Vestibular Paroxysmia with Neurovascular Cross Compression and Antiepileptic Drugs: A Systematic Review and Discussion of Physiopathology
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Quality Assessment
3. Results
3.1. Search Strategy and Study Selection
3.2. Data Extraction: Antiepileptic Drugs for VP and NVCC
3.3. Quality Assessment: Efficacy of Antiepileptic Drugs in the Treatment of VP and NVCC
4. Discussion
4.1. Physiopathology of VP
- Hyperventilation Induced Nystagmus
- Cochlear Symptoms: The Typewriter Tinnitus
4.2. VP with No Radiologic Criteria for NVCC
4.3. Arguments for VP Classification
4.4. Arguments for OXC as First Line VP Treatment?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gowers, W.R. The diagnosis and treatment of auditory nerve vertigo. Br. Med. J. 1877, 1, 418–420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schultze, F. XXX. Linksseitiger Facialiskrarapf in Folge eines Aneurysma der Arteria vertebralis sinistra. In Band 65; De Gruyter: Berlin, Germany; Boston, MA, USA, 1875; pp. 385–391. [Google Scholar] [CrossRef]
- Jannetta, P.J. Neurovascular cross-compression in patients with hyperactive dysfunction symptoms of the eighth cranial nerve. Surg. Forum. 1975, 26, 467–469. [Google Scholar]
- Jannetta, P.J.; Møller, M.B.; Møller, A.R. Disabling positional vertigo. N. Engl. J. Med. 1984, 310, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.; Dieterich, M. Vestibular paroxysmia. Vascular compression of the eighth nerve? Lancet 1994, 343, 798–799. [Google Scholar] [CrossRef] [PubMed]
- Strupp, M.; Lopez-Escamez, J.A.; Kim, J.S.; Straumann, D.; Jen, J.C.; Carey, J.; Bisdorff, A.; Brandt, T. Vestibular paroxysmia: Diagnostic criteria. J. Vestib. Res. 2016, 26, 409–415. [Google Scholar] [CrossRef]
- Haller, S.; Etienne, L.; Kövari, E.; Varoquaux, A.D.; Urbach, H.; Becker, M. Imaging of Neurovascular Compression Syndromes: Trigeminal Neuralgia, Hemifacial Spasm, Vestibular Paroxysmia, and Glossopharyngeal Neuralgia. AJNR Am. J. Neuroradiol. 2016, 37, 1384–1392. [Google Scholar] [CrossRef]
- Hüfner, K.; Barresi, D.; Glaser, M.; Linn, J.; Adrion, C.; Mansmann, U.; Brandt, T.; Strupp, M. Vestibular paroxysmia: Diagnostic features and medical treatment. Neurology 2008, 71, 1006–1014. [Google Scholar] [CrossRef]
- Best, C.; Gawehn, J.; Krämer, H.H.; Thömke, F.; Ibis, T.; Müller-Forell, W.; Dieterich, M. MRI and neurophysiology in vestibular paroxysmia: Contradiction and correlation. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1349–1356. [Google Scholar] [CrossRef]
- Lang, J. Anatomy, length and blood vessel relations of “central” and “peripheral” paths of intracisternal cranial nerves. Zentralblatt Neurochir. 1982, 43, 217–258. [Google Scholar]
- McDermott, A.L.; Dutt, S.N.; Irving, R.M.; Pahor, A.L.; Chavda, S.V. Anterior inferior cerebellar artery syndrome: Fact or fiction. Clin. Otolaryngol. Allied Sci. 2003, 28, 75–80. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Fu, Q.; Luo, H.; Xu, Y. Independent episodes of vestibular paroxysmia and hemifacial spasm due to a distorted vertebral artery in one patient. Acta Neurol. Belg. 2024, 124, 1679–1681. [Google Scholar] [CrossRef]
- Davis, T.C.; Thedinger, B.A.; Greene, G.M. Osteomas of the internal auditory canal: A report of two cases. Am. J. Otol. 2000, 21, 852–856. [Google Scholar] [PubMed]
- Suzuki, J.; Takata, Y.; Miyazaki, H.; Yahata, I.; Tachibana, Y.; Kobayashi, T.; Kawase, T.; Katori, Y. Osteoma of the internal auditory canal mimicking vestibular schwannoma: Case report and review of 17 recent cases. Tohoku J. Exp. Med. 2014, 232, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Reynard, P.; Ionescu, E.; Karkas, A.; Ltaeif-Boudrigua, A.; Thai-Van, H. Unilateral cochleovestibular nerve compression syndrome in a patient with bilateral IAC osteoma. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 213–216. [Google Scholar] [CrossRef]
- Brandt, T. Vertigo, Its Multisensory Syndromes, 2nd ed.; Springer: London, UK, 1999. [Google Scholar]
- Arbusow, V.; Strupp, M.; Dieterich, M.; Jager, L.; Hischa, A.; Schulz, P.; Brandt, T. Alternating episodes of vestibular nerve excitation and failure. Neurology 1998, 51, 1480–1483. [Google Scholar] [CrossRef]
- Chen, C.C.; Lee, T.Y.; Lee, H.H.; Kuo, Y.H.; Bery, A.K.; Chang, T.P. Vestibular paroxysmia: Long-term clinical outcome after treatment. Front. Neurol. 2022, 13, 1036214. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, K.; Becker-Bense, S.; Strobl, R.; Grill, E.; Seelos, K.; Huppert, D. Vestibular paroxysmia: Clinical characteristics and long-term course. J. Neurol. 2022, 269, 6237–6245. [Google Scholar] [CrossRef]
- Alexander, G.E.; Moses, H., 3rd. Carbamazepine for hemifacial spasm. Neurology 1982, 32, 286–287. [Google Scholar] [CrossRef]
- Eyal, S. Tuning Sodium Channel Blockers to the Near-Atomic Level. Epilepsy Curr. 2024, 17, 123–125. [Google Scholar] [CrossRef]
- Moller, M.B. Results of microvascular decompression of the eighth nerve as treatment for disabling positional vertigo. Ann. Otol. Rhinol. Laryngol. 1990, 99, 724–729. [Google Scholar] [CrossRef]
- Brandt, T.; Strupp, M.; Dieterich, M. Vestibular paroxysmia: A treatable neurovascular cross-compression syndrome. J. Neurol. 2016, 263 (Suppl. S1), S90–S96. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Moher, D.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bayer, O.; Brémová, T.; Strupp, M.; Hüfner, K. A randomized double-blind, placebo-controlled, cross-over trial (Vestparoxy) of the treatment of vestibular paroxysmia with oxcarbazepine. J. Neurol. 2018, 265, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Wenping, X.; Hui, X.; Xin, H.; Xiue, L.; Jun, Z.; Shangyong, G. Efficacy and acceptability of oxcarbazepine vs. carbamazepine with betahistine mesilate tablets in treating vestibular paroxysmia: A retrospective review. Postgrad. Med. 2016, 128, 492–495. [Google Scholar] [CrossRef]
- Xue, H.; Xiang, W.; Yu, Y.; Liu, G.; Chong, Y.; Zhou, J. Randomized trial of betahistine mesilate tablets as augmentation for oxcarbazepine and carbamazepine in treating vestibular paroxysmia. Drug Des. Dev. Ther. 2018, 12, 837–843. [Google Scholar] [CrossRef]
- Hanskamp, L.A.J.; Schermer, T.R.; van Leeuwen, R.B. Long-term Prognosis of Vertigo Attacks and Health-related Quality of Life Limitations in Patients With Vestibular Paroxysmia. Otol. Neurotol. 2022, 43, e475–e481. [Google Scholar] [CrossRef]
- Li, J.H. Efficacy of carbamazepine combined with mesilate in treatment of vestibular paroxysmia. Chin. J. Trauma Disabil. Med. 2013, 21, 41–42. [Google Scholar]
- Strupp, M.; Dieterich, M.; Brandt, T.; Feil, K. Therapy of vestibular paroxysmia, superior oblique myokymia, and ocular neuromyotonia. Curr. Treat. Options Neurol. 2016, 18, 34. [Google Scholar] [CrossRef]
- Arvanitaki, A. Effects evoked in an axon by the activity of a contiguous one. J. Neurophysiol. 1942, 5, 89–108. [Google Scholar] [CrossRef]
- Prabhu, P. Is tinnitus a major concern in individuals with auditory neuropathy spectrum disorder?—Questionnaire based study. World J. Otorhinolaryngol. Head Neck Surg. 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Schwaber, M.K.; Hall, J.W. Cochleovestibular nerve compression syndrome. I. Clinical features and audiovestibular findings. Laryngoscope 1992, 102, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; McDonald, W.I. Spontaneous and mechanically evoked activity due to central demyelinating lesion. Nature 1980, 286, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Yamamoto, S.; Sugiyama, K.; Nozue, M. Neurovascular compression syndrome of the eighth cranial nerve. What are the most reliable diagnostic signs? Acta Neurochir. 1998, 140, 1279–1286. [Google Scholar] [CrossRef]
- De Ridder, D.; Heijneman, K.; Haarman, B.; van der Loo, E. Tinnitus in vascular conflict of the eighth cranial nerve: A surgical pathophysiological approach to ABR changes. Prog. Brain Res. 2007, 166, 401–411. [Google Scholar] [CrossRef]
- Rommer, P.S.; Wiest, G.; Kronnerwetter, C.; Zach, H.; Loader, B.; Elwischger, K.; Trattnig, S. 7-Tesla MRI demonstrates absence of structural lesions in patients with vestibular paroxysmia. Front. Neuroanat. 2015, 9, 81. [Google Scholar] [CrossRef]
- Kierig, E.; Gerb, J.; Boegle, R.; Ertl-Wagner, B.; Dieterich, M.; Kirsch, V. Vestibular paroxysmia entails vestibular nerve function, microstructure and endolymphatic space changes linked to root-entry zone neurovascular compression. J. Neurol. 2023, 270, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Minor, L.B.; Haslwanter, T.; Straumann, D.; Zee, D.S. Hyperventilation-induced nystagmus in patients with vestibular schwannoma. Neurology 1999, 53, 2158–2168. [Google Scholar] [CrossRef]
- Choi, K.D.; Kim, J.S.; Kim, H.J.; Koo, J.W.; Kim, J.H.; Kim, C.Y.; Oh, C.W.; Kee, H.J. Hyperventilation-induced nystagmus in peripheral vestibulopathy and cerebellopontine angle tumor. Neurology 2007, 69, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Califano, L.; Melillo, M.G.; Vassallo, A.; Mazzone, S. Hyperventilation-induced nystagmus in a large series of vestibular patients. Acta Otorhinolaryngol. Ital. 2011, 31, 17–26. [Google Scholar]
- Sakellari, V.; Bronstein, A.M.; Corna, S.; Hammon, C.A.; Jones, S.; Wolsley, C.J. The effects of hyperventilation on postural control mechanisms. Brain 1997, 120, 1659–1673. [Google Scholar] [CrossRef]
- Bance, M.L.; O’Driscoll, M.; Patel, N.; Ramsden, R.T. Vestibular disease unmasked by hyperventilation. Laryngoscope 1998, 108, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.J.; Kim, H.J.; Choi, J.Y.; Kim, J.S. Vestibular paroxysmia associated with typewriter tinnitus: A case report and literature review. J. Neurol. 2021, 268, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.A. Typewriter tinnitus: A carbamazepine-responsive syndrome related to auditory nerve vascular compression. J. Otorhinolaryngol. Relat. Spec. 2006, 68, 43–46. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Adriaensens, I.; Lee, A.P.; van de Heyning, P.; Möller, A. Vascular compression of the cochlear nerve and tinnitus: A pathophysiological investigation. Acta Neurochir. 2012, 154, 807–813. [Google Scholar] [CrossRef]
- Mardini, M. Ear-clicking “tinnitus” responding to carbamazepine. N. Eng. J. Med. 1987, 317, 1542. [Google Scholar]
- Sivarasan, N.; Touska, P.; Murdin, L.; Connor, S. MRI findings in vestibular paroxysmia—An observational study. J. Vestib. Res. 2019, 29, 137–145. [Google Scholar] [CrossRef]
- The, C.S.; Noordiana, S.H.; Shamini, S.; Prepageran, N. Vascular Loops: The Innocent Bystander for Vestibular Paroxysmia. Ann. Otol. Rhinol. Laryngol. 2022, 131, 604–608. [Google Scholar] [CrossRef]
- Idriss, S.A.; Thai-Van, H.; Altaisan, R.; Ltaief-Boudrigua, A.; Reynard, P.; Ionescu, E.C. The Narrowed Internal Auditory Canal: A Distinct Etiology of Pediatric Vestibular Paroxysmia. J. Clin. Med. 2022, 11, 4300. [Google Scholar] [CrossRef]
- Ionescu, E.C.; Reynard, P.; Idriss, S.A.; Ltaief-Boudriga, A.; Joly, C.A.; Thai-Van, H. The “Near”-Narrowed Internal Auditory Canal Syndrome in Adults: Clinical Aspects, Audio-Vestibular Findings, and Radiological Criteria for Diagnosis. J. Clin. Med. 2023, 12, 7580. [Google Scholar] [CrossRef]
- Valvassori, G.E. The internal auditory canal revisited. The high-definition approach. Otolaryngol. Clin. N. Am. 1995, 28, 431–451. [Google Scholar] [CrossRef]
- Verbiest, H. Stenosis of the lumbar vertebral canal and sciatica. Neurosurg. Rev. 1980, 3, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.B.; Fundaun, J.; Tampin, B. Entrapment neuropathies: A contemporary approach to pathophysiology, clinical assessment, and management. Pain Rep. 2020, 5, e829. [Google Scholar] [CrossRef]
- Sakina, M.S.; Goh, B.S.; Abdullah, A.; Zulfiqar, M.A.; Saim, L. Internal auditory canal stenosis in congenital sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 2093–2097. [Google Scholar] [CrossRef] [PubMed]
- Reynard, P.; Ionescu, E.; Ltaief-Boudrigua, A.; Thai-Van, H. A Message from a Narrowed Internal Auditory Canal in a Patient with a Hyperpneumatized Petrous Bone. J. Int. Adv. Otol. 2020, 16, 485–488. [Google Scholar] [CrossRef]
- Tunes, C.; Flønes, I.; Helland, C.; Wilhelmsen, K.; Goplen, F.; Wester, K.G. Pre- and post-operative dizziness and postural instability in temporal arachnoid cyst patients. Acta Neurol. Scand. 2014, 129, 335–342. [Google Scholar] [CrossRef]
- Ahle, G.; Visser, W.; Juergens, A.; Schlegel, U. Successful treatment of hyperventilation-induced nystagmus in vestibular schwannoma with oxcarbazepine. J. Neurol. 2008, 255, 1093. [Google Scholar] [CrossRef] [PubMed]
- Nunez, M.; Ruprecht, M.T.; Aguirre, A.S.; Torres, A. A Complex Presentation of Vestibular Paroxysmia in an Adolescent With Wolff- Parkinson-White Syndrome. Pediatr. Neurol. 2024, 161, 26–27. [Google Scholar] [CrossRef]
- Strupp, M.; Elger, C.; Goldschagg, N. Treatment of vestibular paroxysmia with lacosamide. Neurol. Clin. Pract. 2019, 9, 539–541. [Google Scholar] [CrossRef]
- Russell, D.; Baloh, R.W. Gabapentin responsive audiovestibular paroxysmia. J. Neurol. Sci. 2009, 281, 99–100. [Google Scholar] [CrossRef]
- Zakrzewska, J.M.; McMillan, R. Trigeminal neuralgia: The diagnosis and management of this excruciating and poorly understood facial pain. Postgrad. Med. J. 2011, 87, 410–416. [Google Scholar] [CrossRef]
- Wang, Q.P.; Bai, M. Topiramate versus carbamazepine for the treatment of classical trigeminal neuralgia: A meta-analysis. CNS Drugs 2011, 25, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Elger, C.E. What is the evidence that oxcarbazepine and carbamazepine are distinctly different antiepileptic drugs? Epilepsy Behav. 2004, 5, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Friis, M.L.; Kristensen, O.; Boas, J.; Dalby, M.; Deth, S.H.; Gram, L.; Mikkelsen, M.; Pedersen, B.; Sabers, A.; Worm-Petersen, J.; et al. Therapeutic experiences with 947 epileptic out-patients in oxcarbazepine treatment. Acta Neurol. Scand. 1993, 87, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Rouan, M.C.; Lecaillon, J.B.; Godbillon, J.; Menard, F.; Darragon, T.; Meyer, P.; Kourilsky, O.; Hillion, D.; Aldigier, J.C.; Jungers, P. The effect of renal impairment on the pharmacokinetics of oxcarbazepine and its metabolites. Eur. J. Clin. Pharmacol. 1994, 47, 161–167. [Google Scholar] [CrossRef]
- Preuss, C.V.; Randhawa, G.; Wy, T.J.P.; Saadabadi, A. Oxcarbazepine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]

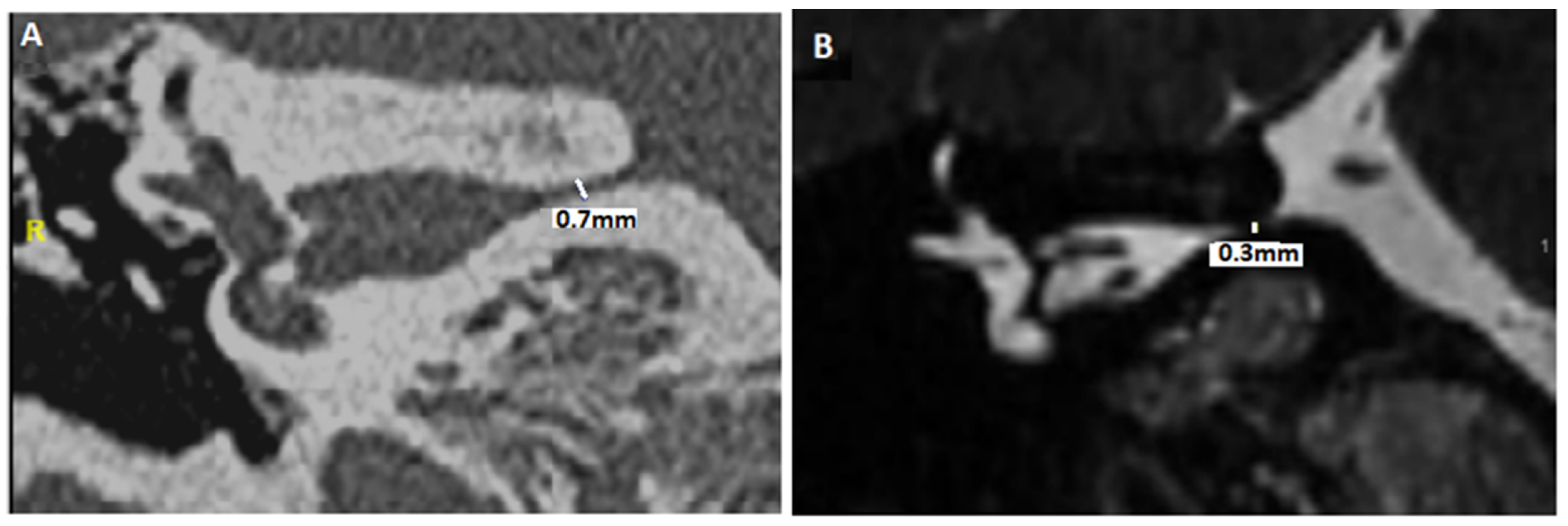
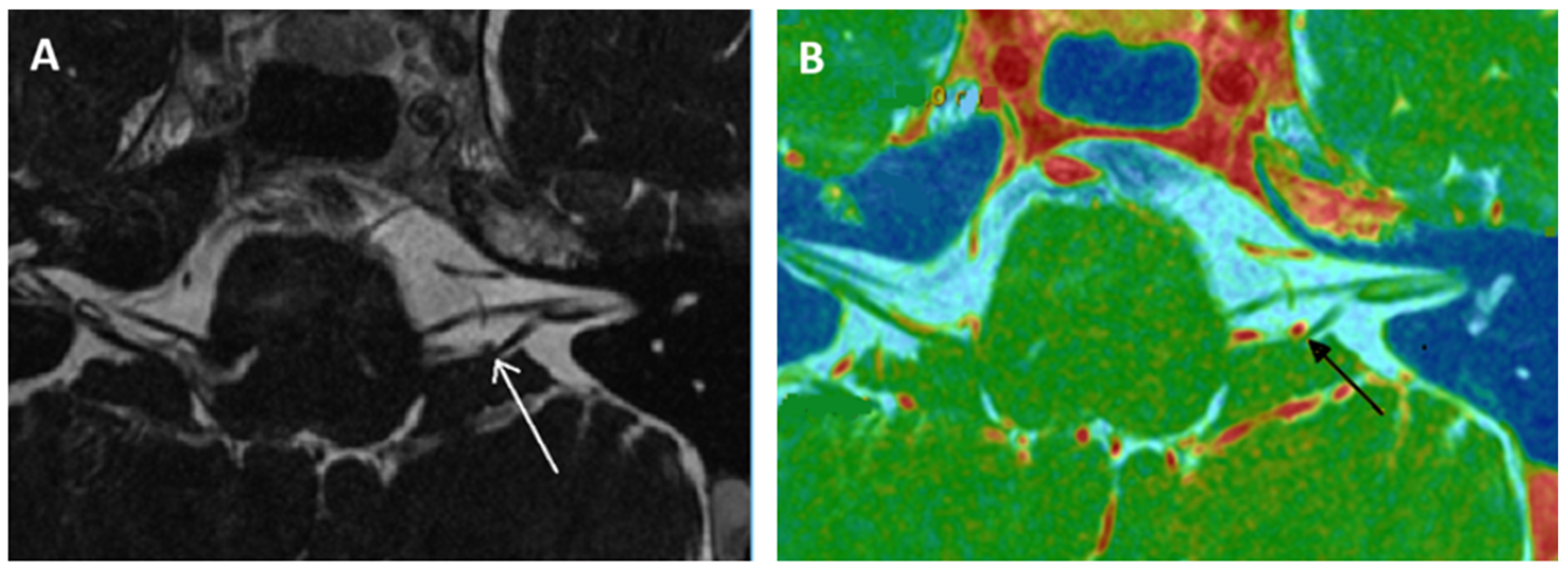
| Reference [X] Level of Evidence | Aim of the Study | Intervention | Results | Bias and Weaknesses |
|---|---|---|---|---|
| Hufner et al. (2008) [8] 4 | Assessment of CBZ and OXC in VP patients | - Follow-up study of 32 patients over a mean period of 31 months - 25 patients accepted treatment (mean treatment period 25 months) - Patients treated with CBZ (mean dose 568 mg) or OXC (mean dose 870 mg) | - Significant decrease in attack frequency down to 10% - Reduction in intensity (15%) and duration of the attacks (11%) - No difference between patients - No effect of gender (p = 0.07) or age (p = 0.060). - No serious side effects occurred - Controlled double-blind trial needed | - Limited number of patients - Lack of verified diagnostic criteria at the beginning of the study - Probable and certain VP included with variable criteria |
| Yi et al. (2016) [26] 4 | Efficacy and acceptability of CBZ and OXC in combination with BMT in the treatment of VP | - Retrospective study - 3 groups: CBZ alone (n = 73), CBZ + BMT (n = 65), OXC + BMT (n = 58) - Doses of CBZ: 100–300 mg, OXC: 300–450 mg, BMT: 10–20 mg, twice daily | - After 12 weeks: frequency of vertigo, duration of vertigo and vertigo scores decreased in all three groups (p = 0.002), no difference between the 3 groups - Difference in response rate between the 3 groups was not significant - Incidence of side effects: 30.1% for CBZ, 18.5% for CBZ + BMT, 8.6% for OXC + BMT | - No randomized controlled trial - Selection bias (same recruitment center) - Only short-term effects evaluation - Relatively small number of recruited patients (separate analysis of short and long vertigo duration to be cautiously interpreted |
| Xue et al. (2018) [27] 2 | CBZ and OXC: similar efficacy and acceptability? - Improvement enhanced by increasing BMT dose | - RCT; double-blind - CBZ + BMT (n = 92) and OXC + BMT (n = 93) - BMT 12 mg/time, or BMT 18 mg/time | - After 12 weeks: similar vertigo frequency, score, duration, and response rate - Incidence of side effects higher in CBZ + BMT group (p = 0.04) - 18 mg of BMT: greater reductions, higher response rates | - Recruitment from the same site (selection bias?) - Application oOXC/CBZ and higher doses of BMT not analysed - Only short-term effects evaluation - Patients included before current VP criteria (Bárány 2016) - No studies on CBZ or OXC dose reduction with BMT (18 mg) |
| Bayer et al. (2018) [25] 2 | To provide further evidence on the efficacy of OXC in VP patients - Patients with definite or probable VP | - RCT, double-blind, crossover - 18 patients randomized - OXC (first period) and placebo (second period), or vice versa - 1st week: 300 mg daily, 2nd: 600 mg daily, >3rd: 900 mg daily) | - Risk of at least one dizzy spell /day = 0.41 with OXC and 0.62 with placebo, relative risk of 0.67 (p = 0.025) - Ratio of the number of attacks was 0.53 (p < 0.001) for OXC versus placebo - Median seizure duration was 4 s with OXC and 3 s with placebo - No serious adverse events identified during the trial | - High drop-out rate (adverse events) - No assessment of subjective relief of symptoms or quality of life |
| Hanskamp et al. (2022) [28] 4 | - Evaluation of outcomes in patients with VP according to Bárány clinical criteria | - Follow-up study; retrospective (61 patients) - Patients with probable or definite VP - Only 12 treated with CBZ (dosage?) | - Mean follow-up: 3.4 years. - 7 patients: improvement in frequency (n = 1) or in frequency and intensity (n = 5) | - Partly retrospective study (recall bias?) - Small sample size: only 12 patients treated - Only carbamazepine - Selection bias |
| Steinmetz et al. (2022) [19] 4 | - To describe clinical symptoms and laboratory findings in large patient cohort of definite or probable VP - To evaluate the long-term course over years in definite VP | - Retrospective study - Series of 73 probable VP patients - Series of 73 definite VP patients treated with CBZ (200–1000 mg daily) or OXC (300–900 mg daily) | - 70%: no accompanying symptoms; 30%: mild unilateral cochlear symptoms - 13 patients changed medication (side effects) to gabapentin (n = 7), lacosamide (n = 5), or phenytoin (n = 1) - All: treated for an average of 6.5 months; 74% asymptomatic during long-term course (56% without medication, 44% with continuous treatment); 26% still had symptoms, frequency significantly reduced over long term (45% and 55% with and without medication) | - Selection bias due to the referral of patients to a specialized tertiary center (offset by the rigorousness of data collection) - No OXC-CBZ comparison |
| Chen et al. (2022) [18] 4 | - To study the long-term treatment outcome of VP | - 29 VP patients - OXC (n = 26), pregabalin (n = 2), gabapentin (n = 1) at least 3 months (dosage? variable) - Follow-up 6 months | - Improvement with or without NVCC is not different (93/100% at 2/4 weeks) - At 8–56 months, 84.6% maintained a good response (300 and 600 mg/day). - Complete remission (no medication) >1 month (n = 11), with remission >12 months (n = 6) - 19 patients = NVCC (presence did not predict results) | - Retrospective study. - Duration of follow-up differed among patients - Recall bias (self recalled dizziness frequency) - No use of formal scales (such as DHI) - Only oxcarbazepine was used |
| ROBINS-I Tool Risk of Bias (RoB) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Study Design | Sample Size | Bias Due to Confounding | Bias in Selection of Participants | Bias in Classification of Interventions | Deviation from Intended Intervention | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of Reported Results |
| Hufner et al. [8] | PCS | 32 | ○ | ● | ○ | Ø | ○ | NA | ○ |
| Yi et al. [26] | RCS | 196 | ○ | ● | ○ | ○ | Ø | ◐ | ○ |
| Xue et al. [27] | RanCtT | 185 | ○ | ● | ○ | ○ | ○ | ○ | ○ |
| Bayer et al. [25] | RanCtT | 18 | ○ | ○ | ● | ● | ● | ○ | ○ |
| Hanskamp et al. [28] | RCS | 61 | ● | ● | ● | ○ | ● | ◐ | ● |
| Steinmetz et al. [19] | RCS | 146 | ○ | ● | ● | Ø | Ø | ◐ | ○ |
| Chen et al. [18] | RCS | 29 | ○ | ○ | ● | ● | ○ | ◐ | ○ |
| HRCT Diagnosis Steps | MRI Diagnosis Steps |
|---|---|
| - Evaluation of the smallest anteroposterior and craniocaudal IAC diameters (after measuring IAC length) - Description of bony abnormalities of the IAC walls (normal bone, fibrous dysplasia, meningeal calcifications, and/or osteoma of exostosis) - Evaluation of any significant angulation or deformation of the IAC (anteroposterior and craniocaudal planes) | - Assessment of the perineural fluid environment in the IAC - Angulation of the CVN? - Presence of NVCC? - Analysis of fusion images between high-resolution T2 and HRCT of the temporal bones - Evaluation of anteroposterior and craniocaudal diameters of the IAC |
| I—Classical VP | II—Secondary VP | III—Idiopathic VP |
|---|---|---|
| - Neurovascular cross compression (NVCC) | - Schwannoma, meningioma - Osteoma, other bony compression - Meningocele - Narrowed internal auditory canal - Arachnoid cysts of the posterior fossa | - Primary vestibular neuropathy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynard, P.; Thai-Van, H.; Neagu, A.; Ionescu, E.C. Vestibular Paroxysmia with Neurovascular Cross Compression and Antiepileptic Drugs: A Systematic Review and Discussion of Physiopathology. Audiol. Res. 2025, 15, 28. https://doi.org/10.3390/audiolres15020028
Reynard P, Thai-Van H, Neagu A, Ionescu EC. Vestibular Paroxysmia with Neurovascular Cross Compression and Antiepileptic Drugs: A Systematic Review and Discussion of Physiopathology. Audiology Research. 2025; 15(2):28. https://doi.org/10.3390/audiolres15020028
Chicago/Turabian StyleReynard, Pierre, Hung Thai-Van, Alexandra Neagu, and Eugen Constant Ionescu. 2025. "Vestibular Paroxysmia with Neurovascular Cross Compression and Antiepileptic Drugs: A Systematic Review and Discussion of Physiopathology" Audiology Research 15, no. 2: 28. https://doi.org/10.3390/audiolres15020028
APA StyleReynard, P., Thai-Van, H., Neagu, A., & Ionescu, E. C. (2025). Vestibular Paroxysmia with Neurovascular Cross Compression and Antiepileptic Drugs: A Systematic Review and Discussion of Physiopathology. Audiology Research, 15(2), 28. https://doi.org/10.3390/audiolres15020028







