The Navigation Ability Test (NAT 2.0): From Football Player Performance to Balance Rehabilitation in Chronic Unilateral Vestibular Loss
Abstract
:1. Introduction
2. Materials and Methods
- Group 1 (GR 1): 48 patients affected by chronic unilateral vestibular loss, 24 males and 24 females, average age 71 years (range: 58–87). All patients presented dizziness and chronic imbalance and they were diagnosed by means of a video head impulse test (VHIT) in order to assess the amplitude of the vestibulo-oculomotor reflex (VOR) at a high frequency of impulsive stimulus of the head. Relative VOR gain values of the lateral semicircular canal (LSC) were considered. Inclusion criteria of subjects into GR 1 were: unilateral LSC-VOR amplitude less than 0.8 at VHIT; chronic vestibular hypofunction of more than 6 months. Exclusion criteria were: Previous otosurgery, Benign Paroxysmal Positional Vertigo (BPPV), Meniere Disease, neurological diseases, and chronic use of Benzodiazepines and/or Neuroleptics.
- Group 2 (GR 2): 60 healthy patients, not sportsmen, 30 males and 30 females, average age 48 ears (range 24–58). They were all asymptomatic and presented normal LSC-VOR at VHIT.
- Group 3 (GR 3): 60 professional football players (Brescia Football men Division B, Brescia Football Women’s Division B and Cremonese Football men Division under 21), 32 males and 28 females average age of 25 years, (range 16–31). They were all asymptomatic and presented normal LSC-VOR at VHIT.
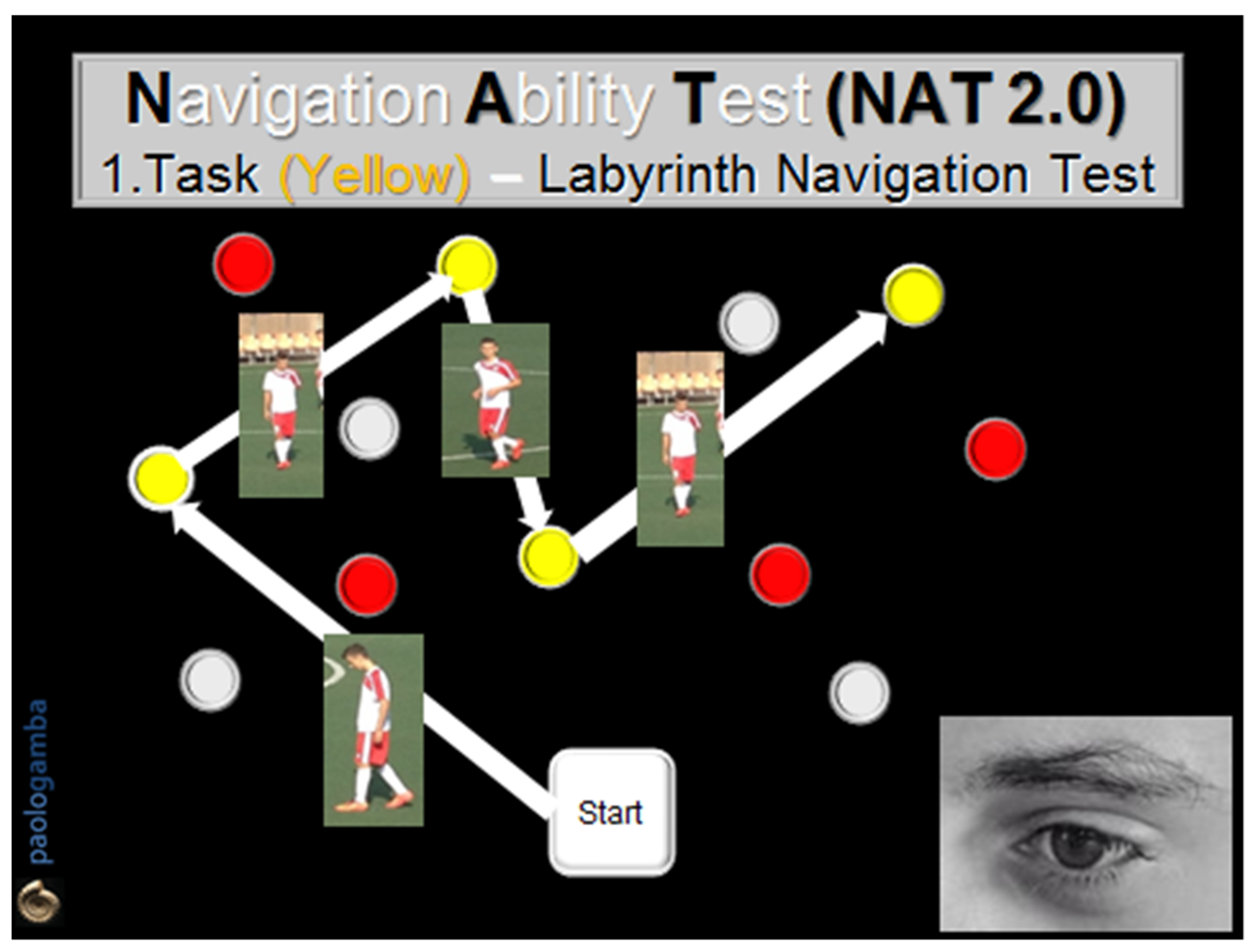
- The 1st task (Labyrinth Navigation Task—LNT, yellow-colored path): in a two-step modality, at a distance of 1 m, the subject was first invited to memorize a yellow-colored path on the ground and then to walk along it with closed eyes 2 consecutive times, in both directions, reaching one obstacle after another, according to the numerical order previously visually detected (Figure 1).
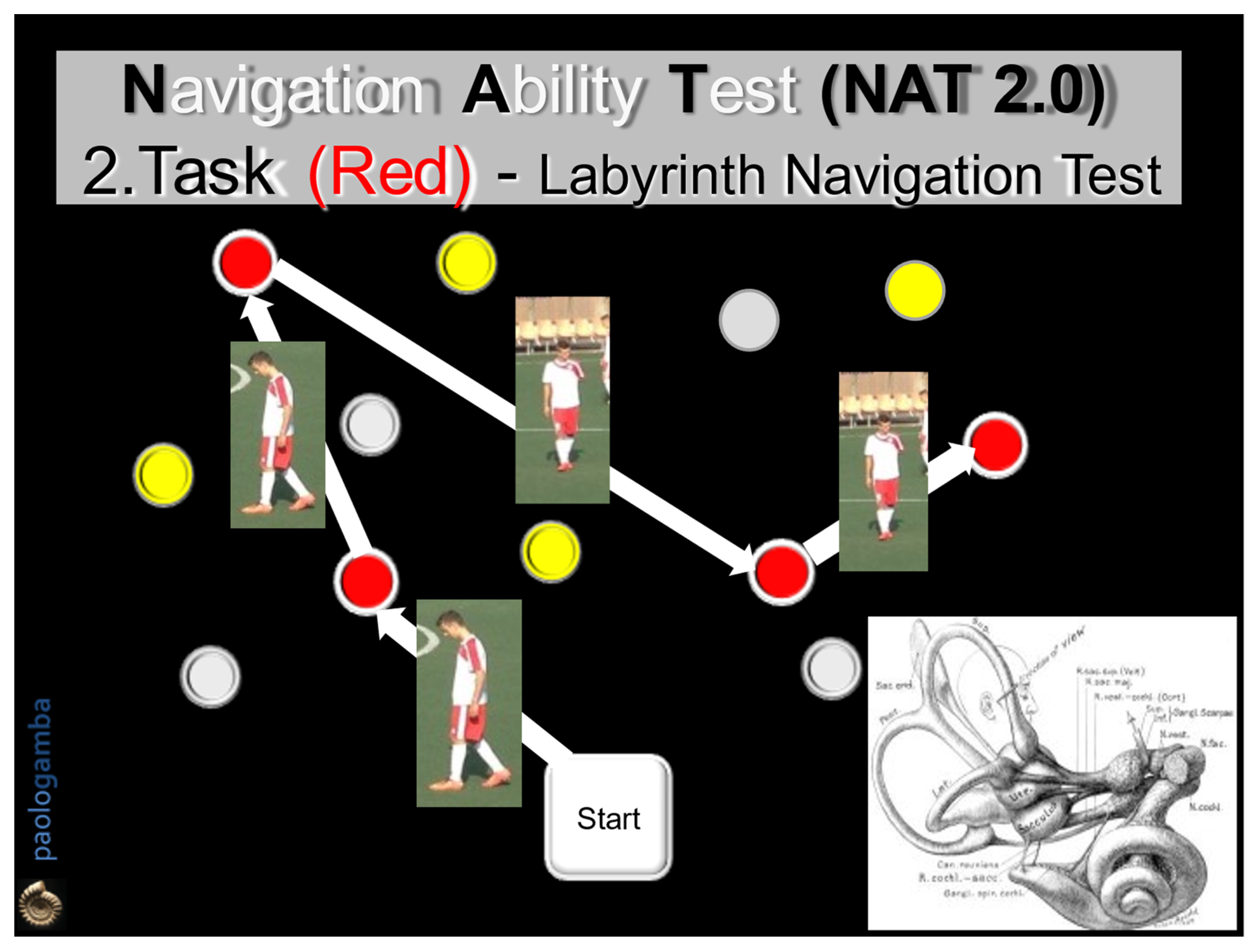
- The 2nd task (Labyrinth Navigation Task—LNT, Red-colored path): in a three-step modality, the subject was invited to memorize a second red-colored path on the ground, then to walk along it first with open eyes in both directions 2 consecutive times and then with closed eyes. In the closed-eyes condition, moment by moment, the subject reconstructs the previously memorized map, exploiting only the labyrinthine information—an expression of the SWM (Figure 2).
3. Results
- GR1 (Dizzy subjects): scores 2 and 2.3, respectively, for the LNT yellow-colored path test and re-test; scores 1.5 and 2.1, respectively, for the LNT red-colored path test and re-test;
- GR2 (Healthy non-sports subjects): scores 3.1 and 3, respectively, for the LNT yellow-colored path test and re-test; scores 2.4 and 2.6, respectively, for the LNT red-colored path test and re-test;
- GR3 (Football player subjects): scores 3.1 and 3, respectively, for the LNT yellow-colored test and re-test; scores 2.6 and 3, respectively, for the LNT red-colored test and re-test.
- Progressively better scores from GR1 to GR3 for both Task 1 (LNT yellow-colored path) and Task 2 (LNT red-colored path), suggesting that both visual and cognitive rehabilitation is essential in vestibular training;
- Test and re-test scores of Task 1 (LNT yellow-colored path) are increased only in GR3, suggesting fast and better results in trained persons;
- Test and re-test scores of Task 2 (LNT red-colored path) are increased in all three groups of subjects, suggesting that pre-programmed and memorized visual information allows for improvement of motor skills in any clinical or training condition;
- Age (significantly higher range in GR1 than in GR2 and GR3) did not affect the performance results in the LNT-Tasks in the three groups of examined subjects: these data suggest that NAT rehabilitation allows safer and effective motor performances, improving balance control in subjects of any age and in any physical training condition (Figure 3).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandt, T.; Dieterich, M. The vestibular cortex. Its locations, functions, and disorders. Ann. N. Y. Acad. Sci. 1999, 871, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Hitier, M.; Besnard, S.; Smith, P.F. Vestibular pathways involved in cognition. Front. Integr. Neurosci. 2014, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Moser, E.I.; Kropff, E.; Moser, M.B. Place cells, grid cells, and the brain’s spatial representation system. Ann. Rev. Neurosci. 2008, 31, 69–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargolini, F.; Fyhn, M.; Hafting, T.; McNaughton, B.L.; Witter, M.P.; Moser, M.B.; Moser, E.I. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 2006, 312, 758–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidetti, G.; Guidetti, R.; Manfredi, M.; Manfredi, M. Vestibular pathology and spatial working memory. Acta Otorhinolaryngol. Ital. 2020, 40, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gamba, P.; Guidetti, R.; Guidetti, G. Navigation Ability Test: A new specific test to asses spatial orientation ability in football players and healthy. J. Sports Med. Phys. Fit. 2020, 60, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.T. Measures of short-term memory: A historical review. Cortex 2007, 43, 635–650. [Google Scholar] [CrossRef]
- Bigelow, R.T.; Agrawal, Y. Vestibular involvement in cognition: Visuospatial ability, attention, executive function, and memory. J. Vestib. Res. 2015, 25, 73–89. [Google Scholar] [CrossRef]
- Gamba, P. Vestibular-limbic relationships: Brain mapping. Insights Depress. Anxiety 2018, 2, 7–13. [Google Scholar]
- Arslan, M. Sui Meccanismi Biologici della Sensibilità Spaziale; Società Italiana di Laringologia, Otologia e Rinologia, Gruppo Otorinolaringologico dell’Alta Italia: Padova, Italy, 1961. [Google Scholar]
- Baddeley, A.D. Working Memory; Oxford University Press: New York, NY, USA, 1986. [Google Scholar]
- Della Sala, S.; Gray, C.; Baddeley, A.; Allamano, N.; Wilson, L. Pattern span: A tool for unwelding visuo-spatial memory. Neuropsychologia 1999, 37, 1189–1199. [Google Scholar] [CrossRef]
- Cesarani, A.; Alpini, D. Terapia delle Vertigini e del Disequilibrio: Il Metodo MCS; Springer: Milan, Italy, 2000. [Google Scholar]
- Guidetti, G.; Guidetti, R.; Manfredi, M.; Manfredi, M.; Lucchetta, A.; Livio, S. Saccades and driving. Acta Otorhinolaryngol. Ital. 2019, 39, 186–196. [Google Scholar] [CrossRef]
- Brandt, T.; Strupp, M.; Dieterich, M. Towards a concept of disorders of “higher vestibular function”. Front. Integr. Neurosci. 2014, 8, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsi, P.M. Human Memory and the Medial Temporal Region of the Brain. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 1972. [Google Scholar]
- Vecchi, T.; Richardson, J.T. Measures of visuospatial short-term memory: The Knox Cube Imitation Test and the Corsi Blocks Test compared. Brain Cogn. 2001, 46, 291–295. [Google Scholar] [CrossRef]
- Brunetti, R.; Del Gatto, C.; Delogu, F. eCorsi: Implementation and testing of the Corsi block-tapping task for digital tablets. Front. Psychol. 2014, 5, 939. [Google Scholar] [CrossRef] [PubMed]
- Lacour, M.; Dutheil, S.; Tighilet, B.; Lopez, C.; Borel, L. Tell me your vestibular deficit, and I’ll tell you how you’ll compensate. Ann. N. Y. Acad. Sci. 2009, 1164, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Damasio, A.R. L’errore di Cartesio. Emozione, Ragione e Cervello Umano; Adelphi: Milan, Italy, 1995. [Google Scholar]
- Rizzolatti, G.; Sinigaglia, C. The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nat. Rev. Neurosci. 2010, 11, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzolatti, G.; Sinigaglia, C. Specchi nel Cervello Come Comprendiamo Gli Altri Dall’interno; Cortina Raffaello: Milan, Italy, 2017. [Google Scholar]
- Rizzolatti, G.; Senigaglia, C. So Quel Che Fai. Il Cervello Che Agisce e i Neuroni Specchio; Cortina Raffaello: Milan, Italy, 2006. [Google Scholar]
- Nielsen, J.B.; Cohen, L.G. The Olympic brain. Does corticospinal plasticity play a role in acquisition of skills required for high performance sports? J. Physiol. 2008, 586, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Lee, K.J.; Han, J.W.; Lee, N.J.; Lee, W.T.; Park, K.A.; Rhyu, I.J. Experience-dependent plasticity of cerebellar vermis in basketball players. Cerebellum 2009, 8, 334–339. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Yokoyama, S.; Kasahara, S.; Yomogida, Y.; Takeuchi, H.; Ogawa, T.; Taki, Y.; Niwa, S.-I.; Kawashima, R. Neural bases of a specific strategy for visuospatial processing in rugby players. Med. Sci. Sports Exerc. 2011, 43, 1857–1862. [Google Scholar] [CrossRef]
- Tapper, A.; Gonzalez, D.; Ro, E.; Niechwiej-Szwedo, E. Executive function deficits in team sport athletes with a history of concussion revealed by a visual-auditory dual task paradigm. J. Sports Sci. 2017, 35, 231–240. [Google Scholar] [CrossRef]
- Alpini, D. The italian vestibular rehabilitation protocols. Neurol. Newsl. 1994, 1, 54–56. [Google Scholar]
- Gufoni, M.; Guidetti, G.; Nuti, D.; Pagnini, P.; Vicini, C.; Tinelli, C.; Mira, E. The relationship between cognitive impairment, anxiety-depression symptoms and balance and spatial orientation complaints in the elderly. Acta Otorhinolaryngol. Ital. 2005, 25, 12–21. [Google Scholar] [PubMed]
- Yarrow, K.; Brown, P.; Krakauer, J.W. Inside the brain of an elite athlete: The neural processes that support high achievement in sports. Nat. Rev. Neurosci. 2009, 10, 585–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taube, J.S.; Goodrige, J.P.; Golob, E.J.; Dudchenko, P.A.; Stackman, R.W. Processing the head direction cell signal: A review and commentary. Brain Res. Bull. 1996, 40, 477–484. [Google Scholar] [CrossRef]
- Taube, J.S. Head direction cell firing properties and behavioural performance in 3-D space. J. Physiol. 2011, 589, 835–841. [Google Scholar] [CrossRef]
- Taube, J.S. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J. Neurosci. 1995, 15, 70–86. [Google Scholar] [CrossRef]
- Taube, J.S. Head direction cells and the neurophysiological basis for a sense of direction. Prog. Neurobiol. 1998, 55, 225–256. [Google Scholar] [CrossRef]
- Taube, J.S. The head direction signal: Origins and sensory-motor integration. Ann. Rev. Neurosci. 2007, 30, 181–207. [Google Scholar] [CrossRef] [Green Version]
- Taube, J.S.; Bassett, J.P. Persistent neural activity in head direction cells. Cereb. Cortex 2003, 13, 1162–1172. [Google Scholar] [CrossRef] [Green Version]
- Marcelli, V.; Esposito, F.; Aragri, A.; Furia, T.; Riccardi, P.; Tosetti, M.; Biagi, L.; Marciano, E.; Di Salle, F. Spatio-temporal pattern of vestibular information processing after brief caloric stimulation. Eur. J. Radiol. 2009, 70, 312–316. [Google Scholar] [CrossRef]
- O’Keefe, J.; Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971, 34, 171–175. [Google Scholar] [CrossRef]
- Halmagyi, G.M.; Chen, L.; MacDougall, H.G.; Weber, K.P.; McGarvie, L.A.; Curthoy, I.S. The Video Head Impulse Test. Front. Neurol. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronstein, A.M.; Brandt, T.; Woollacott, M.H. Clinical Disorders of Balance, Posture and Gait; Arnold Ed: London, UK, 1996. [Google Scholar]
- Guidetti, G. The role of cognitive process in vestibular disorders. Hear. Balance Commun. 2013, 11, 3–35. [Google Scholar] [CrossRef]
- Guidetti, G.; Sgalla, R.; Guidetti, R. The saccadic training for driving safety. Hear. Balance Commun. 2019, 16, 197–207. [Google Scholar] [CrossRef]
- Tramontano, M.; Princi, A.A.; De Angelis, S.; Indovina, I.; Manzari, L. Vestibular rehabilitation in patients with persistent postural-perceptual dizziness: A scoping review. Hear. Balance Commun. 2021, 19, 282–290. [Google Scholar] [CrossRef]
- Teggi, R.; Caldirola, D.; Perna, G.; Bellodi, L.; Bussi, M. Vestibular testing in patient with panic disorders and chronic dizziness. Acta Otorhinolaryngol. Ital. 2007, 27, 243–247. [Google Scholar]
- Bordin, A.; Pazzaglia, F.; Busato, V.; Mazzanti, S.; Calore, G.; Insolia, M.; Basso, R.; Martinelli, M. Training program for the improvement of sense of direction and spatial orientation in aged people. G. Gerontol. 2011, 59, 81–88. [Google Scholar]
- Péruch, P.; Borel, L.; Gaunet, F.; Thinus-Blanc, G.; Magnan, J.; Lacour, M. Spatial performance of unilateral vestibular defective patients in non-visual vs visual navigation. J. Vestib. Res. 1999, 9, 37–47. [Google Scholar] [CrossRef]


| Tasks | GR1 (Dizzy) (n = 48) | GR2 (Heathy) (n = 60) | GR3 (Players) (n = 60) |
|---|---|---|---|
| Task 1: LNT | |||
| LNT-1 (test) | 2 | 3.1 | 3.1 |
| LNT-2 (re-test) | 2.3 | 3 | 3 |
| Task 2: LNT | |||
| LNT-1 (test) | 1.5 | 2.4 | 2.6 |
| LNT-2 (re-test) | 2.1 | 2.6 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamba, P.; Guidetti, R.; Balzanelli, C.; Bavazzano, M.; Laborai, A. The Navigation Ability Test (NAT 2.0): From Football Player Performance to Balance Rehabilitation in Chronic Unilateral Vestibular Loss. Audiol. Res. 2022, 12, 249-259. https://doi.org/10.3390/audiolres12030026
Gamba P, Guidetti R, Balzanelli C, Bavazzano M, Laborai A. The Navigation Ability Test (NAT 2.0): From Football Player Performance to Balance Rehabilitation in Chronic Unilateral Vestibular Loss. Audiology Research. 2022; 12(3):249-259. https://doi.org/10.3390/audiolres12030026
Chicago/Turabian StyleGamba, Paolo, Riccardo Guidetti, Cristiano Balzanelli, Maurizio Bavazzano, and Andrea Laborai. 2022. "The Navigation Ability Test (NAT 2.0): From Football Player Performance to Balance Rehabilitation in Chronic Unilateral Vestibular Loss" Audiology Research 12, no. 3: 249-259. https://doi.org/10.3390/audiolres12030026







