The Influence of Carotid and Vertebral Doppler Ultrasonography and Brain MRI Abnormalities on Hearing Levels, Tinnitus Intensities and Frequencies
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Medical Imaging
2.3. Pure-Tone Audiometry and Tinnitus Pitch-Matching
2.4. Statistical Analysis
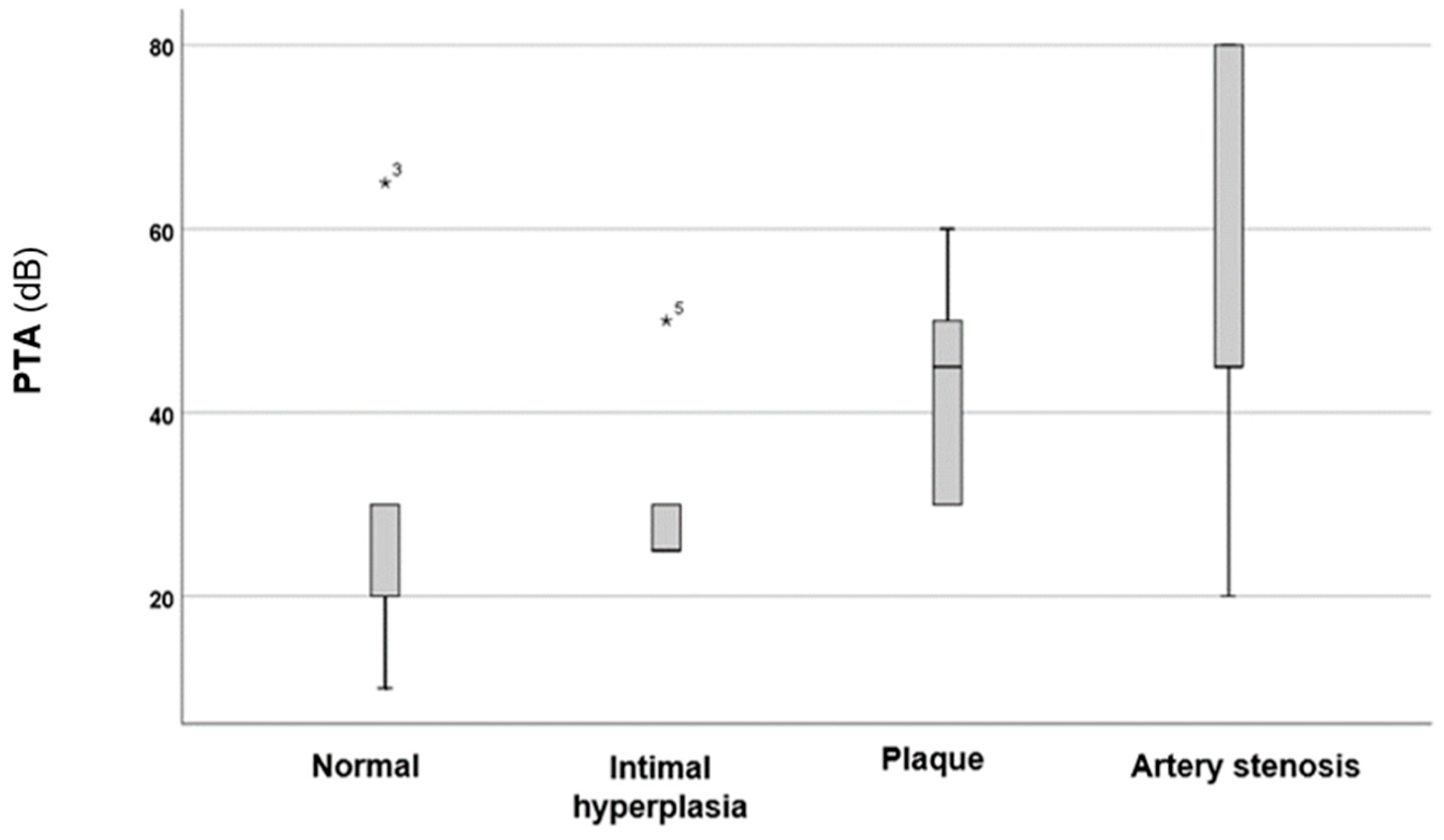
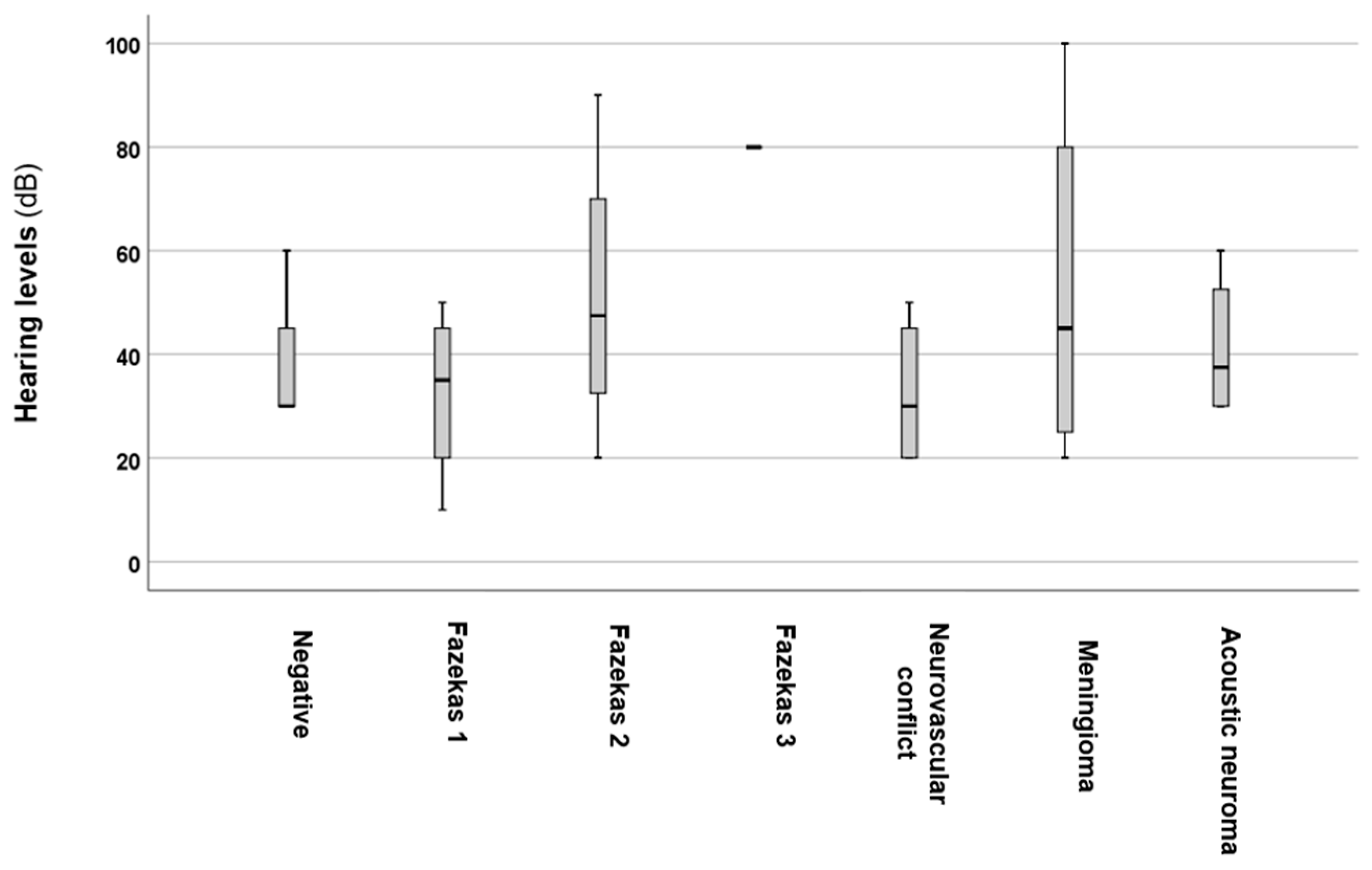
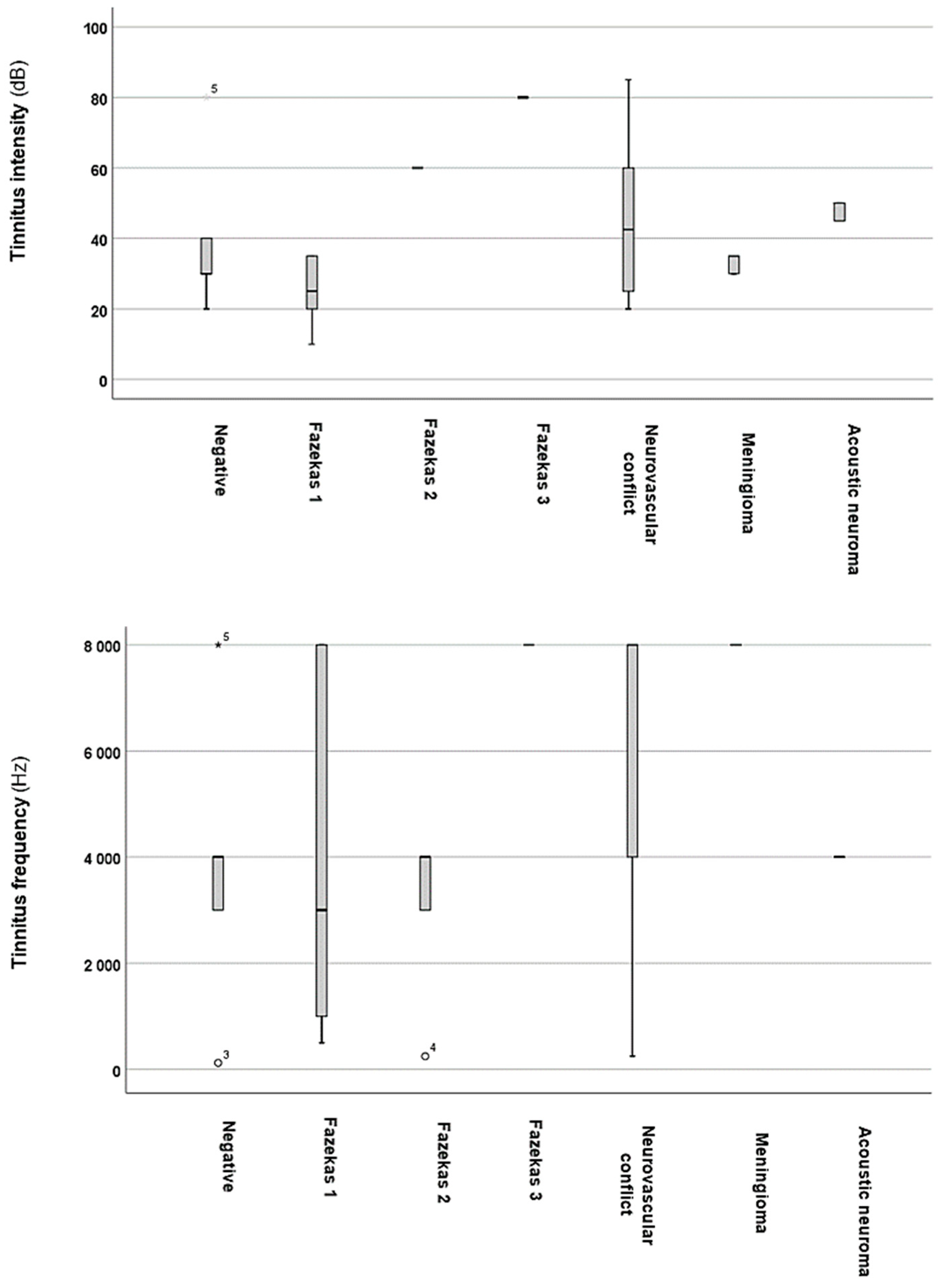
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AICA | anterior inferior cerebellar artery |
| ABR | auditory brainstem response |
| BAEP | brainstem auditory evoked potential |
| dB | decibel |
| DWI | diffusion-weighted imaging |
| FLAIR | fluid-attenuated inversion recovery |
| Hz | Hertz |
| IBM | International Business Machines Corporation |
| IQR | interquartile range |
| MRA | magnetic resonance angiography |
| MRI | magnetic resonance imaging |
| PTA | pure-tone average |
| SPSS | Statistical Package for the Social Sciences |
| Q1 | first quartile |
| Q3 | third quartile |
| SWI | susceptibility-weighted imaging |
References
- Lee, H.Y.; Jung, D.J. Recent Updates on Tinnitus Management. J. Audiol. Otol. 2023, 27, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Jarach, C.M.; Lugo, A.; Scala, M.; van den Brandt, P.A.; Cederroth, C.R.; Odone, A.; Garavello, W.; Schlee, W.; Langguth, B.; Gallus, S. Global Prevalence and Incidence of Tinnitus: A Systematic Review and Meta-analysis. JAMA Neurol. 2022, 79, 888–900. [Google Scholar] [CrossRef]
- Park, H.M.; Jung, J.; Kim, J.K.; Lee, Y.J. Tinnitus and Its Association With Mental Health and Health-Related Quality of Life in an Older Population: A Nationwide Cross-Sectional Study. J. Appl. Gerontol. 2022, 41, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef]
- Tan, C.M.; Lecluyse, W.; McFerran, D.; Meddis, R. Tinnitus and patterns of hearing loss. J. Assoc. Res. Otolaryngol. 2013, 14, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Deshmukh, P. Sudden Sensorineural Hearing Loss: A Review. Cureus 2022, 14, e29458. [Google Scholar] [CrossRef]
- Terzi, S.; Arslanoğlu, S.; Demiray, U.; Eren, E.; Cancuri, O. Carotid Doppler ultrasound evaluation in patients with pulsatile tinnitus. Indian J. Otolaryngol. Head Neck Surg. 2015, 67, 43–47. [Google Scholar] [CrossRef]
- Koyuncu, M.; Celik, O.; Lüleci, C.; Inan, E.; Oztürk, A. Doppler sonography of vertebral arteries in patients with tinnitus. Auris Nasus Larynx 1995, 22, 24–28. [Google Scholar] [CrossRef]
- Mutlu, A.; Cam, I.; Dasli, S.; Topdag, M. Doppler ultrasonography can be useful to determine the etiology of idiopathic sudden sensorineural hearing loss. Auris Nasus Larynx 2018, 45, 456–460. [Google Scholar] [CrossRef]
- Dorobisz, K.; Dorobisz, T.; Janczak, D.; Krecicki, T. The evaluation of the sense of hearing in patients with carotid artery stenosis within the extracranial segments. Acta Neurol. Belg. 2019, 119, 385–392. [Google Scholar] [CrossRef]
- Maslan, J.T.; Lack, C.M.; Zapadka, M.; Gasser, T.G.; Oliver, E. High resolution T2 MRI in the diagnosis of cerebellopontine angle and internal auditory canal lesions. Clin. Imaging 2017, 45, 8–11. [Google Scholar] [CrossRef]
- Carnevale, L.; Lembo, G. Imaging the cerebral vasculature at different scales: Translational tools to investigate the neurovascular interfaces. Cardiovasc. Res. 2024; cvae165, online ahead of print. [Google Scholar] [CrossRef]
- Schwaber, M.K. Medical evaluation of tinnitus. Otolaryngol. Clin. N. Am. 2003, 36, 287–292, vi. [Google Scholar] [CrossRef] [PubMed]
- Schleuning, A.J., 2nd. Management of the patient with tinnitus. Med. Clin. N. Am. 1991, 75, 1225–1237. [Google Scholar] [CrossRef]
- Papanikolaou, V.; Khan, M.H.; Keogh, I.J. Incidental findings on MRI scans of patients presenting with audiovestibular symptoms. BMC Ear Nose Throat Disord. 2010, 10, 6. [Google Scholar] [CrossRef]
- de Groot, J.C.; de Leeuw, F.E.; Oudkerk, M.; van Gijn, J.; Hofman, A.; Jolles, J.; Breteler, M.M. Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Ann. Neurol. 2000, 47, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Barkhof, F.; Wahlund, L.O.; Pantoni, L.; Erkinjuntti, T.; Scheltens, P.; Schmidt, R. CT and MRI rating of white matter lesions. Cerebrovasc. Dis. 2002, 13, 31–36. [Google Scholar] [CrossRef]
- Carhart, R.; Jerger, J. Preferred Method For Clinical Determination of Pure-Tone Thresholds. J. Speech Lang. Hear. Res. 1959, 24, 330–345. [Google Scholar] [CrossRef]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngol. Head Neck Surg. 2019, 161, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Koops, E.A.; Renken, R.J.; Lanting, C.P.; van Dijk, P. Cortical Tonotopic Map Changes in Humans Are Larger in Hearing Loss Than in Additional Tinnitus. J. Neurosci. 2020, 40, 3178–3185. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Hu, H.Y.; Chiu, Y.C.; Lee, F.P.; Huang, H.M. Association of Sudden Sensorineural Hearing Loss With Vertebrobasilar Insufficiency. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 672–675. [Google Scholar] [CrossRef]
- Nakata, T.; Okada, M.; Hanari, T.; Takagi, T.; Fujiwara, T.; Hato, N. Association of the prognosis and severity of idiopathic sudden sensorineural hearing loss with cervical ultrasonographic findings. Auris Nasus Larynx 2021, 48, 1074–1080. [Google Scholar] [CrossRef]
- Carlin, R.E.; McGraw, D.J.; Anderson, C.B. Objective tinnitus resulting from internal carotid artery stenosis. J. Vasc. Surg. 1997, 25, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Cowley, P.O.; Jones, R.; Tuch, P.; McAuliffe, W. Pulsatile tinnitus from reversal of flow in an aberrant occipital artery: Resolved after carotid artery stenting. AJNR Am. J. Neuroradiol. 2009, 30, 995–997. [Google Scholar] [CrossRef] [PubMed]
- Saxby, C.; Koumpa, F.; Mohamed, S.; Singh, A. The use of magnetic resonance imaging in the investigation of patients with unilateral non-pulsatile tinnitus without asymmetrical hearing loss. J. Laryngol. Otol. 2021, 135, 680–683. [Google Scholar] [CrossRef]
- Hoekstra, C.E.; Prijs, V.F.; van Zanten, G.A. Diagnostic yield of a routine magnetic resonance imaging in tinnitus and clinical relevance of the anterior inferior cerebellar artery loops. Otol. Neurotol. 2015, 36, 359–365. [Google Scholar] [CrossRef]
- Shweel, M.; Hamdy, B. Diagnostic utility of magnetic resonance imaging and magnetic resonance angiography in the radiological evaluation of pulsatile tinnitus. Am. J. Otolaryngol. 2013, 34, 710–717. [Google Scholar] [CrossRef]
- Choi, K.J.; Sajisevi, M.B.; Kahmke, R.R.; Kaylie, D.M. Incidence of Retrocochlear Pathology Found on MRI in Patients With Non-Pulsatile Tinnitus. Otol. Neurotol. 2015, 36, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Amoako-Tuffour, Y.; Faiz, K.; Shankar, J.J.S. Diagnostic Yield of MRI for Sensorineural Hearing Loss—An Audit. Can. J. Neurol. Sci. 2020, 47, 656–660. [Google Scholar] [CrossRef]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Age-related hearing loss and cognitive decline: MRI and cellular evidence. Ann. N. Y Acad. Sci. 2021, 1500, 17–33. [Google Scholar] [CrossRef]

| Category | Values |
|---|---|
| Age (median years; IQR, Q1–Q3) | 51 (24; 41–65) |
| Sex (men/women) | 184/239 |
| Tinnitus onset (median months; IQR, Q1–Q3) | 12 (33; 3–9) |
| Tinnitus location right, n (%) left, n (%) bilateral, n (%) | 103 (24.3%) 134 (31.7%) 186 (44%) |
| PTA (median dB; IQR, Q1–Q3) | 30 (25; 20–45) |
| Tinnitus intensity (median dB; IQR, Q1–Q3) | 30 (27.5; 20–47.5) |
| Tinnitus frequency (median Hz; IQR, Q1–Q3) | 4000 (6000; 2000–8000) |
| Dependent | Predictor | β | Std. Error | p-Value | OR | 95% CI (Lower Bound) | 95% CI (Upper Bound) |
|---|---|---|---|---|---|---|---|
| Sensorineural hearing loss | Carotid–vertebral ultrasonography alteration | 5.891 | 0.399 | 0.000 * | 361.596 | 165.303 | 790.982 |
| Brain MRI alteration | 5.854 | 0.396 | 0.000 * | 348.777 | 160.589 | 757.496 | |
| Sex | −0.262 | 0.330 | 0.428 | 0.770 | 0.403 | 1.471 | |
| Chronic symptoms | −0.533 | 0.368 | 0.148 | 0.587 | 0.285 | 1.208 |
| Dependent | Predictor | β | Std. Error | p-Value | OR | 95% CI (Lower Bound) | 95% CI (Upper Bound) |
|---|---|---|---|---|---|---|---|
| Tinnitus intensity (higher intensity = over 30 dB) | Carotid–vertebral ultrasonography alteration | −2.896 | 1.094 | 0.008 * | 0.055 | 0.006 | 0.472 |
| Brain MRI alteration | 0.197 | 0.601 | 0.744 | 1.217 | 0.375 | 3.955 | |
| Sex | −0.199 | 0.552 | 0.718 | 0.819 | 0.278 | 2.418 | |
| Chronic symptoms | −0.604 | 0.619 | 0.330 | 0.547 | 0.162 | 1.841 | |
| Right-sided tinnitus | −0.603 | 0.649 | 0.352 | 0.547 | 0.153 | 1.950 | |
| Left-sided tinnitus | −1.231 | 0.723 | 0.089 | 0.292 | 0.071 | 1.205 | |
| Bilateral tinnitus | 0.230 | 0.328 | 0.000 * | 1.259 | 0.662 | 2.395 | |
| Tinnitus frequency (higher frequency = over 4000 Hz) | Carotid–vertebral ultrasonography alteration | 0.336 | 0.488 | 0.491 | 1.400 | 0.538 | 3.642 |
| Brain MRI alteration | 0.100 | 0.447 | 0.824 | 1.105 | 0.460 | 2.655 | |
| Sex | −0.074 | 0.423 | 0.861 | 0.929 | 0.406 | 2.126 | |
| Chronic symptoms | 0.075 | 0.509 | 0.883 | 1.078 | 0.398 | 2.921 | |
| Right-sided tinnitus | −0.021 | 0.578 | 0.971 | 0.979 | 0.315 | 3.039 | |
| Left-sided tinnitus | 0.615 | 0.367 | 0.094 | 1.849 | 0.900 | 3.799 | |
| Bilateral tinnitus | −0.634 | 0.496 | 0.201 | 0.530 | 0.201 | 1.402 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnár, A.; Molnár, V.; Mavrogeni, P.; Maihoub, S. The Influence of Carotid and Vertebral Doppler Ultrasonography and Brain MRI Abnormalities on Hearing Levels, Tinnitus Intensities and Frequencies. Audiol. Res. 2025, 15, 29. https://doi.org/10.3390/audiolres15020029
Molnár A, Molnár V, Mavrogeni P, Maihoub S. The Influence of Carotid and Vertebral Doppler Ultrasonography and Brain MRI Abnormalities on Hearing Levels, Tinnitus Intensities and Frequencies. Audiology Research. 2025; 15(2):29. https://doi.org/10.3390/audiolres15020029
Chicago/Turabian StyleMolnár, András, Viktória Molnár, Panayiota Mavrogeni, and Stefani Maihoub. 2025. "The Influence of Carotid and Vertebral Doppler Ultrasonography and Brain MRI Abnormalities on Hearing Levels, Tinnitus Intensities and Frequencies" Audiology Research 15, no. 2: 29. https://doi.org/10.3390/audiolres15020029
APA StyleMolnár, A., Molnár, V., Mavrogeni, P., & Maihoub, S. (2025). The Influence of Carotid and Vertebral Doppler Ultrasonography and Brain MRI Abnormalities on Hearing Levels, Tinnitus Intensities and Frequencies. Audiology Research, 15(2), 29. https://doi.org/10.3390/audiolres15020029







