Brachial Tunneled Peripherally Inserted Central Catheters and the Risk of Catheter Complications: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. PICOS Questions
2.2. Data Sources and Searches
2.3. Study Selection
2.4. Data Extraction and Quality Assessment
2.5. Data Synthesis and Analysis
2.6. Assessment of Heterogeneity
3. Results
3.1. Study Characteristics
3.2. Risk of Bias and Applicability
3.3. Results of Individual Studies
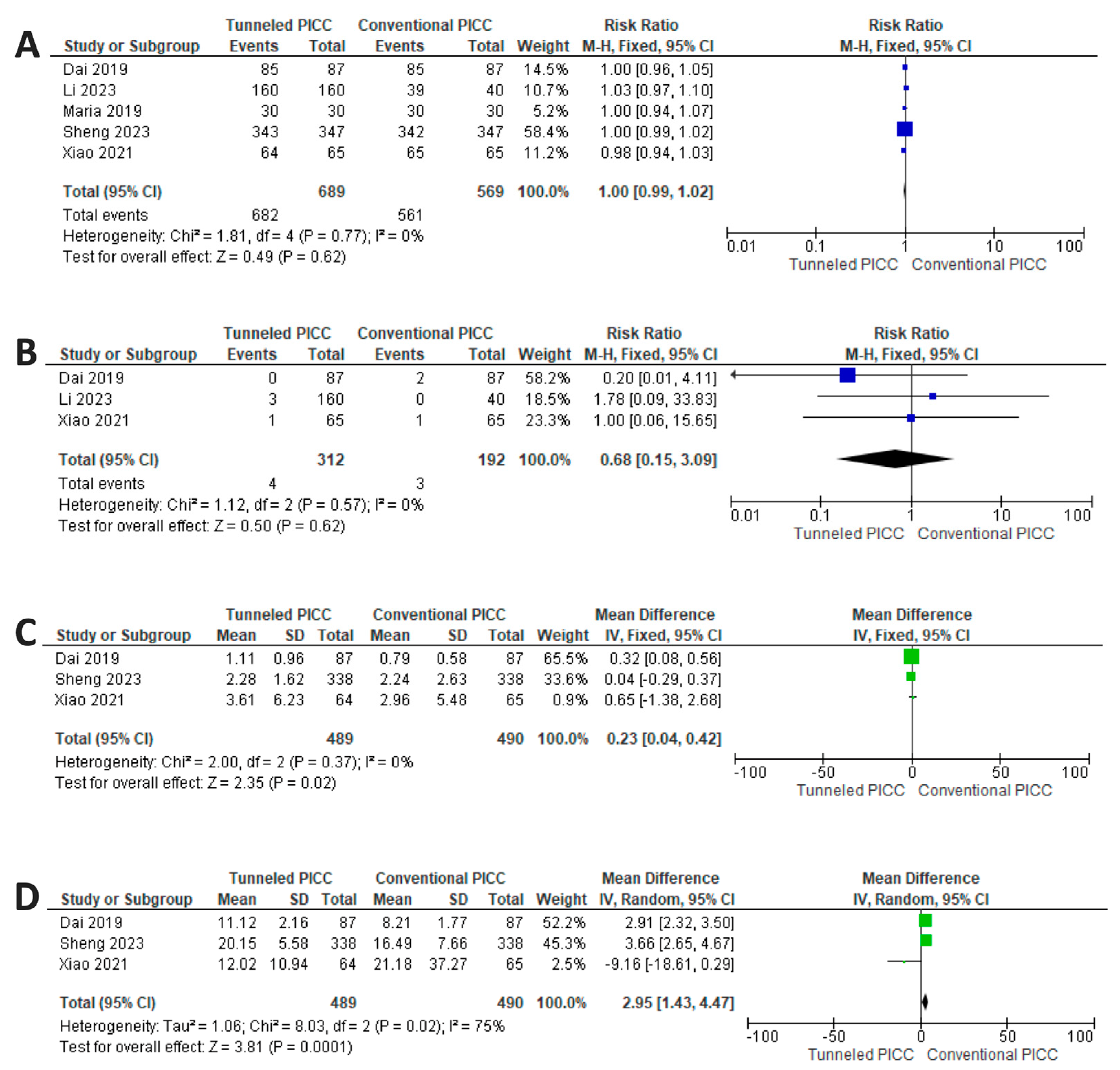
3.4. Results of Syntheses: Safety Profile
3.5. Results of Synthesis: Efficacy Profile
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Conflicts of Interest
References
- McMullen, K.M.; Smith, B.A.; Rebmann, T. Impact of SARS-CoV-2 on hospital-acquired infection rates in the United States: Predictions and early results. Am. J. Infect. Control 2020, 48, 1409–1411. [Google Scholar] [CrossRef]
- Gidaro, A.; Vailati, D.; Gemma, M. Retrospective survey from vascular access team Lombardy net in COVID-19 era. J. Vasc. Access 2022, 23, 532–537. [Google Scholar] [CrossRef]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin. Infect. Dis. 2011, 52, 1087–1099. [Google Scholar] [CrossRef]
- Broviac, J.W.; Cole, J.J.; Scribner, B.H. A silicone rubber atrial catheter for prolonged parenteral alimentation. Surg. Gynecol. Obstet. 1973, 136, 602–606. [Google Scholar]
- Hickman, R.O.; Buckner, C.D.; Clift, R.A.; Sanders, J.E.; Stewart, P.; Thomas, E.D. A modified right atrial catheter for access to the venous system in marrow transplant recipients. Surg. Gynecol. Obstet. 1979, 148, 871–875. [Google Scholar]
- Heberlein, W. Principles of tunneled cuffed catheter placement. Tech. Vasc. Interv. Radiol. 2011, 14, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Loveday, H.P.; Wilson, J.A.; Pratt, R.J.; Golsorkhi, M.; Tingle, A.; Bak, A.; Browne, J.; Prieto, J.; Wilcox, M. epic3: National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J. Hosp. Infect. 2014, 86 (Suppl. S1), S1–S70. [Google Scholar] [CrossRef]
- Selby, J.B., Jr.; Cohn, D.J.; Koenig, G. Peripherally inserted tunnelled catheters: A new option for venous access. Minim. Invasive Ther. Allied Technol. 2001, 10, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Giustivi, D.; Gidaro, A.; Baroni, M.; Paglia, S. Tunneling technique of PICCs and Midline catheters. J. Vasc. Access 2022, 23, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Elli, S.; Abbruzzese, C.; Cannizzo, L.; Vimercati, S.; Vanini, S.; Lucchini, A. ‘Extended subcutaneous route’ technique: A quick subcutaneous tuneling technique for PICC insertion. J. Vasc. Access 2017, 18, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Ostroff, M.D.; Moureau, N.L. Report of Modification for Peripherally Inserted Central Catheter Placement: Subcutaneous Needle Tunnel for High Upper Arm Placement. J. Infus. Nurs. Off. Publ. Infus. Nurses Soc. 2017, 40, 232–237. [Google Scholar] [CrossRef]
- Brescia, F.; Pittiruti, M.; Spencer, T.R.; Dawson, R.B. The SIP protocol update: Eight strategies, incorporating Rapid Peripheral Vein Assessment (RaPeVA), to minimize complications associated with peripherally inserted central catheter insertion. J. Vasc. Access 2024, 25, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Ostroff, M.D.; Moureau, N.; Pittiruti, M. Rapid Assessment of Vascular Exit Site and Tunneling Options (RAVESTO): A new decision tool in the management of the complex vascular access patients. J. Vasc. Access 2023, 24, 311–317. [Google Scholar] [CrossRef]
- Gorski, L.A.; Hadaway, L.; Hagle, M.E.; Broadhurst, D.; Clare, S.; Kleidon, T.; Meyer, B.M.; Nickel, B.; Rowley, S.; Sharpe, E.; et al. Infusion Therapy Standards of Practice, 8th Edition. J. Infus. Nurs. Off. Publ. Infus. Nurses Soc. 2021, 44, S1–S224. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaf, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Risk of Bias Tool 2. Available online: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed on 1 December 2022).
- Schunemann, H.B.J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. The GRADE Working Group. Updated October 2013. Available online: https://guidelinedevelopment.org/handbook (accessed on 23 September 2021).
- Higgins, J.P.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011). The Cochrane Collaboration. 2011. Available online: http://handbook.cochrane.org (accessed on 23 September 2021).
- McQuay, H.J.; Moore, R.A. Using numerical results from systematic reviews in clinical practice. Ann. Intern. Med. 1997, 126, 712–720. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. J. Intell. Inf. Syst. 2006, 27, 159–184. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, E.T.; Cho, S.B.; Lee, J.H.; Shim, D.J. Subcutaneous tuneling versus conventional insertion of peripherally inserted central catheters in hospitalized patients (TUNNEL-PICC): A study protocol for a randomized controlled trial. Trials 2022, 23, 781. [Google Scholar] [CrossRef]
- Kim, I.J.; Shim, D.J.; Lee, J.H.; Kim, E.T.; Byeon, J.H.; Lee, H.J.; Cho, S.G. Impact of subcutaneous tunnels on peripherally inserted catheter placement: A multicenter retrospective study. Eur. Radiol. 2019, 29, 2716–2723. [Google Scholar] [CrossRef]
- Saijo, F.; Odaka, Y.; Mutoh, M.; Katayose, Y.; Tokumura, H. A novel technique of axillary vein puncture involving peripherally inserted central venous catheters for a small basilic vein. J. Vasc. Access 2018, 19, 311–315. [Google Scholar] [CrossRef]
- Low, X.Z.; Tay, K.H.; Leong, S.; Lo, R.H.G.; Zhuang, K.D.; Chua, J.M.E.; Too, C.W. Repurposing the power injectable peripherally inserted central catheter as a tunnelled, non-cuffed, centrally inserted central venous catheter in oncological patients for short- to mid-term vascular access: A pilot study. J. Vasc. Access 2021, 22, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Lingegowda, D.; Gehani, A.; Sen, S.; Mukhopadhyay, S.; Ghosh, P. Centrally inserted tunnelled peripherally inserted central catheter: Off-label use for venous access in oncology patients. J. Vasc. Access 2020, 21, 773–777. [Google Scholar] [CrossRef]
- Gupta, N.; Gandhi, D.; Sharma, S.; Goyal, P.; Choudhary, G.; Li, S. Tunneled and routine peripherally inserted central catheters placement in adult and pediatric population: Review, technical feasibility, and troubleshooting. Quant. Imaging Med. Surg. 2021, 11, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Ostroff, M.D.; Goldberg, D.; Bauhman, G.; Korb, A.C. Venous catheter at alternate exit site in a 2-year-old requiring long-term antibiotics for osteomyelitis: A case report. J. Vasc. Access 2021, 22, 1013–1016. [Google Scholar] [CrossRef]
- Fabiani, A.; Dreas, L.; Sanson, G. Tuneling a midline catheter: When the traffic light shifts from yellow to green. J. Vasc. Access 2018, 19, 667–671. [Google Scholar] [CrossRef]
- Dai, C.; Li, J.; Li, Q.M.; Guo, X.; Fan, Y.Y.; Qin, H.Y. Effect of tunneled and nontunneled peripherally inserted central catheter placement: A randomized controlled trial. J. Vasc. Access 2020, 21, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.F.; Xiao, C.Q.; Li, J.; Dai, C.; Fan, Y.Y.; Cao, H.J.; Qin, H.Y. Subcutaneous tunneling technique to improve outcomes for patients undergoing chemotherapy with peripherally inserted central catheters: A randomized controlled trial. J. Int. Med. Res. 2021, 49, 3000605211004517. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Z.; Lin, X.; Huang, W.; Huang, C.; Luo, J.; Li, L.; Zhang, X.; Qin, H. A Randomized Controlled Trial to Compare Peripherally Inserted Central Catheter Tunnel Lengths in Adult Patients with Cancer. Clin. J. Oncol. Nurs. 2023, 27, 295–304. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, L.H.; Wu, Y.; Gao, W.; Dongye, S.Y. Implementation of Tunneled Peripherally Inserted Central Catheters Placement in Cancer Patients: A Randomized Multicenter Study. Clin. Nurs. Res. 2023, 31, 10547738231194099. [Google Scholar] [CrossRef]
- Maria, K.; Theodoros, K.; Maria, B.; Panagiotis, K.; Emmanouil, S.; Evangelos, K.A. Implementation of tunneled versus not tunneled peripherally inserted central catheters. J. Vasc. Nurs. 2019, 37, 132–134. [Google Scholar] [CrossRef]
- Annetta, M.G.; Bertoglio, S.; Biffi, R.; Brescia, F.; Giarretta, I.; Greca, A.; Panocchia, N.; Passaro, G.; Perna, F.; Pinelli, F.; et al. Management of antithrombotic treatment and bleeding disorders in patients requiring venous access devices: A systematic review and a GAVeCeLT consensus statement. J. Vasc. Access 2022, 23, 660–671. [Google Scholar] [CrossRef]
- Elli, S.; Cannizzo, L.; Giannini, L.; Romanato, F.; Trimarco, C.; Pessina, M.; Lucchini, A.; Foti, G.; Rondelli, E. Femorally inserted central catheters with exit site at mid-thigh: A low risk alternative for central venous catheterization. J. Vasc. Access 2022, 11297298221132073. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, A.; Donadoni, M.; Quici, M.; Rizzi, G.; La Cava, L.; Foschi, A.; Calloni, M.; Casella, F.; Martini, E.; Taino, A.; et al. Safety of mid-thigh exit site venous catheters in multidrug resistant colonized patients. J. Vasc. Access 2023, 11297298231188150. [Google Scholar] [CrossRef] [PubMed]
- Balsorano, P.; Virgili, G.; Villa, G.; Pittiruti, M.; Romagnoli, S.; De Gaudio, A.R.; Pinelli, F. Peripherally inserted central catheter-related thrombosis rate in modern vascular access era-when insertion technique matters: A systematic review and meta-analysis. J. Vasc. Access 2020, 21, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.G.; Wu, O.; Bodenham, A.R.; Agarwal, R.; Menne, T.F.; Jones, B.L.; Heggie, R.; Hill, S.; Dixon-Hughes, J.; Soulis, E.; et al. Central venous access devices for the delivery of systemic anticancer therapy (CAVA): A randomised controlled trial. Lancet 2021, 398, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Govindan, S.; Snyder, A.; Flanders, S.A.; Chopra, V. Peripherally Inserted Central Catheters in the ICU: A Retrospective Study of Adult Medical Patients in 52 Hospitals. Crit. Care Med. 2018, 46, e1136–e1144. [Google Scholar] [CrossRef] [PubMed]



| Author, Years | Country, Type, and Time of Enrollment | Tunneling Tech. | Skin Closure | PICC Dressing * | Definition of Wound Oozing | Definition of Thrombosis | Definition of Infection | Definition of Dislodgement |
|---|---|---|---|---|---|---|---|---|
| Dai et al., 2019 [31] | China Monocentric from July 2018 through May 2019 (11 months) | Large peripheral cannula 14 G | Wound closure strip | Gauze and transparent membrane | Wound oozing as an important outcome was recorded when oozing lasted more than 24 h. Grade 1 was a small amount of oozing lasting for 2–3 days, Grade 2 was oozing lasting for 4–5 days, and Grade 3 was oozing lasting more than 6 days | Venous thrombosis was classified as symptomatic or asymptomatic and confirmed as having an association with the PICC or occurring within 5 days of extubation | Infections resulting from the use of catheters were defined according to national infection-control guidelines. | Catheter dislodgement was recorded when the tip moved more than 2 cm. |
| Xiao et al., 2021 [32] | China Monocentric from July 2019 through January 2020 (7 months) | Blunt tunneler | Wound closure strip | Gauze and transparent membrane | Oozing that lasted >24 h after placement. Classified into three grades according to severity: Grade 1 (bleeding lasting for 2 to 3 days), Grade 2 (bleeding lasting for 4 to 5 days), and Grade 3 (bleeding lasting >6 days). | The presence of an intraluminal thrombus as confirmed by color Doppler ultrasound. Classified as symptomatic or asymptomatic (symptomatic thrombosis was diagnosed when symptoms occurred). | Defined according to the Centers for Disease Control and Prevention and classified as local infection or central line-associated bloodstream infection. | Exposed portion of PICC prolapsed by >2 cm. |
| Li et al., 2023 [33] | China Monocentric from March 2021 through August 2021 (6 months) | Blunt tunneler | Nylon sutures | Gauze and transparent membrane | Oozing that lasted for more than 24 h. | Venous thrombosis was identified by pain and swelling of the arm and confirmed by B-mode ultrasound | Infections included local skin infections and CLABSI, which were diagnosed by clinical physicians and confirmed by blood culture results. | The catheter shifted more than 2 cm |
| Sheng et al., 2023 [34] | China Multicenter (three hospital) from August 2011 through December 2021 (5 months) | Blunt tunneler | Octyl cyanoacrylate skin adhesive | Glue and transparent membrane | N/A | CRT was confirmed by ultrasound or CT examination showing the presence of a thrombus in the vein with a catheter | Infections were defined according to Infectious Diseases Society of America criteria | Catheter malposition was defined as exposed length prolapsed ≥5 cm |
| Maria et al., 2019 [35] | Greece Monocentric from August 2014 through February 2015 (7 months) | Large peripheral cannula 14 G | Nylon sutures | Glue and transparent membrane | N/A | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giustivi, D.; Donadoni, M.; Elli, S.M.; Casella, F.; Quici, M.; Cogliati, C.; Cavalli, S.; Rizzi, G.; La Cava, L.; Bartoli, A.; et al. Brachial Tunneled Peripherally Inserted Central Catheters and the Risk of Catheter Complications: A Systematic Review and Meta-Analysis. Nurs. Rep. 2024, 14, 455-467. https://doi.org/10.3390/nursrep14010035
Giustivi D, Donadoni M, Elli SM, Casella F, Quici M, Cogliati C, Cavalli S, Rizzi G, La Cava L, Bartoli A, et al. Brachial Tunneled Peripherally Inserted Central Catheters and the Risk of Catheter Complications: A Systematic Review and Meta-Analysis. Nursing Reports. 2024; 14(1):455-467. https://doi.org/10.3390/nursrep14010035
Chicago/Turabian StyleGiustivi, Davide, Mattia Donadoni, Stefano Maria Elli, Francesco Casella, Massimiliano Quici, Chiara Cogliati, Silvia Cavalli, Giulia Rizzi, Leyla La Cava, Arianna Bartoli, and et al. 2024. "Brachial Tunneled Peripherally Inserted Central Catheters and the Risk of Catheter Complications: A Systematic Review and Meta-Analysis" Nursing Reports 14, no. 1: 455-467. https://doi.org/10.3390/nursrep14010035
APA StyleGiustivi, D., Donadoni, M., Elli, S. M., Casella, F., Quici, M., Cogliati, C., Cavalli, S., Rizzi, G., La Cava, L., Bartoli, A., Martini, E., Taino, A., Perego, M., Foschi, A., Castelli, R., Calloni, M., & Gidaro, A. (2024). Brachial Tunneled Peripherally Inserted Central Catheters and the Risk of Catheter Complications: A Systematic Review and Meta-Analysis. Nursing Reports, 14(1), 455-467. https://doi.org/10.3390/nursrep14010035









