683–2695. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Matsui, T.; Furukawa, H.; Yao, T.; Sakurai, T.; Mitsuyasu, T. A New Stereoscopic Endoscopy System: Accurate 3-Dimensional Measurement in Vitro and in Vivo with Distortion-Correction Function. Gastrointest. Endosc. 2002, 55, 412–420. [Google Scholar] [CrossRef]
- Bernal, E.; Casado, S.; Grasa, Ó.G.; Montiel, J.M.M.; Gil, I. Computer Vision Distance Measurement from Endoscopic Sequences: Prospective Evaluation in Laparoscopic Ventral Hernia Repair. Surg. Endosc. 2014, 28, 3506–3512. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).