Effectiveness of Virtual Reality for Managing Pain, Fear, and Anxiety in Children and Adolescents Undergoing Needle-Related Procedures: Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
2.2. Data Extraction
2.3. Quality and Bias Risk Assessment
2.4. Data Synthesis and Statistical Analysis
3. Results
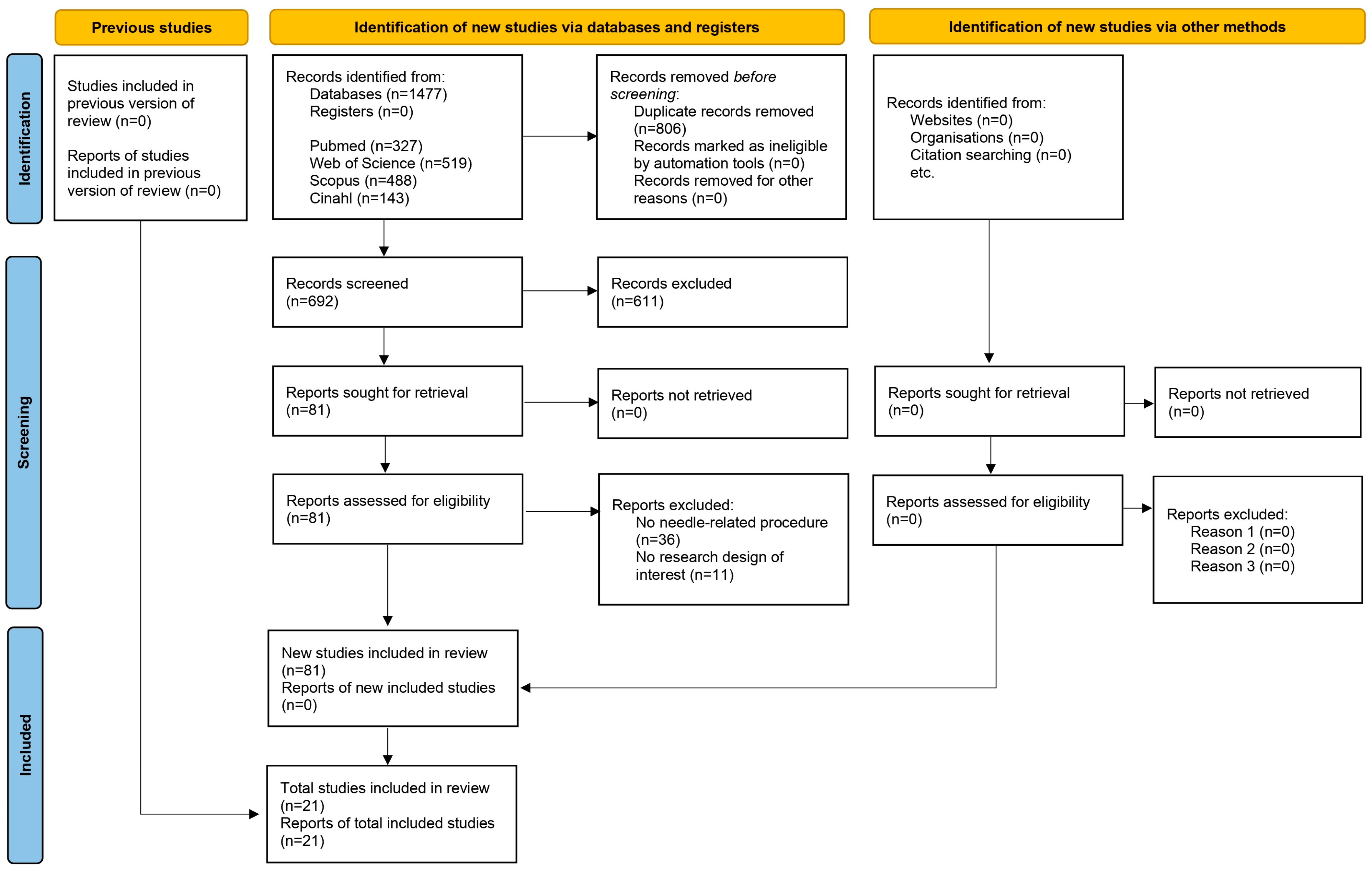
3.1. Results Obtained in the Selection of Articles
3.2. Descriptive Analysis of the Results Found
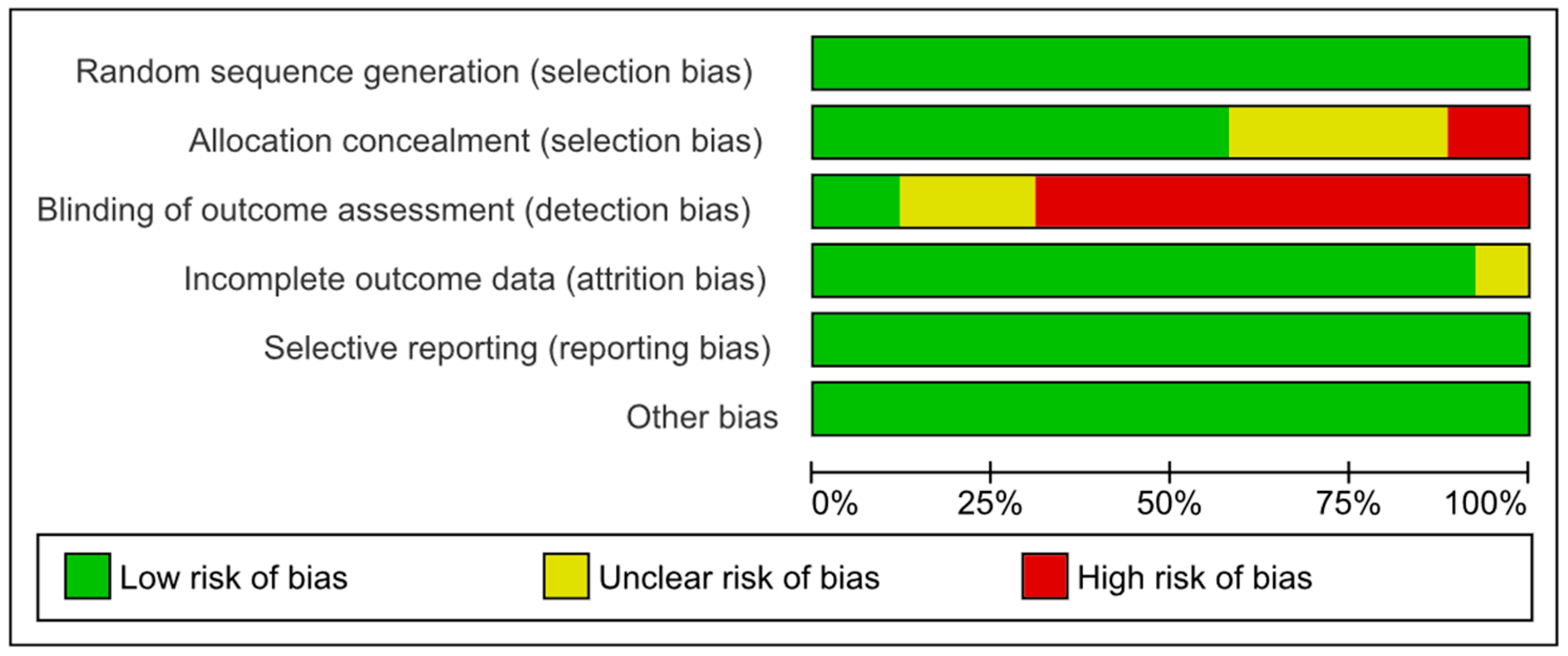
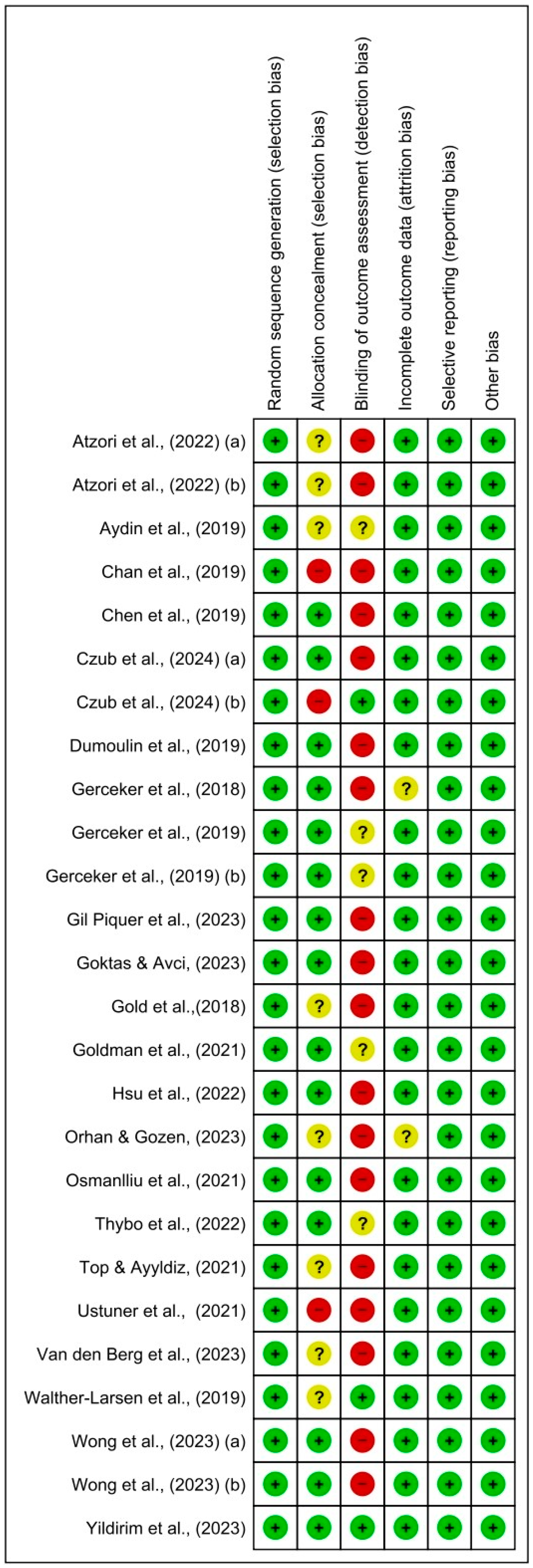
3.3. Assessment of Risk of Bias in Selected Studies and Publication Bias
3.4. Results of Meta-Analysis
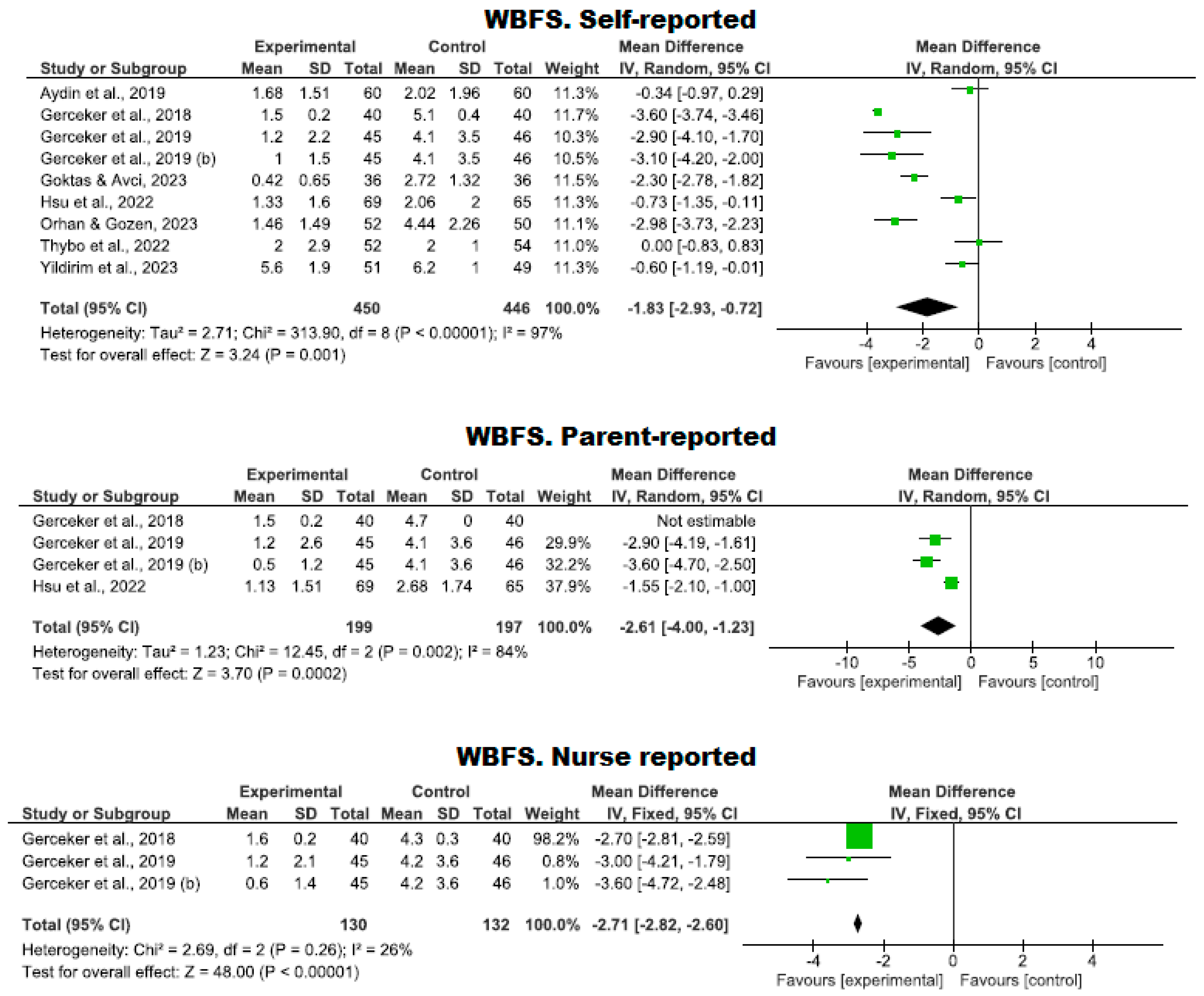
3.4.1. Assessment of Pain Using Virtual Reality
Use of the WBFS Scale
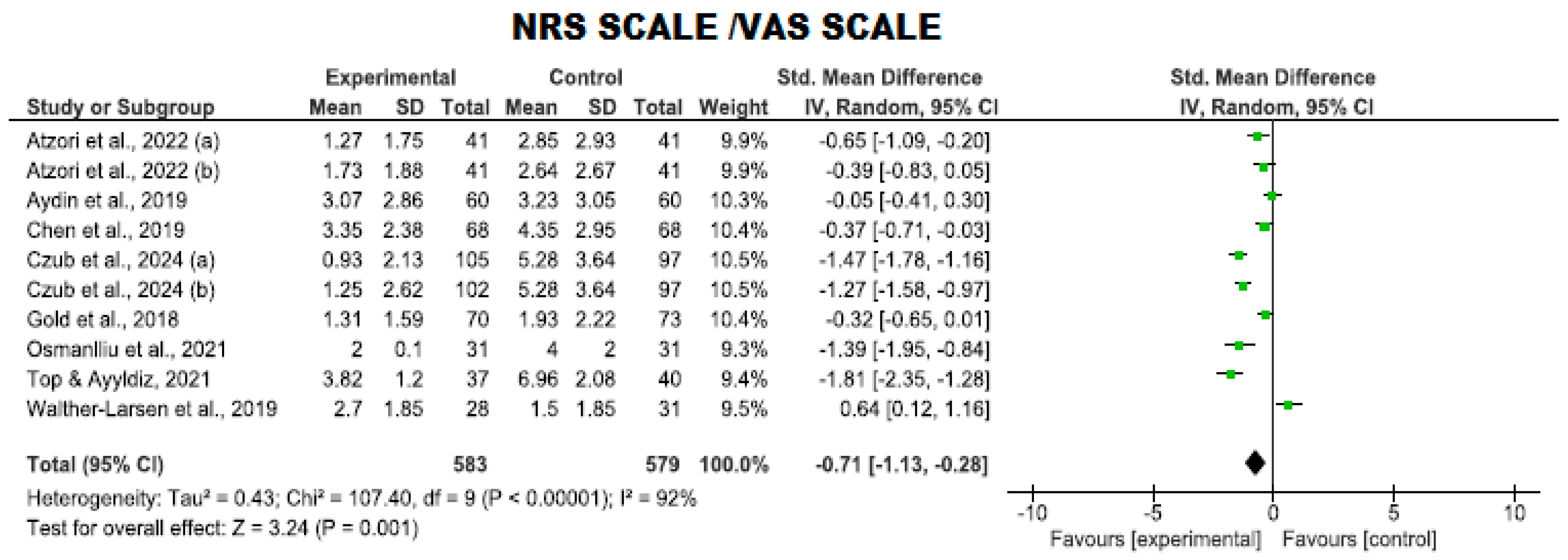
Use of the NRS and VAS Scales
Other Pain Assessment Scales
3.4.2. Assessment of Anxiety Using Virtual Reality
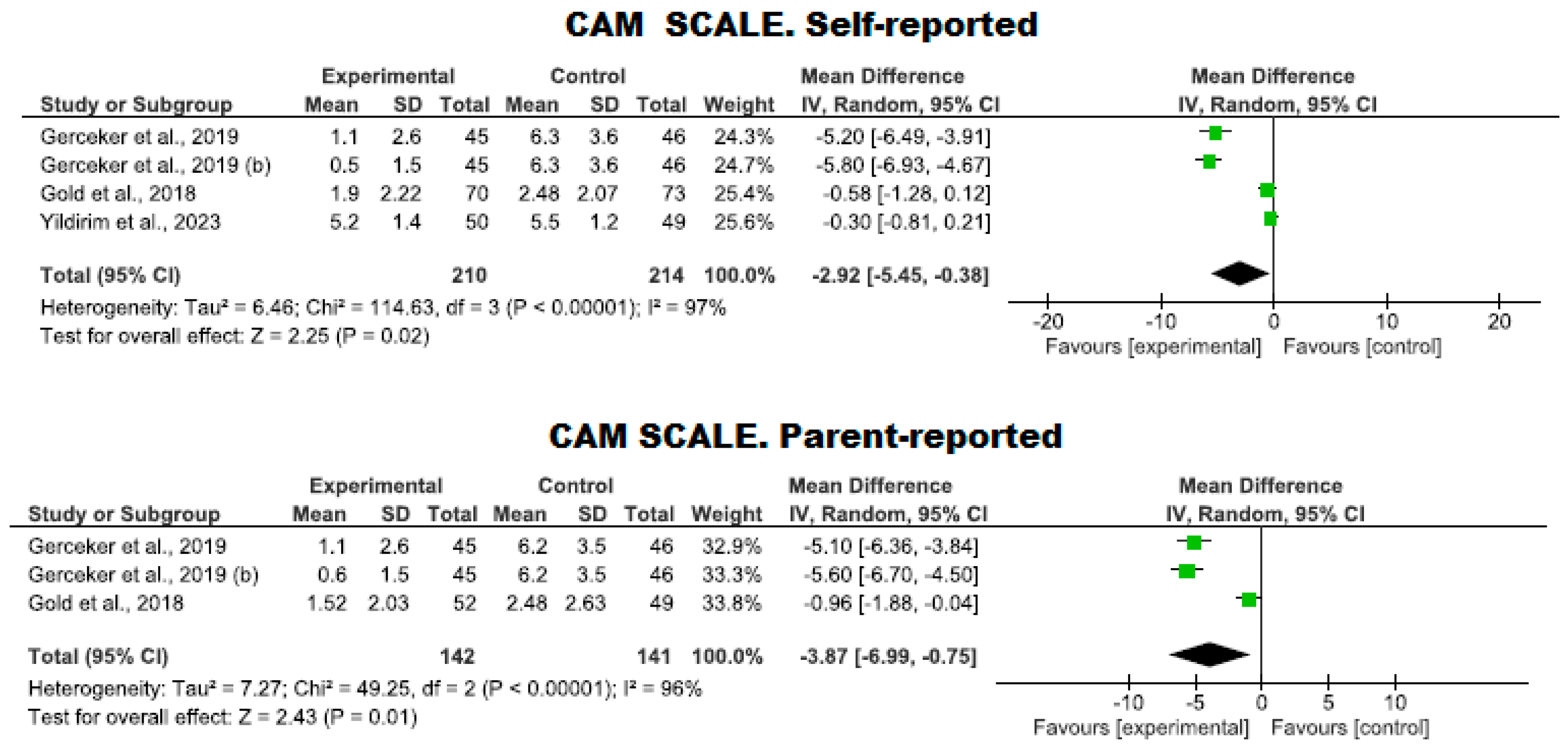
Use of the CAM-S Scale
Use of Other Scales to Assess Anxiety
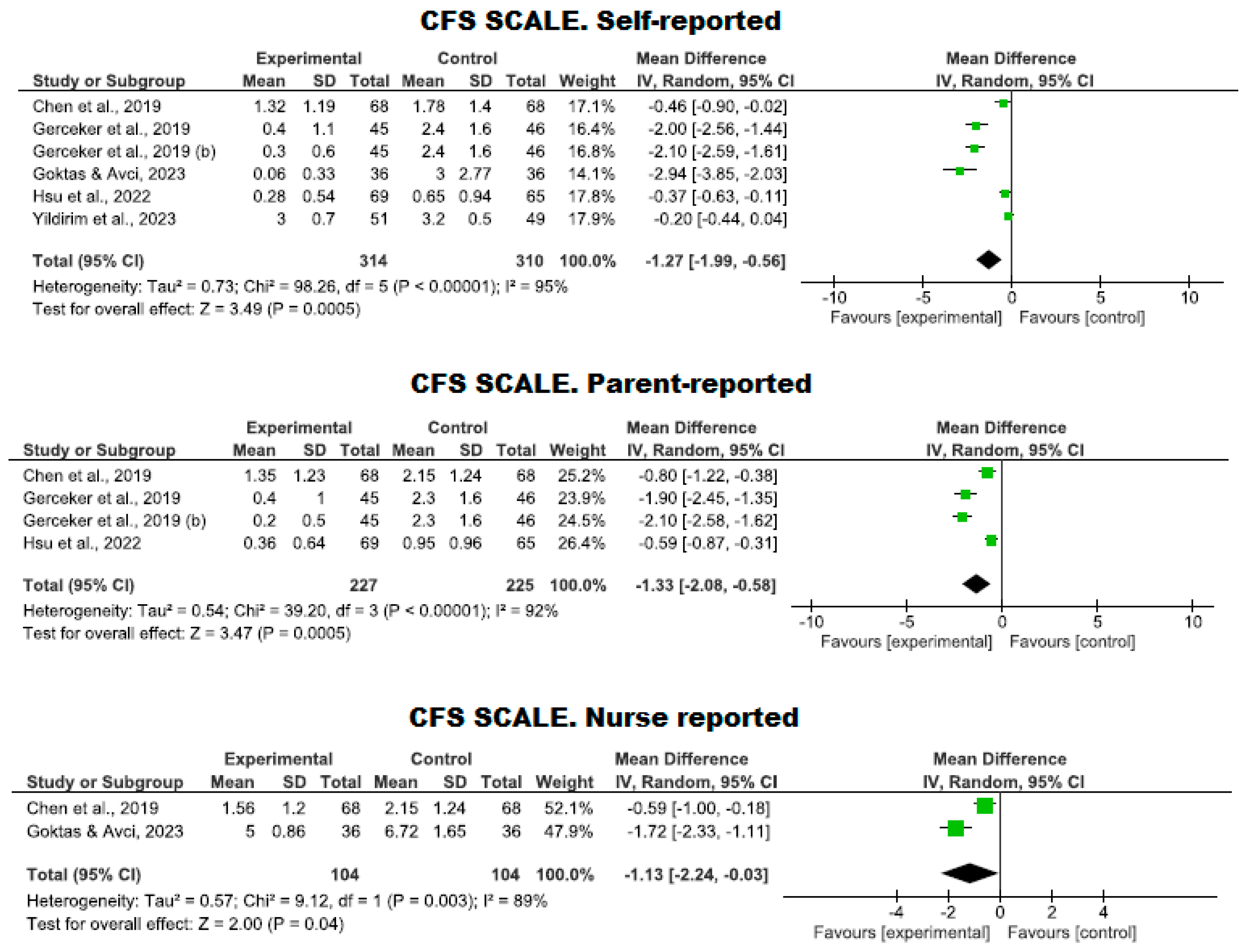
3.4.3. Management of Fear Using Virtual Reality
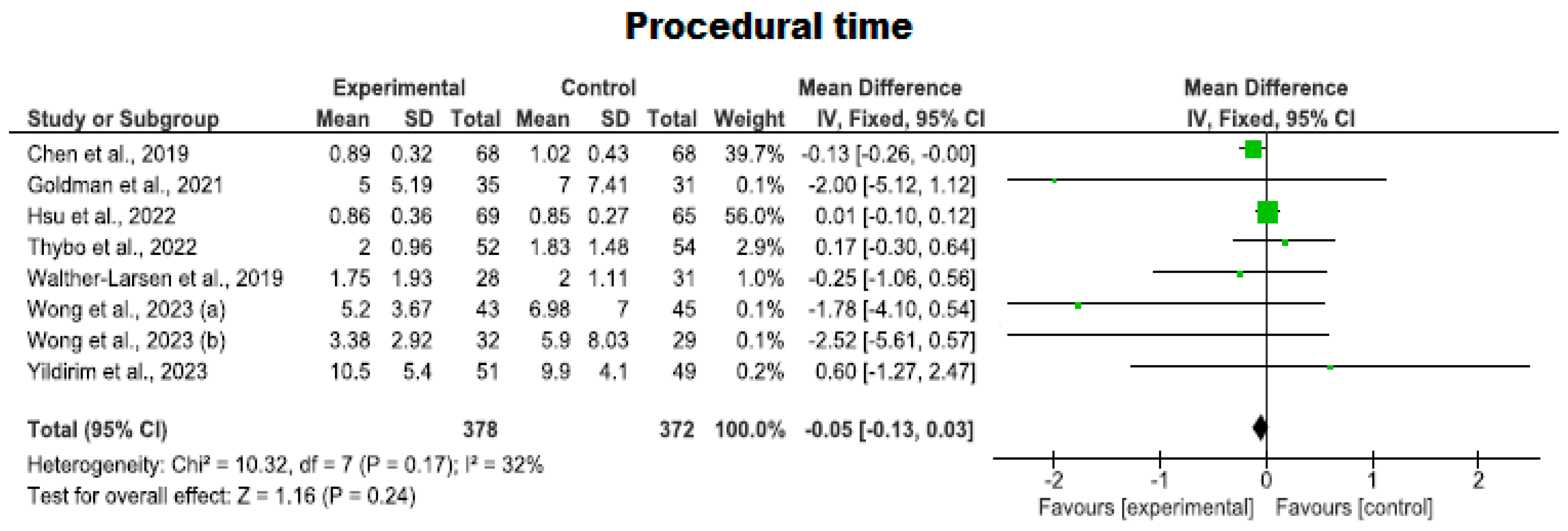
3.4.4. Procedural Time Using Virtual Reality
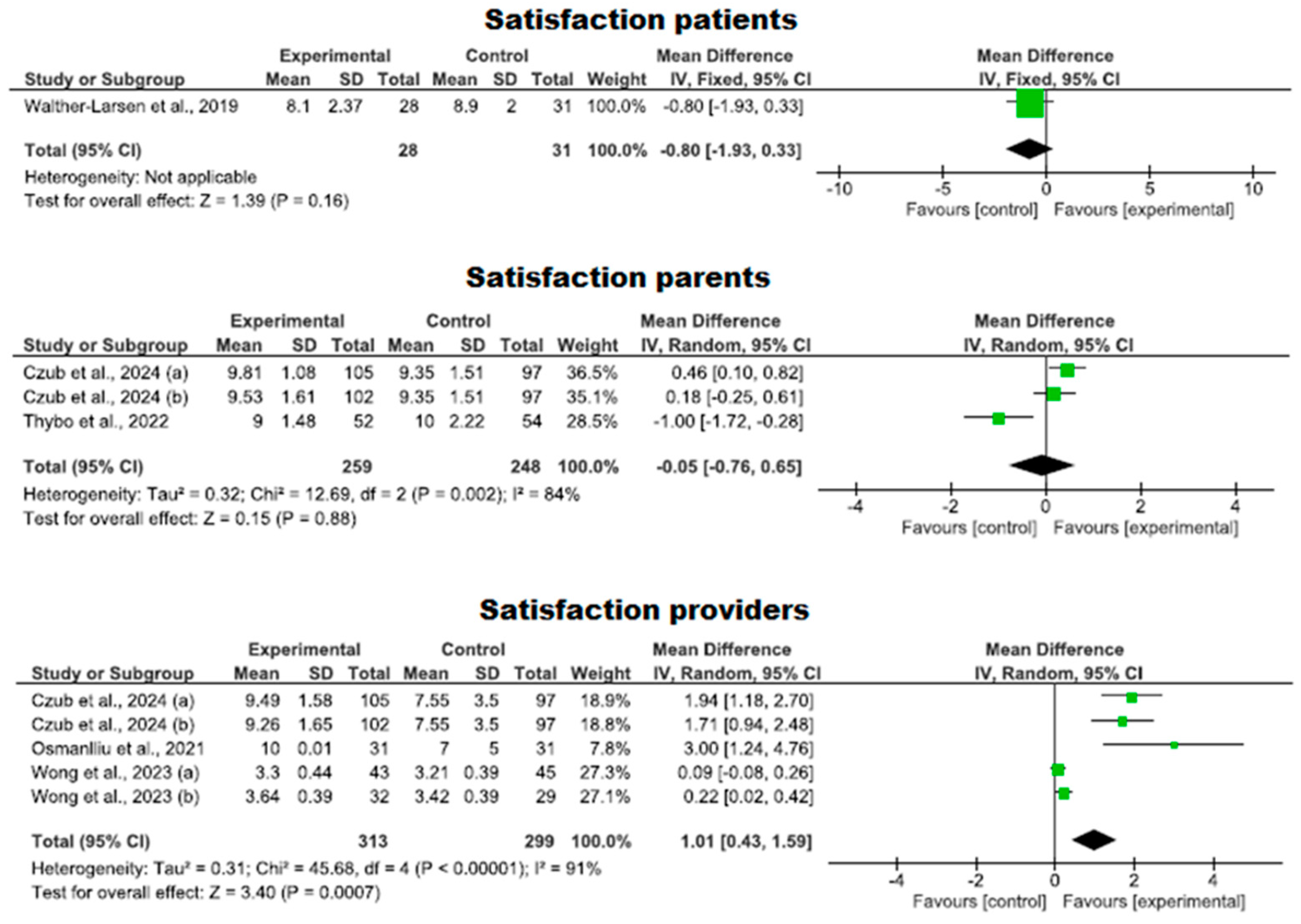
3.4.5. Degree of Satisfaction with the Use of Virtual Reality
4. Discussion
4.1. Limitations and Strengths
4.2. Prospective Lines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Use of Artificial Intelligence
Conflicts of Interest
References
- Walther-Larsen, S.; Petersen, T.; Friis, S.M.; Aagaard, G.; Drivenes, B.; Opstrup, P. Immersive Virtual Reality for Pediatric Procedural Pain: A Randomized Clinical Trial. Hosp. Pediatr. 2019, 9, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.I.; SooHoo, M.; Laikin, A.M.; Lane, A.S.; Klein, M.J. Effect of an Immersive Virtual Reality Intervention on Pain and Anxiety Associated With Peripheral Intravenous Catheter Placement in the Pediatric Setting: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2122569. [Google Scholar] [CrossRef]
- Gerçeker, G.Ö.; Bektaş, M.; Aydınok, Y.; Ören, H.; Ellidokuz, H.; Olgun, N. The effect of virtual reality on pain, fear, and anxiety during access of a port with huber needle in pediatric hematology-oncology patients: Randomized controlled trial. Eur. J. Oncol. Nurs. 2021, 50, 101886. [Google Scholar] [CrossRef]
- Saliba, T.; Schmartz, D.; Fils, J.-F.; Van Der Linden, P. The use of virtual reality in children undergoing vascular access procedures: A systematic review and meta-analysis. J. Clin. Monit. Comput. 2022, 36, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- International Association for the Study of Pain. International Association for the Study of Pain (IASP). 2020. Available online: https://www.iasp-pain.org/resources/terminology/ (accessed on 1 July 2024).
- Ran, L.; Zhao, N.; Fan, L.; Zhou, P.; Zhang, C.; Yu, C. Application of virtual reality on non-drug behavioral management of short-term dental procedure in children. Trials 2021, 22, 562. [Google Scholar] [CrossRef]
- American Psychiatric Association (Ed.) Diagnóstico y Manual Estadístico de los Trastornos Mentales DSM-5; American Psychiatric Publishing: Washington, DC, USA, 2023. [Google Scholar]
- Atzori, B.; Vagnoli, L.; Graziani, D.; Hoffman, H.G.; Sampaio, M.; Alhalabi, W.; Messeri, A.; Lauro-Grotto, R. An Exploratory Study on the Effectiveness of Virtual Reality Analgesia for Children and Adolescents with Kidney Diseases Undergoing Venipuncture. Int. J. Environ. Res. Public Health 2022, 19, 2291. [Google Scholar] [CrossRef] [PubMed]
- Eijlers, R.; Dierckx, B.; Staals, L.M.; Berghmans, J.M.; van der Schroeff, M.P.; Strabbing, E.M.; Wijnen, R.M.H.; Hillegers, M.H.J.; Legerstee, J.S.; Utens, E.M.W.J. Virtual reality exposure before elective day care surgery to reduce anxiety and pain in children: A randomised controlled trial. Eur. J. Anaesthesiol. 2019, 36, 728–737. [Google Scholar] [CrossRef]
- Hsu, M.-F.; Whu, Y.-W.; Lin, I.-C.; Liu, C.-Y.; Lai, F.-C.; Liu, P.-C.; Chen, C.-W. Effectiveness of Virtual Reality Interactive Play for Children during Intravenous Placement: A Randomized Controlled Trial. Asian Nurs. Res. 2022, 16, 87–93. [Google Scholar] [CrossRef]
- Dumoulin, S.; Bouchard, S.; Ellis, J.; Lavoie, K.L.; Vézina, M.-P.; Charbonneau, P.; Tardif, J.; Hajjar, A. A Randomized Controlled Trial on the Use of Virtual Reality for Needle-Related Procedures in Children and Adolescents in the Emergency Department. Games Health J. 2019, 8, 285–293. [Google Scholar] [CrossRef]
- Chan, E.; Hovenden, M.; Ramage, E.; Ling, N.; Pham, J.H.; Rahim, A.; Lam, C.; Liu, L.; Foster, S.; Sambell, R.; et al. Virtual Reality for Pediatric Needle Procedural Pain: Two Randomized Clinical Trials. J. Pediatr. 2019, 209, 160–167.e4. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Cheng, S.-F.; Lee, P.-C.; Lai, C.-H.; Hou, I.-C.; Chen, C.-W. Distraction using virtual reality for children during intravenous injections in an emergency department: A randomised trial. J. Clin. Nurs. 2020, 29, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Merriam-Webster. [Merriam-Webster]. Merriam-Webster. 2024. Available online: https://www.merriam-webster.com/dictionary/virtual+reality (accessed on 2 July 2024).
- Kumari, S.; Bahuguna, R.; Garg, N.; Yeluri, R. Immersive and Non-Immersive Virtual Reality Distraction on Pain Perception to Intraoral Injections. J. Clin. Pediatr. Dent. 2021, 45, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Shen, J.; Wheeler, K.K.; Patterson, J.; Lever, K.; Armstrong, M.; Shi, J.; Thakkar, R.K.; Groner, J.I.; Noffsinger, D.; et al. Efficacy of Smartphone Active and Passive Virtual Reality Distraction vs. Standard Care on Burn Pain among Pediatric Patients: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2112082. [Google Scholar] [CrossRef]
- Goldman, R.D.; Behboudi, A. Virtual reality for intravenous placement in the emergency department-a randomized controlled trial. Eur. J. Pediatr. 2021, 180, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Estarli, M.; Aguilar-Barrera, E.S.; Martínez-Rodríguez, R.; Baladia, E.; Durán-Agüero, S.; Camacho, S.; Buhring, K.; Herrero-López, A.; Gil-González, D.M. Reference items for publishing protocols of systematic reviews and meta-analyses: PRISMA-P 2015 statement. Rev. Española Nutr. Humana Dietética 2016, 20, 148. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Wong-Baker FACES Foundation. Available online: https://wongbakerfaces.org/ (accessed on 31 June 2024).
- Tsze, D.S.; von Baeyer, C.L.; Pahalyants, V.; Dayan, P.S. Validity and Reliability of the Verbal Numerical Rating Scale for Children Aged 4 to 17 Years with Acute Pain. Ann Emerg. Med. 2018, 71, 691–702.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMurtry, C.M.; Noel, M.; Chambers, C.T.; McGrath, P.J. Children’s fear during procedural pain: Preliminary investigation of the Children’s Fear Scale. Health Psychol. 2011, 30, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Kleiber, C.; McCarthy, A.M. Evaluating instruments for a study on children’s responses to a painful procedure when parents are distraction coaches. J. Pediatr. Nurs. 2006, 21, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; The Cochrane Collaboration: London, UK, 2011; Available online: https://training.cochrane.org/handbook/current (accessed on 31 June 2024).
- GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime; 2022. Available online: https://www.gradepro.org/ (accessed on 10 July 2024).
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Aydın, A.İ.; Özyazıcıoğlu, N. Using a Virtual Reality Headset to Decrease Pain Felt During a Venipuncture Procedure in Children. J. Perianesth. Nurs. 2019, 34, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Czub, M.; Serrano-Ibáñez, E.R.; Piskorz, J.; Esteve, R.; Lydon, H.K.; López-Martínez, A.E.; Mullen, B.; Ramírez-Maestre, C.; Heary, C.; O’Neill, C.; et al. Virtual Reality Distraction for Needle-Related Pain and Distress in Children: A Multicenter Randomized Controlled Trial. Cyberpsychol. Behav. Soc. Netw. 2024, 27, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Gerçeker, G.Ö.; Binay, Ş.; Bilsin, E.; Kahraman, A.; Yılmaz, H.B. Effects of Virtual Reality and External Cold and Vibration on Pain in 7- to 12-Year-Old Children during Phlebotomy: A Randomized Controlled Trial. J. Perianesth. Nurs. 2018, 33, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Özalp Gerçeker, G.; Ayar, D.; Özdemir, E.Z.; Bektaş, M. Effects of virtual reality on pain, fear and anxiety during blood draw in children aged 5–12 years old: A randomised controlled study. J. Clin. Nurs. 2020, 29, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Gil Piquer, R.; Mañes Jiménez, Y.; España Marí, M.; Peris Peris, A.; Solanes Donet, P.; García Lledó, N.; Pons Fernández, N. Usefulness of virtual reality in the management of pain associated with venepuncture: A multicentre randomized clinical trial. An. Pediatr. (Engl. Ed.). 2024, 100, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Goktas, N.; Avci, D. The effect of visual and/or auditory distraction techniques on children’s pain, anxiety and medical fear in invasive procedures: A randomized controlled trial. J. Pediatr. Nurs. 2023, 73, e27–e35. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.I.; Mahrer, N.E. Is Virtual Reality Ready for Prime Time in the Medical Space? A Randomized Control Trial of Pediatric Virtual Reality for Acute Procedural Pain Management. J. Pediatr. Psychol. 2018, 43, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Orhan, E.; Gozen, D. The Effect of Virtual Reality on Pain Experienced by School-Age Children During Venipuncture: A Randomized Controlled Study. Games Health J. 2023, 12, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Osmanlliu, E.; Trottier, E.D.; Bailey, B.; Lagacé, M.; Certain, M.; Khadra, C.; Sanchez, M.; Thériault, C.; Paquin, D.; Côtes-Turpin, C.; et al. Distraction in the Emergency department using Virtual reality for INtravenous procedures in Children to Improve comfort (DEVINCI): A pilot pragmatic randomized controlled trial. Can. J. Emerg. Med. 2021, 23, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Thybo, K.H.; Friis, S.M.; Aagaard, G.; Jensen, C.S.; Dyekjaer, C.D.; Jørgensen, C.H.; Walther-Larsen, S. A randomized controlled trial on virtual reality distraction during venous cannulation in young children. Acta Anaesthesiol. Scand. 2022, 66, 1077–1082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ustuner Top, F.; Kuzlu Ayyıldız, T. Pain management in children during invasive procedures: A randomized clinical trial. Nurs. Forum. 2021, 56, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, S.; Hoogeveen, M.O.; van Winden, T.M.S.; Chegary, M.; Genco, M.S.; Jonkman, N.H. Virtual reality hypnosis for needle-related procedural pain and fear management in children: A non-inferiority randomized trial. Eur. J. Pediatr. 2023, 182, 4421–4430. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Choi, K.C. Effects of an Immersive Virtual Reality Intervention on Pain and Anxiety among Pediatric Patients Undergoing Venipuncture: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e230001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yıldırım, B.G.; Gerçeker, G.Ö. The Effect of Virtual Reality and Buzzy on First Insertion Success, Procedure-Related Fear, Anxiety, and Pain in Children during Intravenous Insertion in the Pediatric Emergency Unit: A Randomized Controlled Trial. J. Emerg. Nurs. 2023, 49, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, Y.; Liu, N.; Fan, L. Effectiveness of virtual reality intervention on reducing the pain, anxiety and fear of needle-related procedures in paediatric patients: A systematic review and meta-analysis. J. Adv. Nurs. 2023, 79, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Bexson, C.; Oldham, G.; Wray, J. Safety of virtual reality use in children: A systematic review. Eur. J. Pediatr. 2024, 83, 2071–2090. [Google Scholar] [CrossRef]
- Czech, O.; Wrzeciono, A.; Rutkowska, A.; Guzik, A.; Kiper, P.; Rutkowski, S. Virtual Reality Interventions for Needle-Related Procedural Pain, Fear and Anxiety—A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jenabi, E.; Bashirian, S.; Salehi, A.M.; Rafiee, M.; Bashirian, M. Virtual reality for pain reduction during intravenous injection in pediatrics: A systematic review and meta-analysis of controlled clinical trials. Clin. Exp. Pediatr. 2023, 66, 533–537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rav, E.; Sheth, R.; Ahmad, A.H. Systemic Lidocaine Infusions for Pediatric Patients with Cancer-Related Pain. Children 2022, 9, 1934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Potts, D.A.; Davis, K.F.; Elci, O.U.; Fein, J.A. A Vibrating Cold Device to Reduce Pain in the Pediatric Emergency Department: A Randomized Clinical Trial. Pediatr. Emerg. Care 2019, 35, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Benini, F.; Castagno, E.; Urbino, A.F.; Fossali, E.; Mancusi, R.L.; Milani, G.P. Pain management in children has significantly improved in the Italian emergency departments. Acta Paediatr. 2020, 109, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Babamohamadi, H.; Ameri, Z.; Asadi, I.; Asgari, M.R. Comparison of the Effect of EMLA™ Cream and the Valsalva Maneuver on Pain Severity during Vascular Needle Insertion in Hemodialysis Patients: A Controlled, Randomized, Clinical Trial. Evid. Based Complement. Alternat. Med. 2022, 2022, 8383021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moore, D.J.; Keogh, E.; Eccleston, C. The effect of threat on attentional interruption by pain. Pain 2013, 154, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Triberti, S.; Repetto, C.; Riva, G. Psychological factors influencing the effectiveness of virtual reality-based analgesia: A systematic review. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Uman, L.S.; Birnie, K.A.; Noel, M.; Parker, J.A.; Chambers, C.T.; McGrath, P.J.; Kisely, S.R. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst. Rev. 2013, 10, CD005179, Update in Cochrane Database Syst. Rev. 2018, 10, CD005179. [Google Scholar] [CrossRef] [PubMed]
- Le May, S.; Hupin, M.; Khadra, C.; Ballard, A.; Paquin, D.; Beaudin, M.; Bouchard, S.; Cotes-Turpin, C.; Noel, M.; Guingo, E.; et al. Decreasing Pain and Fear in Medical Procedures with a Pediatric Population (DREAM): A Pilot Randomized Within-Subject Trial. Pain Manag. Nurs. 2021, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.K.S.; McCracken, L.M. Virtual reality as a tool for pain management: A systematic review of clinical trials. Pain Med. 2020, 21, 1137–1148. [Google Scholar] [CrossRef]
- Won, A.S.; Bailey, J.; Bailenson, J.; Tataru, C.; Yoon, I.A.; Golianu, B. Immersive Virtual Reality for Pediatric Pain. Children 2017, 4, 52. [Google Scholar] [CrossRef]
- Gold, J.I.; Kim, S.H.; Kant, A.J.; Joseph, M.H.; Rizzo, A.S. Effectiveness of virtual reality for pediatric pain distraction during IV placement. CyberPsychology Behav. 2006, 9, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ahmad, S. The emerging role of virtual reality in pediatric pain management: A systematic review. J. Pediatr. Psychol. 2022, 47, 500–511. [Google Scholar] [CrossRef]
- Indovina, P.; Barone, D.; Gallo, L.; Chirico, A.; De Pietro, G.; Giordano, A. Virtual reality as a distraction intervention to relieve pain and distress during medical procedures: A comprehensive literature review. Clin. J. Pain 2018, 34, 858–877. [Google Scholar] [CrossRef] [PubMed]
| Study/Country /Procedure/Setting/ Level of Evidence | Design/Sample (Gender/Mean Age ± SD, Years or Median with IQR) | Intervention (During the Procedure) | Outcomes/Instruments/Measurement’s Time | Experimental Group(s) Finding (Mean ± SD or Mean Difference or CI) | Control Group(s) Finding (Mean ± SD or Mean Difference or CI) | p-Value Main Finding |
|---|---|---|---|---|---|---|
| Atzori et al. [8] (2022)/Italy VENIPUNCTURE (Children’s hospital) 1+ | RCT n = 82 VRG = 41 (20 F, 21 M; 11.39 ± 2.73) CG = 41 (18 F, 23 M; 12.17 ± 2.62) | VRG: immersive VR with Snow World software, using VR equipment consisting of a VR helmet and the personal 3D viewer Sony: HMZ T-2, supported by a laptop CG: standard treatment as usual | Pain (worst and emotional): NRS Post-intervention | Pain- NRS 1.56 ± 1.83 (Worst) 1.17 ± 1.80 (Emotional) | Pain-NRS 2.74 ± 2.76 (Worst) 2.41 ± 2.94 (Emotional) | VRG vs. CG Pain (VNRS) <0.05 (Worst) <0.05 (Emotional) |
| Aydin and Özyazıcıoğlu [29] (2019)/USA VENIPUNCTURE (Clinic) 1+ | RCT n = 67 VRG = 60 (30 F, 30 M; 10.50 ± 1.14) CG = 60 (29 F, 31 M; 10.30 ± 12) | VRG: 3D ‘‘Aquarium VR’’ application (simulates a submarine journey to discover things underlying the virtual aquarium) via a virtual reality headset CG: no interventional procedure | Pain: VAS, WBSPF Post-intervention | Pain -VAS: 3.07 ± 2.86 WBSPF: 1.68 ± 1.51 | Pain-VAS: 2.02 ± 1.96 WBSPF: 3.23 ± 3.05 | VRG vs. CG Pain (VAS, WBSPF) 0.006, 0.039 |
| Chan et al. [12] (2019)/Australia VENIPUNCTURE INTRAVENOUS CANNULATION (Emergency department) 1− | RCT Emergency department study: n = 123 VRG = 64 (29 F, 35M; 7.9 ± 1.5), CG = 59 (27 F, 32 M; 8.2 ± 2.4) | VRG: virtual reality sequence that consists of an interactive underwater adventure, using the “Google Pixel XL” device CG: standard care | Pain: FPS-R Anxiety: visual analog thermometer Child distress (caregiver’s rating): VAS Post-intervention | Pain-FPS-R: −1.39 (−2.42 to –0.36) Anxiety-VAT: −2.2 (−3.20 to –1.20) Child distress (VAS 1 (0–5)) | Pain-FPS-R: 0.39 (−1.45 to −0.67) Anxiety-VAT: −0.46 (−1.36 to 0.45) Child distress-VAS: 4 (1–8) | VRG vs. CG Pain (FPS-R): 0.018 Anxiety (VAT): 0.011 Child distress (VAS): 0.004 |
| Chen et al. [13] (2020)/Taiwan INTRAVENOUS INJECTION (Emergency department) 1+ | RCT n = 136 VRG = 68 (3 F, 38M; 9.3 ± 1.7) CG = 68 (29 F, 39 M; 9.0 ± 1.7) | VRG: immersive VR using an iPhone device and a head-mounted display (Xiaozhai V4) with four virtual environments CG: regular intravenous injection | Pain: WBSPF Fear: CFS Post-intervention | Pain-WBSPF Child: 3.35 ± 2.38 Primary caregiver: 3.26 ± 2.37 Nurse: 3.29 ± 2.01 Fear-CFS Child: 1.32 ± 1.19 Primary caregiver: 1.35 ± 1.23 Nurse: 1.56 ± 1.20 | Pain-WBSPF Child: 4.35 ± 2.95 Primary caregiver: 4.29 ± 2.70 Nurse: 4.29 ± 2.52 Fear-CFS Child: 1.78 ± 1.40 Primary caregiver: 2.03 ± 1.36 Nurse: 2.15 ± 1.24 | VRG vs. CG Pain (WBSPF) Child: 0.031 Caregiver:.020 Nurse: 0.12 Fear (CFS) Child: 0.043 Caregiver: 0.003 Nurse: 0.006 |
| Czub et al. [30] (2024)/Poland, Ireland, Spain VENOUS BLOOD DRAW (Pediatric phlebotomy clinics) 1+ | RCT n = 312 (304 analyzed) VRG = 105 (39 F, 66 M; 6.8 ± 1.4) GMG = 102 (49 F, 53 M; 6.6 ± 1.3) CG = 97 (50 F, 47 M; 6.6 ± 1.5) | VRG: Magic Spheres game using a Samsung Gear VR system with a Galaxy S8 phone (created by the authors of the study) GMG: two-dimensional version of the Magic Spheres game on a mobile phone screen CG: usual care | Pain: FPS-R, NRS Anxiety: VAS Post-intervention | Pain-FPS-R VRG: 0.933 ± 2.132 GMG: 1.248 ± 2.621 NRS VRG: 1.886 ± 2.411 GMG: 2.110 ± 2.589 Anxiety-VAS VRG: 4.486 ± 3.680 GMG: 4.157 ± 3.506 | Pain-FPS-R 3.691 ± 2.132 NRS 5.278 ± 3.63 Anxiety-VAS 3.722 ± 3.744 | Pain-(FPS-R) VRG vs. CG: <0.001 VRG vs. GMG: 0.719 GMG vs. CG: 0.001 Correlation FPS-R with NRS: >0.001 |
| Dumoulin et al. [11] (2019)/Canada VENIPUNCTURE IV PLACEMENT (Emergency department of a children’s hospital) 1+ | RCT n = 59 VRG = 20 (6 F, 14M; 13.85 ± 2.80) TVCG = 24 (6 F, 9M; 12.65 ± 3.33) CG = 15 (9 F, 9 M; 13.81 ± 2.43) | VRG: immersive real-time game developed by the UQO Cyberpsychology Lab using Virtools 4 TVCG: watch television CG: standard care conditions carried out with the Child Life program intervention | Pain: VAS Fear of pain: VAS Post-intervention | Pain-VAS: 21.75 ± 20.96 Fear of pain-VAS: 19.75 ± 21.18 | Pain-VAS TVCG: 35.43 ± 32.65 CG: 25.33 ± 25.25 Fear of pain-VAS TVCG: 35.42 ± 35.93 CG: 29.33 ± 34.48 | Pain (VAS) VRG Pre-Post: <0.05 TVCG Pre-Post: <0.05 CG Pre-Post: <0.05 Fear of pain (VAS) VRG Pre-Post: <0.01 TVCG Pre-Post: <0.05 CG Pre-Post: <0.05 |
| Gerçeker.et al. [31] (2018)/Turkey PHLEBOTOMY (Phlebotomy Unit) 1+ | RCT n = 147 (121 analyzed) VRG = 40 (20 F, 20 M; 9.7 ± 1.5) BCG = 41(20 F, 21 M; 9.7 ± 1.6) CG = 15 (20 F, 20 M; 8.9 ± 1.3) | VRG: Virtual reality intervention through three videos (Magic English Disney Family, Princess Sofia’s Secret Library, and dinosaur cartoons; PANGEA) using Samsung Gear Oculus with headphones and a Samsung Galaxy S5 Note BCG: Buzzy device with cold and vibration application, starting just before the procedure CG: standard care | Pain: WBSPF Post-intervention | Pain-WBSPF Self-reported: 1.5 ± 0.2 Parent-reported: 1.5 ± 0.2 Nurse-reported: 1.6 ± 0.2 Research-reported: 1.3 ± 0.2 | Pain-WBSPF Self-reported BCG: 2.0 ± 0.2 CG: 5.1 ± 0.4 Parent-reported BCG: 2.0 ± 0.2 CG: 4.7 ± 0.4 Nurse-reported BCG: 1.8 ± 0.1 CG: 4.3 ± 0.3 Research-reported BCG: 1.8 ± 0.2 CG: 5.4 ± 0.4 | VRG vs. BCG vs. CG Pain (WBSPF) Self-reported, Parent-reported, Nurse-reported, Research-reported: 0.000 |
| Gerçeker et al. [32] (2020)/Turquía BLOOD DRAW (University hospital special draw unit) 1++ | RCT n = 141 (136 analyzed) VRRG = 45 (22 F, 23 M; n.d.) VRORG = 45 (21 F, 24 M; n.d.) CG = 46 (20 F, 26 M; n.d.) | VRRG: VR application with a rollercoaster game using a Samsung Galaxy S5 Note mobile phone connected to a virtual headset VRORG: VR application with a submarine experience game, using a Samsung Galaxy S5 Note mobile phone connected to a virtual headset CG: standard care | Pain: WBSPF Fear: CFS Anxiety: CAM-S Post-intervention | Pain-WBSPF Self-reported VRRG: 1.2 ± 2.2 VRORG:1.0 ± 1.5 Parent-reported VRRG: 1.2 ± 2.6 VRORG:0.5 ± 1.2 Nurse-reported VRRG: 1.2 ± 2.1 VRORG: 0.6 ± 1.4 Research-reported VRRG: 1.0 ± 2.2 VRORG: 0.4 ± 1.2 Fear-CFS Self-reported VRRG: 0.4 ± 1.1 VRORG: 0.3 ± 0.6 Parent-reported VRGR: 0.4 ± 1.0 VRGOR: 0.2 ± 0.5 Research-reported VRGR: 0.4 ± 1.0 VRGOR: 0.2 ± 0.5 Anxiety-Self-reported VRGR: 1.1 ± 2.6 VRGOR: 0.5 ± 1.5 Parent-reported VRGR: 1.1 ± 2.6 VRGOR: 0.2 ± 0.5 Research-reported VRGR: 1.0 ± 2.4 VRGOR: 0.6 ± 1.7 | Pain-BSPF Self-reported: 4.1 ± 3.5 Parent-reported: 4.1 ± 3.6 Nurse-reported: 4.2 ± 3.6 Research-reported: 4.0 ± 3.6 Fear-CFS Self-reported: 2.4 ± 1.6 Parent-reported: 2.3 ± 1.6 Research-reported: 2.1 ± 1.4/2.3 ± 1.6 Anxiety-CAM-S Self-reported: 6.3 ± 3.6 Parent-reported: 6.2 ± 3.5 Research-reported: 6.3 ± 3.5 | VRRG vs. VRORG vs. CG Pain (WBSPF) Self-reported, Parent-reported, Nurse-reported, Research-reported: 0.000 Fear (CFS) Self-reported, Parent-reported, Research-reported VRGR vs. VRGOR vs. CG Post: 0.000 Anxiety (CAM-S) Self-reported, Parent-reported, Research-reported: 0.000 |
| Gil Piquer et al. [33] (2023)/Spain BLOOD DROW (Care centers) 1+ | RCT n = 83 VRG = 43 (21 F, 22 M; 10, 7–12) CG = 40 (16 F, 24 M; 9, 7–12) | VRG: watch a movie titled “Henry” through an Oculus Quest 2 VR headset device (from the preparation to the moment of the puncture) CG: usual care | Pain: VAS Anxiety: GDS, NS Procedural time: minutes Post-intervention | Pain-VAS: 2.23 ± n.d. Anxiety-GDS Child: 1.58 ± n.d. NS-Nurse: 3.1 ± n.d. Procedural time: 1 (1–3) | Pain-VAS: 3.37 ± n.d. Anxiety-GDS Child: 2 ± n.d. NS-Nurse CG: 3.2 ± n.d. Procedural time minutes: 1 (1–2) | VRG vs. CG Pain (VAS): 0.012 Anxiety (GDS, NS): 0.081, 0.13 Procedural time (minutes): 0.77 |
| Goktas and Avci [34] (2023)/Turkey DELTOID VACCINATION, VENIPUNCTURE, PHLEBOTOMY (Emergency department) 1++ | RCT n = 144 VRG = 36 (18 F, 18 M; n.d.) KCG = 36 (18 F, 18 M; n.d.) MCG = 36 (18 F, 18 M; n.d.) CG = 36 (18 F, 18 M; n.d.) | VRG: VR distraction with the audio video “The Spacewalker”, using the dispositive Zore G07E VR Shinecon 3D Virtual Reality Glasses”, which includes a headset (starting 3 min before the procedure) KCG: a visual distraction technique with a kaleidoscope during the invasive procedure (starting 3 min before the procedure) MCG: a music distraction technique using twenty current popular songs played through the “S16 Bluetooth Over-Ear Wireless Headphones with Microphone” (starting 3 min before the procedure) CG: standard procedure | Pain: WBFPRS Anxiety: CAMS Fear: CMFS Post-intervention | Pain-WBFPRS: 0.42 ± 0.65 Anxiety-CAMS: 8.06 ± 1.90 Fear-CMFS: 14.78 ± 2.65 | Pain-WBFPRS KCG: 0.67 ± 0.93 MCG: 0.67 ± 0.93 CG: 2.72 ± 1.32 Anxiety-CAMS KCG: 8.31 ± 1.74 MCG: 8.03 ± 2.57 CG: 7.47 ± 1.73 Fear-CMFS KCG: 14.81 ± 2.62 MCG: 14.14 ± 1.91 CG: 13.94 ± 2.16 | VRG vs. KCG vs. MCG vs. CG Pain (WBFPRS): <0.001 Anxiety (CAMS): <0.001 Fear (CMFS): <0.001 |
| Gold et al. [35] (2018)/USA BLOOD DRAW (Children’s hospital) 1+ | RCT n = 143 (107 analyzed) VRG = 70 (33 F, 37 M; 15.79 ± 3) CG = 73 (38 F, 35 M; 15.06 ± 3.23) | VRG: VR game “Bear Blast” (applied VRä) using the Samsung Galaxy S6 mobile-based Gear VR goggles (aged 13–21) or the Google Pixel mobile-based Merge VR goggles (aged 10–12) CG: standard of care | Pain: VAS, CAS Anxiety: VAS, FAS Post-intervention | Pain VAS, CAS, FPS-R Patient-report 1.31 ± 1.59, 1.58 ± 2.02, 1.40 ± 0.73 Caregiver-report 1.06 ± 1.72, 1.19 ± 1.57, 1.54 ± 0.88 Anxiety VAS, FAS Patient-report 1.90 ± 2.22, 0.28 ± 0.22 Caregiver-report 1.52 ± 2.03, 0.33 ± 0.22 | Pain VAS, CAS, FPS-R Patient-report 1.93 ± 2.22, 2.00 ± 2.10, 1.70 ± 1.13 Caregiver-report 2.26 ± 2.68, 2.29 ± 2.38, 2.02 ± 1.30 Anxiety VAS, FAS Patient-report 2.48 ± 2.07, 0.40 ± 0.24 Caregiver-report 2.48 ± 2.63, 0.38 ± 0.25 | Pain (VAS, CAS, FPS-R) Patient-report VRG: 0.001, <0.001, <0.001 CG: 0.053, 0.095, <0.05 Caregiver-report VRG: <0.001, 0.001, 0.001 CG: <0.01, <0.01, 0.07 Anxiety (VAS, FAS) Patient-report VRG: <0.001, <0.001 CG: <0.01, <0.001 Caregiver-report VRG: <0.001, <0.001 CG: <0.01, <0.05 |
| Goldman and Behboudi [17] (2021)/Canada IV CATHETERIZATION PROCEDURE (Emergency department) 1++ | RCT n = 66 VRG = 35 (13 F, 22 M; 4.4 ± 0.9) CG = 31 (17 F, 14 M; 4.7 ± 0.75) | VRG: VR game (Roller Coaster app) played using an Asus Zenfone 2 ZE551ML mobile device, VR goggles, and a virtual reality headset CG: standard of care | Pain: FPS-R Anxiety: VSA Procedural time: minutes Post-intervention | Pain-FPS-R: 2 (0–4) Anxiety-VSA: 2.4 (0–8) Procedural Time minutes: 5 (3–10) | Pain-FPS-R: 4 (2–6) Anxiety-VSA: 2.4 (0–7) Procedural Time: 7 (3–13) | VRG vs. CG Pain (FPS-R): 0.004 Anxiety (VSA): 0.51 Procedural Time: 0.34 |
| Hsu et al. [10] (2022)/ Taiwan INTRAVENOUS PLACEMENT (Pediatric ward) 1++ | RCT n = 134 VRG = 69 (37 F, 32 M; 9.81 ± 1.70) CG = 65 (43 F, 22 M; 10.22 ± 1.70) | VRG: immersive VR with HTC Vive VR headset and head-mounted display device of the VR Cosmos helmet. Two types of interactive sessions in the VR environment were used: an instructional play session (pre-injection) and an emotional catharsis play session (post-injection) CG: before the procedure, an educational picture book was provided on intravenous placement | Pain: WBSPF Fear: CFS Post-intervention | Pain-WBSPF Child: 1.33 ± 1.60 Caregiver: 1.13 ± 1.51 Fear-CFS Child: 0.28 ± 0.54 Caregiver: 0.36 ± 0.64 | Pain-WBSPF Child: 2.06 ± 2.00 Caregiver: 2.68 ± 1.74 Fear-CFS Child: 0.65 ± 0.94 Caregiver: 0.95 ± 0.96 | VRG vs. CG Pain (WBSPF) Child: 0.028 Caregiver: <0.001 Fear (CFS) Child: 0.004 Caregiver: <0.001 |
| Orhan et al. [36] (2023)/Turkey VENIPUNCTURE (Pediatric outpatient clinic) 1− | RCT n = 102 VRG = 52 (32 F, 20 M; 9.75 ± 1.56) CG = 50 (30 F, 20 M; 9.94 ± 1.39) | VRG: VR game “‘VR Feel the Nature” with 4 different options (sightseeing in a village, park, forest, and windmill) using a VR headset (Bobo VR Z4 mini) connected to a mobile phone CG: routine practice | Pain: FPS-R Anxiety: STAIC Post-intervention | Pain-FPS-R: 1.46 ± 1.49 Anxiety-STAIC: 31.48 ± 7.30 | Pain-FPS-R: 4.44 ± 2.26 Anxiety-STAIC: 32.4 ± 69.22 | VRG vs. CG Pain (FPS-R): 0.001 Anxiety (STAIC): 0.553 |
| Osmanlliu et al. [37] (2020)/Canada VENIPUNCTURE (IV PLACEMENT) (Emergency department) 1+ | RCT n = 62 VRG = 31 (20 F, 11 M; 11.1 ± 2.9) CG = 31 (18 F, 13 M; 12.3 ± 3.0) | VRG: VR distraction using a videogame Dreamland Oculus Rift®® with the Oculus Rift ® device, in a sitting position (starting 3 min before the procedure) + local standard of care CG: local standard of care | Pain: NRS Anxiety: CFS Post-intervention | Pain-VNRS: 3 (1,6;2,4) Anxiety-CFS: 1 (0, 2; 1, 1) | Pain-VNRS: 3 (1,5.5;2,5) Anxiety-CFS: 2 (0, 3; 1, 3) | VRG vs. CG Pain (VNRS): 0.75 Anxiety (CFS): n.d. |
| Thybo et al. [38] (2022)/Denmark VENOUS CANNULATION (University hospital) 1++ | RCT n = 106 VRG = 52 (16 F, 36 M; 5.9 ± 1.4) CG = 54 (10 F, 44 M; 5.8 ± 1.4) | VRG: a three-dimensional VR interactive game named “Freddy the Frog (3D)”, using the Oculus Go VR goggles CG: dual-dimensional game with a tablet or smartphone | Pain: WBSPF Procedural time: minutes Post-intervention | Pain- WBSPF: 20 (0–40) Procedural time minutes: 2 (1–2.7) | Pain-WBSPF: 20 (0–55) Procedural time (minutes): 1.83 (1–2) | VRG vs. CG Pain (WBSPF): 0.19 Procedural time: 0.72 |
| Top and Ayyildiz [39] (2021)/Turkey BLOOD DRAW (Blood draw unit) 1− | RCT n = 80 (77 analyzed) VRG = 37 (19 F, 18 M; 4.86 ± 0.99) CG = 40 (22 F, 18 M; 4.70 ± 0.09) | VRG: a video watching by virtual reality glasses 2 min before starting the procedure, and continued until the end of the blood draw CG: usual procedure in the blood test room | Pain: FPS-R Procedural time: minutes Post-intervention | Pain-FPS-R: 3.82 ± 1.20 | Pain-FPS-R: 6.96 ± 2.08 | VRG vs. CG Pain (FPS-R): < 0.001 |
| Van den Berg et al. [40] (2023)/Netherlands VENIPUNCTURE, INTRAVENOUS CATHETER, INJECTION (Pediatric clinic) 1− | RCT n = 138 (114 analyzed) VRG = 60 (32 F, 28 M; n.d.) CG = 54 (30 F, 24 M; n.d.) | VRG: medical hypnosis (audio script) through VR, using the G2 VR headset from PICO (software provided by SyncVR Medical) CG: medical hypnosis induced by a trained healthcare provider (audio script) | Pain: WBSPF Observed reported pain: NRS Fear: CFS Post-intervention | Pain-WBSPF: 0.0 (0.0–3.5) Observed reported pain-NRS: 0.0 (0.0–1.0) Fear CFS 0.0 (0.0–0.75) Observed reported 0.0 (0.0–0.0) | Pain-WBSPF: 2.0 (0.0–4.0) Observed reported pain-NRS: 0.5 (0.0–1.0) Fear-CFS Child: 0.0 (0.0–1.0) Observer reported: 0.0 (0.0–1.0) | VRG vs. CG Pain (WBSPF): 0.289 Observed reported pain (NRS): 0.062 Fear (CFS) Child: 0.203 Observed reported: 0.231 |
| Walther-Larsen et al. [1] (2019)/USA VENIPUNCTURE (Pediatric unit) 1+ | RCT n = 64 (59 analyzed) GVR = 28 (1 F, 27 M; a 10.9 ± 2.8) GC = 31 (6 F, 25 M; a 10.1 ± 2.2) | VRG: VR game “Seagull Splash” using Samsung Galaxy S6 mobile-based Gear VR goggles and controller in the hang not assigned for the procedure GC: Standard care. Topical numbing cream, positioning, and distraction | Pain: VAS Procedural time: minutes Satisfaction: 0 to 10 scale Adverse event: number Post-intervention | Pain-VAS: 27 (0–33) Procedural time: 1.75 (1.0–3.6) Satisfaction: 81 (68–100) | Pain-VAS: 15 (5–30) Procedural time: 2.0 (1.0–2.5) Satisfaction: 89 (73–100) | VRG vs. CG Pain-VAS: 0.23 Procedural time: 0.58 Satisfaction: 0.82 |
| Wong and Choi [41] (2023)/China VENIPUNCTURE (Pediatric unit) 1++ | RCT n = 149 VRG = 75(40 F, 35 M; 7.21 ± 2.45) CG = 74 (46 F, 28 M; 7.21 ± 2.49) | VRG: immersive VR intervention with 2 age-appropriate VR scenarios (self-designed cartoon character DD who was going to undergo venipuncture), carried out with a disposable headset and a head-mounted display connected to a smartphone CG: standard care | Pain: FPS-R Anxiety: CSAS-C Length of procedure: minutes Post-intervention | Pain-FPS-R: 2.24 ± 2.81 Anxiety- CSAS-C: −0.10 ± 1.2 Length of procedure minutes: 4.43 ± 3.47 | Pain-FPS-R: 4.99 ± 3.95 Anxiety-CSAS-C: 0.10 ± 1.02 Length of procedure minutes: 6.56 ± 7.39 | VRG vs. CG Pain (FPS-R): <0.001 Anxiety (CSAS-C): 0.03 Procedural time: 0.03 |
| Yildirim and Gerçeker [42] (2023)/Turkey IV INSERTION (Pediatric emergency department) 1++ | RCT n = 150 VRG = 51 (24 F, 27 M; 6.4 ± 1.6) BCG = 50 (23 F, 27 M; 6.7 ± 1.7) CG = 49 (24 F, 25 M; 6.4 ± 1.6) | VRG: immersive VR, wearing a virtual headset and using Oculus Rift VR and a Samsung Galaxy S7 mobile phone BCG: cold Buzzy device attached to the arm and ending 5 min after IV insertion was completed CG: no distraction device. Distraction carried out by asking questions | Pain: WBSPF, CAS-C Anxiety: CAM-S Fear: CFS Post-intervention | Pain-WBSPF: 5.6 ± 1.9 CAS: 5.9 ± 1.5 Anxiety-CAM-S: 5.2 ± 1.4 Fear-CFS: 3.0 ± 0.7 | Pain-WBSPF BCG: 5.9 ± 1.6 CG: 6.2 ± 1.0 CAS BCG: 5.8 ± 1.5 CG: 6.0 ± 1.1 Anxiety-CAM-S BCG: 5.1 ± 1.5 CG: 5.5 ± 1.2 Fear-CFS BCG: 2.9 ± 0.5 CG: 3.2 ± 0.5 | VRG vs. BCG vs. CG Pain (WBSPF, CAS) 0.206, 0.897 Anxiety (CAM-S): 0.246 Fear (CFS): 0.033 |
| Certainty Assessment | № of Patients | Effect | Certainty | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirect Evidence | Imprecision | Other Considerations | Virtual Reality | Control | Relative (95% CI) | Absolute (95% CI) | ||
| 9 | RCTs | Serious | It is not serious | Serious | It is not serious | Strong association | 450 | 446 | - | MD −1.83 (−2.93 to −0.72) | ⨁⨁⨁◯ Moderate | WBFS. Self-reported |
| 4 | RCTs | Serious | It is not serious | Serious | It is not serious | - | 199 | 197 | - | MD −2.61 (−4.00 to −1.23) | ⨁⨁◯◯ Low | WBFS. Parent-reported |
| 3 | RCTs | Serious | It is not serious | Serious | It is not serious | - | 130 | 132 | - | MD −2.71 (−2.82 to −2.6) | ⨁⨁◯◯ Low | WBFS. Nurse reported |
| 10 | RCTs | Serious | It is not serious | Serious | It is not serious | Very strong association | 583 | 579 | - | SMD −0.71 (−1.13 to −0.28) | ⨁⨁⨁⨁ High | NRS |
| 4 | RCTs | Serious | It is not serious | Serious | It is not serious | - | 210 | 214 | - | MD −2.92 (−5.45 to −0.38) | ⨁⨁◯◯ Low | CAM. Self-reported |
| 3 | RCTs | Serious | It is not serious | Serious | It is not serious | - | 142 | 141 | - | MD −3.87 (−6.99 to −0.75) | ⨁⨁◯◯ Low | CAM. Parent-reported |
| 6 | RCTs | Serious | It is not serious | Serious | It is not serious | Strong association | 314 | 310 | - | MD −1.27 (−1.99 to −0.56) | ⨁⨁⨁◯ Moderate | CFS. Self-reported |
| 4 | RCTs | Serious | It is not serious | Serious | It is not serious | - | 227 | 225 | - | MD −1.33 (−2.08 to −0.58) | ⨁⨁◯◯ Low | CFS. Parent-reported |
| 2 | RCTs | Serious | It is not serious | Serious | It is not serious | - | 104 | 104 | - | MD −1.13 (−2.24 to −0.03) | ⨁⨁◯◯ Low | CFS. Nurse reported |
| 8 | RCTs | Serious | It is not serious | Serious | It is not serious | Strong association | 378 | 372 | - | MD −0.05 (−0.13 to 0.03) | ⨁⨁⨁◯ Moderate | Procedural time |
| 5 | RCTs | Serious | It is not serious | Serious | It is not serious | Strong association | 313 | 299 | - | MD 0.05 (−0.76 to 0.65) | ⨁⨁⨁◯ Moderate | Satisfaction providers |
| 3 | RCTs | Serious | It is not serious | Serious | It is not serious | - | 259 | 248 | - | MD 0.81 (0.26 to 1.37) | ⨁⨁◯◯ Low | Satisfaction parents |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres-Matos, R.; Castillo-García, M.; Magni, E.; Pabón-Carrasco, M. Effectiveness of Virtual Reality for Managing Pain, Fear, and Anxiety in Children and Adolescents Undergoing Needle-Related Procedures: Systematic Review with Meta-Analysis. Nurs. Rep. 2024, 14, 2456-2484. https://doi.org/10.3390/nursrep14030182
Cáceres-Matos R, Castillo-García M, Magni E, Pabón-Carrasco M. Effectiveness of Virtual Reality for Managing Pain, Fear, and Anxiety in Children and Adolescents Undergoing Needle-Related Procedures: Systematic Review with Meta-Analysis. Nursing Reports. 2024; 14(3):2456-2484. https://doi.org/10.3390/nursrep14030182
Chicago/Turabian StyleCáceres-Matos, Rocío, Mario Castillo-García, Eleonora Magni, and Manuel Pabón-Carrasco. 2024. "Effectiveness of Virtual Reality for Managing Pain, Fear, and Anxiety in Children and Adolescents Undergoing Needle-Related Procedures: Systematic Review with Meta-Analysis" Nursing Reports 14, no. 3: 2456-2484. https://doi.org/10.3390/nursrep14030182
APA StyleCáceres-Matos, R., Castillo-García, M., Magni, E., & Pabón-Carrasco, M. (2024). Effectiveness of Virtual Reality for Managing Pain, Fear, and Anxiety in Children and Adolescents Undergoing Needle-Related Procedures: Systematic Review with Meta-Analysis. Nursing Reports, 14(3), 2456-2484. https://doi.org/10.3390/nursrep14030182






