Narrative Review: Glucocorticoids in Alcoholic Hepatitis—Benefits, Side Effects, and Mechanisms
Abstract
1. Introduction
Document Retrieval and Data Mining
2. Efficacy of GCs in sAH Therapy
3. Pharmacological Actions of Different GCs
4. Literature That Supports Beneficial Roles of GCs and Hepatic GR in sAH
5. Adverse Effects of GCs
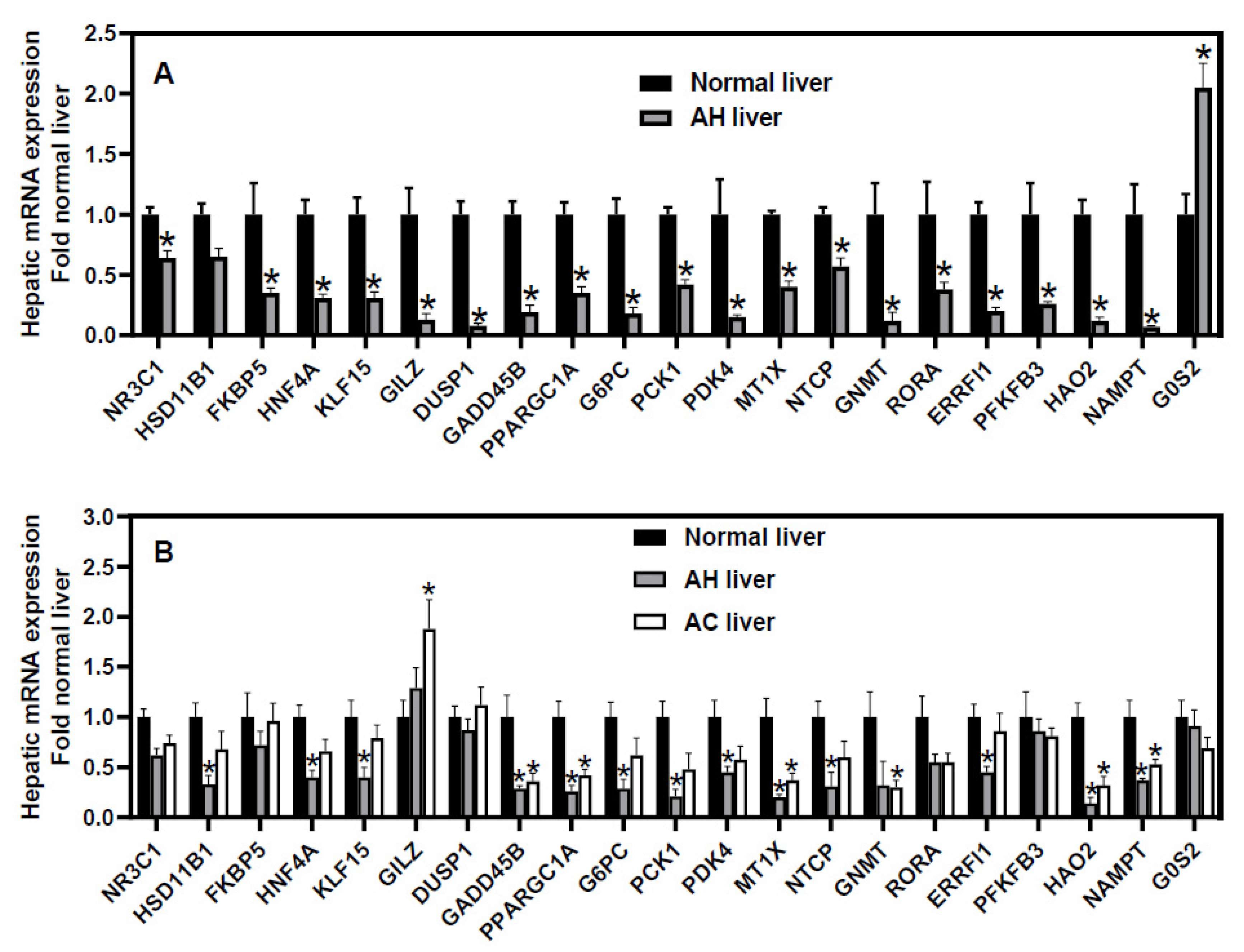
6. Down-Regulation of GR-Target Genes in Human sAH
7. Dysregulation of Non-Canonical GR-Target Genes in sAH
8. GC Resistance/Non-Responsiveness (GCR) as a Limiting Factor in sAH Therapy
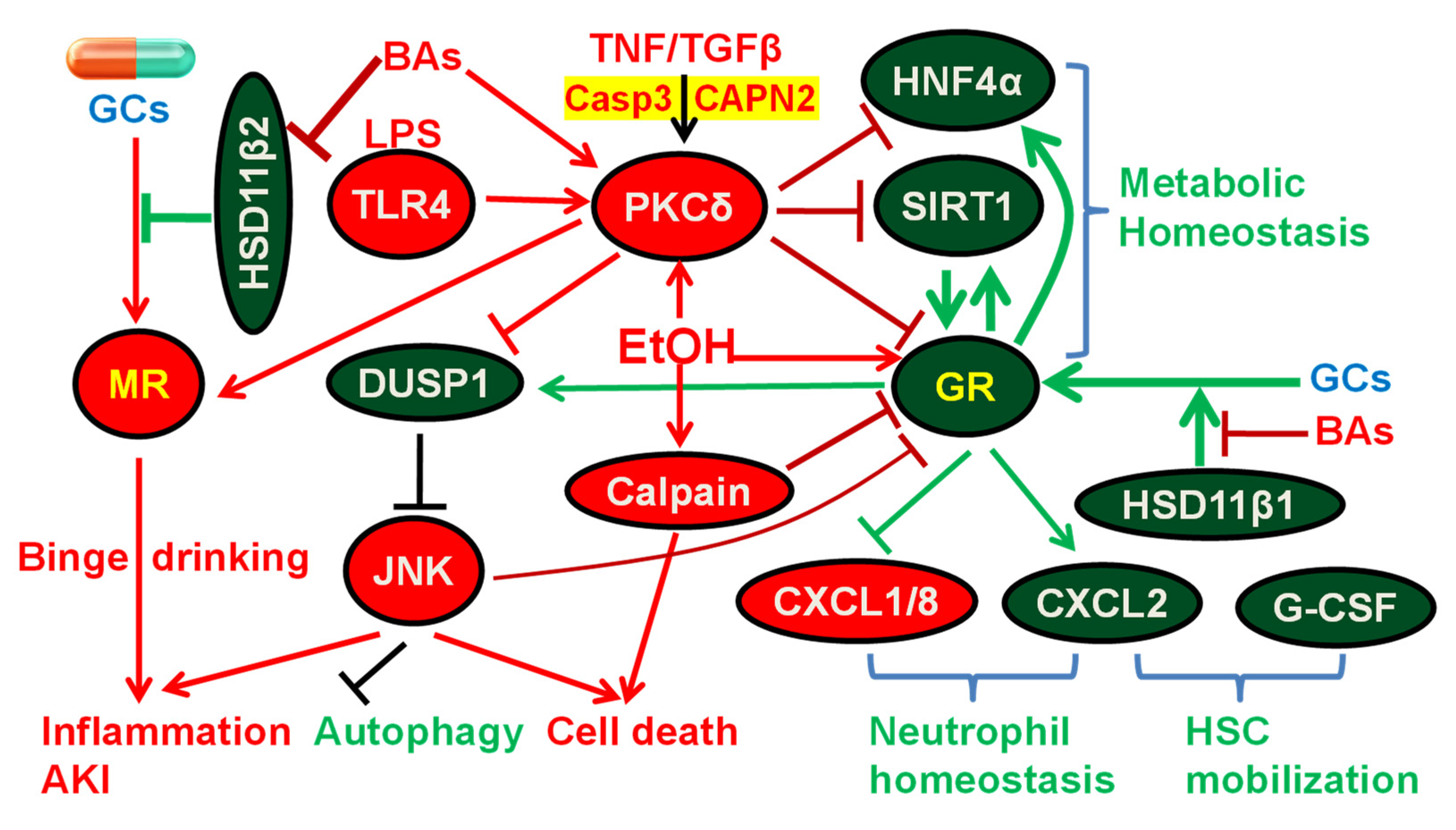
9. Mechanisms of GR Deficiency and GCR in sAH
10. Protein Kinase C δ (PKCδ) and Calpain in Liver Injury and GCR
11. Imbalance of CXC Chemokines in sAH and Infection
12. Summary and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singal, A.K.; Mathurin, P. Diagnosis and Treatment of Alcohol-Associated Liver Disease: A Review. JAMA 2021, 326, 165–176. [Google Scholar]
- Termeie, O.; Fiedler, L.; Martinez, L.; Foster, J.; Perumareddi, P.; Levine, R.S.; Hennekens, C.H. Alarming Trends: Mortality from Alcoholic Cirrhosis in the United States. Am. J. Med. 2022, in press. [CrossRef]
- White, A.M.; Castle, I.P.; Powell, P.A.; Hingson, R.W.; Koob, G.F. Alcohol-Related Deaths During the COVID-19 Pandemic. JAMA 2022, 327, 1704–1706. [Google Scholar] [CrossRef]
- Quagliarini, F.; Mir, A.A.; Balazs, K.; Wierer, M.; Dyar, K.A.; Jouffe, C.; Makris, K.; Hawe, J.; Heinig, M.; Filipp, F.V.; et al. Cistromic Reprogramming of the Diurnal Glucocorticoid Hormone Response by High-Fat Diet. Mol. Cell. 2019, 76, 531–545.e5. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Goyal, O.; Kishore, H.; Sidhu, S. New paradigms in management of alcoholic hepatitis: A review. Hepatol. Int. 2017, 11, 255–267. [Google Scholar]
- Hosseini, N.; Shor, J.; Szabo, G. Alcoholic Hepatitis: A Review. Alcohol Alcohol. 2019, 54, 408–416. [Google Scholar]
- Mellinger, J.L.; Shedden, K.; Winder, G.S.; Tapper, E.; Adams, M.; Fontana, R.J.; Volk, M.L.; Blow, F.C.; Lok, A.S.F. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology 2018, 68, 872–882. [Google Scholar] [CrossRef]
- Shirazi, F.; Singal, A.K.; Wong, R.J. Alcohol-associated Cirrhosis and Alcoholic Hepatitis Hospitalization Trends in the United States. J. Clin. Gastroenterol. 2021, 55, 174–179. [Google Scholar] [CrossRef]
- Thompson, J.A.; Martinson, N.; Martinson, M. Mortality and costs associated with alcoholic hepatitis: A claims analysis of a commercially insured population. Alcohol 2018, 71, 57–63. [Google Scholar] [CrossRef]
- Ladhani, S.; Hirode, G.; Singal, A.K.; Wong, R.J. Impact of Safety-Net Burden on In-Hospital Mortality and Hospitalization Costs Among Patients with Alcoholic Hepatitis and Alcoholic Cirrhosis. Alcohol Alcohol. 2021, 56, 368–375. [Google Scholar] [CrossRef]
- Thursz, M.; Morgan, T.R. Treatment of Severe Alcoholic Hepatitis. Gastroenterology 2016, 150, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Rattan, P.; Shah, V.H. Review article: Current and emerging therapies for acute alcohol-associated hepatitis. Aliment. Pharmacol. Ther. 2022, 56, 28–40. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018, 69, 154–181. [Google Scholar] [CrossRef] [PubMed]
- Foncea, C.G.; Sporea, I.; Lupusoru, R.; Moga, T.V.; Bende, F.; Sirli, R.; Popescu, A. Day-4 Lille Score Is a Good Prognostic Factor and Early Predictor in Assessing Therapy Response in Patients with Liver Cirrhosis and Severe Alcoholic Hepatitis. J. Clin. Med. 2021, 10, 2338. [Google Scholar] [CrossRef] [PubMed]
- Thursz, M.R.; Richardson, P.; Allison, M.; Austin, A.; Bowers, M.; Day, C.P.; Downs, N.; Gleeson, D.; MacGilchrist, A.; Grant, A.; et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N. Engl. J. Med. 2015, 372, 1619–1628. [Google Scholar] [CrossRef]
- Arab, J.P.; Diaz, L.A.; Baeza, N.; Idalsoaga, F.; Fuentes-Lopez, E.; Arnold, J.; Ramirez, C.A.; Morales-Arraez, D.; Ventura-Cots, M.; Alvarado-Tapias, E.; et al. Identification of optimal therapeutic window for steroid use in severe alcohol-associated hepatitis: A worldwide study. J. Hepatol. 2021, 75, 1026–1033. [Google Scholar] [CrossRef]
- Louvet, A.; Thursz, M.R.; Kim, D.J.; Labreuche, J.; Atkinson, S.R.; Sidhu, S.S.; O’Grady, J.G.; Akriviadis, E.; Sinakos, E.; Carithers, R.L., Jr.; et al. Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo-a Meta-analysis of Individual Data From Controlled Trials. Gastroenterology 2018, 155, 458–468.e8. [Google Scholar] [CrossRef]
- Louvet, A.; Labreuche, J.; Artru, F.; Bouthors, A.; Rolland, B.; Saffers, P.; Lollivier, J.; Lemaitre, E.; Dharancy, S.; Lassailly, G.; et al. Main drivers of outcome differ between short term and long term in severe alcoholic hepatitis: A prospective study. Hepatology 2017, 66, 1464–1473. [Google Scholar] [CrossRef]
- Pavlov, C.S.; Varganova, D.L.; Casazza, G.; Tsochatzis, E.; Nikolova, D.; Gluud, C. Glucocorticosteroids for people with alcoholic hepatitis. Cochrane Database Syst. Rev. 2017, 11, CD001511. [Google Scholar] [CrossRef]
- Amjad, W.; Alukal, J.; Doycheva, I.; Zhang, T.; Maheshwari, A.; Yoo, H.; Thuluvath, P.J. A Combination of N-Acetylcysteine and Prednisone Has No Benefit Over Prednisone Alone in Severe Alcoholic Hepatitis: A Retrospective Analysis. Dig. Dis. Sci. 2020, 65, 3726–3733. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, H.J.; Kim, J.H.; Yoo, Y.J.; Kim, T.S.; Kang, S.H.; Suh, S.J.; Joo, M.K.; Jung, Y.K.; Lee, B.J.; et al. Treatment of Severe Alcoholic Hepatitis With Corticosteroid, Pentoxifylline, or Dual Therapy: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2017, 51, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, P.; Maevskaya, M.; Pavlov, A.; Komkova, I.; Pavlov, C.; Ivashkin, V. Prednisolone plus S-adenosil-L-methionine in severe alcoholic hepatitis. Hepatol. Int. 2016, 10, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, E.; Gomez-Sanchez, C.E. The multifaceted mineralocorticoid receptor. Compr. Physiol. 2014, 4, 965–994. [Google Scholar] [PubMed]
- Gathercole, L.L.; Lavery, G.G.; Morgan, S.A.; Cooper, M.S.; Sinclair, A.J.; Tomlinson, J.W.; Stewart, P.M. 11beta-Hydroxysteroid dehydrogenase 1: Translational and therapeutic aspects. Endocr. Rev. 2013, 34, 525–555. [Google Scholar] [PubMed]
- Heier, C.R.; Yu, Q.; Fiorillo, A.A.; Tully, C.B.; Tucker, A.; Mazala, D.A.; Uaesoontrachoon, K.; Srinivassane, S.; Damsker, J.M.; Hoffman, E.P.; et al. Vamorolone targets dual nuclear receptors to treat inflammation and dystrophic cardiomyopathy. Life Sci. Alliance 2019, 2, e201800186. [Google Scholar] [CrossRef]
- Perschel, F.H.; Buhler, H.; Hierholzer, K. Bile acids and their amidates inhibit 11 beta-hydroxysteroid dehydrogenase obtained from rat kidney. Pflug. Arch. 1991, 418, 538–543. [Google Scholar] [CrossRef]
- Escher, G.; Nawrocki, A.; Staub, T.; Vishwanath, B.S.; Frey, B.M.; Reichen, J.; Frey, F.J. Down-regulation of hepatic and renal 11 beta-hydroxysteroid dehydrogenase in rats with liver cirrhosis. Gastroenterology 1998, 114, 175–184. [Google Scholar] [CrossRef]
- Trinchet, J.C.; Gerhardt, M.F.; Balkau, B.; Munz, C.; Poupon, R.E. Serum bile acids and cholestasis in alcoholic hepatitis. Relationship with usual liver tests and histological features. J. Hepatol. 1994, 21, 235–240. [Google Scholar] [CrossRef]
- Alpert, L.; Hart, J. The Pathology of Alcoholic Liver Disease. Clin. Liver Dis. 2016, 20, 473–489. [Google Scholar] [CrossRef]
- Axley, P.; Russ, K.; Singal, A.K. Severe Alcoholic Hepatitis: Atypical Presentation with Markedly Elevated Alkaline Phosphatase. J. Clin. Transl. Hepatol. 2017, 5, 414–415. [Google Scholar] [CrossRef][Green Version]
- Nissenbaum, M.; Chedid, A.; Mendenhall, C.; Gartside, P. Prognostic significance of cholestatic alcoholic hepatitis. VA Cooperative Study Group #119. Dig. Dis. Sci. 1990, 35, 891–896. [Google Scholar] [PubMed]
- Forrest, E.H.; Storey, N.; Sinha, R.; Atkinson, S.R.; Vergis, N.; Richardson, P.; Masson, S.; Ryder, S.; Thursz, M.R.; Allison, M.; et al. Baseline neutrophil-to-lymphocyte ratio predicts response to corticosteroids and is associated with infection and renal dysfunction in alcoholic hepatitis. Aliment. Pharmacol. Ther. 2019, 50, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Diederich, S.; Eigendorff, E.; Burkhardt, P.; Quinkler, M.; Bumke-Vogt, C.; Rochel, M.; Seidelmann, D.; Esperling, P.; Oelkers, W.; Bahr, V. 11beta-hydroxysteroid dehydrogenase types 1 and 2: An important pharmacokinetic determinant for the activity of synthetic mineralo- and glucocorticoids. J. Clin. Endocrinol. Metab. 2002, 87, 5695–5701. [Google Scholar] [CrossRef] [PubMed]
- Rebuffat, A.G.; Tam, S.; Nawrocki, A.R.; Baker, M.E.; Frey, B.M.; Frey, F.J.; Odermatt, A. The 11-ketosteroid 11-ketodexamethasone is a glucocorticoid receptor agonist. Mol. Cell Endocrinol. 2004, 214, 27–37. [Google Scholar] [CrossRef]
- Jacob, K.A.; Leaf, D.E.; Dieleman, J.M.; van Dijk, D.; Nierich, A.P.; Rosseel, P.M.; van der Maaten, J.M.; Hofland, J.; Diephuis, J.C.; de Lange, F.; et al. Dexamethasone for Cardiac Surgery Study, G. Intraoperative High-Dose Dexamethasone and Severe AKI after Cardiac Surgery. J. Am. Soc. Nephrol. 2015, 26, 2947–2951. [Google Scholar] [CrossRef]
- Schirris, T.J.J.; Jansen, J.; Mihajlovic, M.; van den Heuvel, L.P.; Masereeuw, R.; Russel, F.G.M. Mild intracellular acidification by dexamethasone attenuates mitochondrial dysfunction in a human inflammatory proximal tubule epithelial cell model. Sci. Rep. 2017, 7, 10623. [Google Scholar] [CrossRef]
- Garg, A.X.; Chan, M.T.V.; Cuerden, M.S.; Devereaux, P.J.; Abbasi, S.H.; Hildebrand, A.; Lamontagne, F.; Lamy, A.; Noiseux, N.; Parikh, C.R.; et al. Effect of methylprednisolone on acute kidney injury in patients undergoing cardiac surgery with a cardiopulmonary bypass pump: A randomized controlled trial. CMAJ 2019, 191, E247–E256. [Google Scholar] [CrossRef]
- Ahmed, A.; Rabbitt, E.; Brady, T.; Brown, C.; Guest, P.; Bujalska, I.J.; Doig, C.; Newsome, P.N.; Hubscher, S.; Elias, E.; et al. A switch in hepatic cortisol metabolism across the spectrum of non alcoholic fatty liver disease. PLoS ONE 2012, 7, e29531. [Google Scholar]
- Nasiri, M.; Nikolaou, N.; Parajes, S.; Krone, N.P.; Valsamakis, G.; Mastorakos, G.; Hughes, B.; Taylor, A.; Bujalska, I.J.; Gathercole, L.L.; et al. 5alpha-Reductase Type 2 Regulates Glucocorticoid Action and Metabolic Phenotype in Human Hepatocytes. Endocrinology 2015, 156, 2863–2871. [Google Scholar] [CrossRef]
- Rachdaoui, N.; Sarkar, D.K. Effects of alcohol on the endocrine system. Endocrinol. Metab. Clin. N. Am. 2013, 42, 593–615. [Google Scholar] [CrossRef]
- Mueller, K.M.; Themanns, M.; Friedbichler, K.; Kornfeld, J.W.; Esterbauer, H.; Tuckermann, J.P.; Moriggl, R. Hepatic growth hormone and glucocorticoid receptor signaling in body growth, steatosis and metabolic liver cancer development. Mol. Cell Endocrinol. 2012, 361, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y. The Role of Growth Hormone and Insulin-Like Growth Factor-I in the Liver. Int. J. Mol. Sci. 2017, 18, 1447. [Google Scholar] [CrossRef] [PubMed]
- Tronche, F.; Opherk, C.; Moriggl, R.; Kellendonk, C.; Reimann, A.; Schwake, L.; Reichardt, H.M.; Stangl, K.; Gau, D.; Hoeflich, A.; et al. Glucocorticoid receptor function in hepatocytes is essential to promote postnatal body growth. Genes Dev. 2004, 18, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.M.; Kornfeld, J.W.; Friedbichler, K.; Blaas, L.; Egger, G.; Esterbauer, H.; Hasselblatt, P.; Schlederer, M.; Haindl, S.; Wagner, K.U.; et al. Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology 2011, 54, 1398–1409. [Google Scholar] [CrossRef]
- Lu, H.; Lei, X.; Winkler, R.; John, S.; Kumar, D.; Li, W.; Alnouti, Y. Crosstalk of hepatocyte nuclear factor 4a and glucocorticoid receptor in the regulation of lipid metabolism in mice fed a high-fat-high-sugar diet. Lipids Health Dis. 2022, 21, 46. [Google Scholar]
- Massey, V.; Parrish, A.; Argemi, J.; Moreno, M.; Mello, A.; Garcia-Rocha, M.; Altamirano, J.; Odena, G.; Dubuquoy, L.; Louvet, A.; et al. Integrated Multiomics Reveals Glucose Use Reprogramming and Identifies a Novel Hexokinase in Alcoholic Hepatitis. Gastroenterology 2021, 160, 1725–1740.e2. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Chen, J.; Su, Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front. Endocrinol. 2018, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Praestholm, S.M.; Correia, C.M.; Goitea, V.E.; Siersbaek, M.S.; Jorgensen, M.; Havelund, J.F.; Pedersen, T.A.; Faergeman, N.J.; Grontved, L. Impaired glucocorticoid receptor expression in liver disrupts feeding-induced gene expression, glucose uptake, and glycogen storage. Cell Rep. 2021, 37, 109938. [Google Scholar] [CrossRef]
- Glavind, E.; Aagaard, N.K.; Gronbaek, H.; Moller, H.J.; Orntoft, N.W.; Vilstrup, H.; Thomsen, K.L. Alcoholic Hepatitis Markedly Decreases the Capacity for Urea Synthesis. PLoS ONE 2016, 11, e0158388. [Google Scholar]
- Parekh, P.J.; Balart, L.A. Ammonia and Its Role in the Pathogenesis of Hepatic Encephalopathy. Clin. Liver Dis. 2015, 19, 529–537. [Google Scholar] [CrossRef]
- Okun, J.G.; Conway, S.; Schmidt, K.V.; Schumacher, J.; Wang, X.; de Guia, R.; Zota, A.; Klement, J.; Seibert, O.; Peters, A.; et al. Molecular regulation of urea cycle function by the liver glucocorticoid receptor. Mol. Metab. 2015, 4, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Glavind, E.; Aagaard, N.K.; Gronbaek, H.; Orntoft, N.W.; Vilstrup, H.; Thomsen, K.L. Time course of compromised urea synthesis in patients with alcoholic hepatitis. Scand. J. Gastroenterol. 2017, 592–597. [Google Scholar]
- Alvaro, D.; Gigliozzi, A.; Marucci, L.; Alpini, G.; Barbaro, B.; Monterubbianesi, R.; Minetola, L.; Mancino, M.G.; Medina, J.F.; Attili, A.F.; et al. Corticosteroids modulate the secretory processes of the rat intrahepatic biliary epithelium. Gastroenterology 2002, 122, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Zollner, G.; Trauner, M. Nuclear receptors as therapeutic targets in cholestatic liver diseases. Br. J. Pharmacol. 2009, 156, 7–27. [Google Scholar] [PubMed]
- Petrescu, A.D.; Grant, S.; Frampton, G.; Kain, J.; Hadidi, K.; Williams, E.; McMillin, M.; DeMorrow, S. Glucocorticoids Cause Gender-Dependent Reversal of Hepatic Fibrosis in the MDR2-Knockout Mouse Model. Int. J. Mol. Sci. 2017, 18, 2389. [Google Scholar] [CrossRef] [PubMed]
- Gruver-Yates, A.L.; Cidlowski, J.A. Tissue-specific actions of glucocorticoids on apoptosis: A double-edged sword. Cells 2013, 2, 202–223. [Google Scholar] [CrossRef] [PubMed]
- Eloranta, J.J.; Jung, D.; Kullak-Ublick, G.A. The human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator-1alpha, and suppressed by bile acids via a small heterodimer partner-dependent mechanism. Mol. Endocrinol. 2006, 20, 65–79. [Google Scholar] [CrossRef]
- Warskulat, U.; Kubitz, R.; Wettstein, M.; Stieger, B.; Meier, P.J.; Haussinger, D. Regulation of bile salt export pump mRNA levels by dexamethasone and osmolarity in cultured rat hepatocytes. Biol. Chem. 1999, 380, 1273–1279. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, J.M.; Zhou, Y.; Harpavat, S.; Moore, D.D. Glucocorticoids Have Opposing Effects on Liver Fibrosis in Hepatic Stellate and Immune Cells. Mol. Endocrinol. 2016, 30, 905–916. [Google Scholar] [CrossRef]
- Zou, X.; Ramachandran, P.; Kendall, T.J.; Pellicoro, A.; Dora, E.; Aucott, R.L.; Manwani, K.; Man, T.Y.; Chapman, K.E.; Henderson, N.C.; et al. 11Beta-hydroxysteroid dehydrogenase-1 deficiency or inhibition enhances hepatic myofibroblast activation in murine liver fibrosis. Hepatology 2018, 67, 2167–2181. [Google Scholar] [CrossRef]
- Jimenez, V.A.; Walter, N.A.; Shnitko, T.A.; Newman, N.P.; Diem, K.; Vanderhooft, L.; Hunt, H.J.; Grant, K.A. Mifepristone decreases chronic voluntary ethanol consumption in rhesus macaques. J. Pharmacol. Exp. Ther. 2020, 375, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, L.F.; Estey, D.; Goodell, V.; Macshane, L.G.; Logrip, M.L.; Schlosburg, J.E.; McGinn, M.A.; Zamora-Martinez, E.R.; Belanoff, J.K.; Hunt, H.J.; et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J. Clin. Investig. 2015, 125, 3193–3197. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Roh, H.C.; Kumari, M.; Rosen, E.D. Adipocyte glucocorticoid receptor is important in lipolysis and insulin resistance due to exogenous steroids, but not insulin resistance caused by high fat feeding. Mol. Metab. 2017, 6, 1150–1160. [Google Scholar] [CrossRef]
- Narum, S.; Westergren, T.; Klemp, M. Corticosteroids and risk of gastrointestinal bleeding: A systematic review and meta-analysis. BMJ Open 2014, 4, e004587. [Google Scholar] [CrossRef]
- Out, C.; Dikkers, A.; Laskewitz, A.; Boverhof, R.; van der Ley, C.; Kema, I.P.; Wolters, H.; Havinga, R.; Verkade, H.J.; Kuipers, F.; et al. Prednisolone increases enterohepatic cycling of bile acids by induction of Asbt and promotes reverse cholesterol transport. J. Hepatol. 2014, 61, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.H.; Frost, R.A.; Vary, T.C. Regulation of muscle protein synthesis during sepsis and inflammation. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E453–E459. [Google Scholar] [CrossRef] [PubMed]
- Salamone, I.M.; Quattrocelli, M.; Barefield, D.Y.; Page, P.G.; Tahtah, I.; Hadhazy, M.; Tomar, G.; McNally, E.M. Intermittent glucocorticoid treatment enhances skeletal muscle performance through sexually dimorphic mechanisms. J. Clin. Investig. 2022, 132, e149828. [Google Scholar] [CrossRef]
- Gathercole, L.L.; Morgan, S.A.; Bujalska, I.J.; Hauton, D.; Stewart, P.M.; Tomlinson, J.W. Regulation of lipogenesis by glucocorticoids and insulin in human adipose tissue. PLoS ONE 2011, 6, e26223. [Google Scholar] [CrossRef]
- Kwon, H.J.; Won, Y.S.; Park, O.; Feng, D.; Gao, B. Opposing effects of prednisolone treatment on T/NKT cell- and hepatotoxin-mediated hepatitis in mice. Hepatology 2014, 59, 1094–1106. [Google Scholar] [CrossRef]
- van der Goes, A.; Hoekstra, K.; van den Berg, T.K.; Dijkstra, C.D. Dexamethasone promotes phagocytosis and bacterial killing by human monocytes/macrophages in vitro. J. Leukoc. Biol. 2000, 67, 801–807. [Google Scholar] [CrossRef]
- Lim, H.Y.; Muller, N.; Herold, M.J.; van den Brandt, J.; Reichardt, H.M. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology 2007, 122, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bruder-Nascimento, T.; Ferreira, N.S.; Zanotto, C.Z.; Ramalho, F.; Pequeno, I.O.; Olivon, V.C.; Neves, K.B.; Alves-Lopes, R.; Campos, E.; Silva, C.A.; et al. NLRP3 Inflammasome Mediates Aldosterone-Induced Vascular Damage. Circulation 2016, 134, 1866–1880. [Google Scholar] [CrossRef] [PubMed]
- Belden, Z.; Deiuliis, J.A.; Dobre, M.; Rajagopalan, S. The Role of the Mineralocorticoid Receptor in Inflammation: Focus on Kidney and Vasculature. Am. J. Nephrol. 2017, 46, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Durango, N.; Arrese, M.; Hernandez, A.; Jara, E.; Kalergis, A.M.; Cabrera, D. A Mineralocorticoid Receptor Deficiency in Myeloid Cells Reduces Liver Steatosis by Impairing Activation of CD8(+) T Cells in a Nonalcoholic Steatohepatitis Mouse Model. Front. Immunol. 2020, 11, 563434. [Google Scholar] [CrossRef]
- Pizarro, M.; Solis, N.; Quintero, P.; Barrera, F.; Cabrera, D.; Rojas-de Santiago, P.; Arab, J.P.; Padilla, O.; Roa, J.C.; Moshage, H.; et al. Beneficial effects of mineralocorticoid receptor blockade in experimental non-alcoholic steatohepatitis. Liver Int. 2015, 35, 2129–2138. [Google Scholar] [CrossRef]
- Schreier, B.; Wolf, A.; Hammer, S.; Pohl, S.; Mildenberger, S.; Rabe, S.; Gekle, M.; Zipprich, A. The selective mineralocorticoid receptor antagonist eplerenone prevents decompensation of the liver in cirrhosis. Br. J. Pharmacol. 2018, 175, 2956–2967. [Google Scholar] [CrossRef]
- Damsker, J.M.; Cornish, M.R.; Kanneboyina, P.; Kanneboyina, I.; Yu, Q.; Lipson, R.; Phadke, A.; Knoblach, S.M.; Panchapakesan, K.; Morales, M.; et al. Vamorolone, a dissociative steroidal compound, reduces collagen antibody-induced joint damage and inflammation when administered after disease onset. Inflamm. Res. 2019, 68, 969–980. [Google Scholar] [CrossRef]
- Smith, E.C.; Conklin, L.S.; Hoffman, E.P.; Clemens, P.R.; Mah, J.K.; Finkel, R.S.; Guglieri, M.; Tulinius, M.; Nevo, Y.; Ryan, M.M.; et al. Efficacy and safety of vamorolone in Duchenne muscular dystrophy: An 18-month interim analysis of a non-randomized open-label extension study. PLoS Med. 2020, 17, e1003222. [Google Scholar] [CrossRef]
- Almeida, L.E.F.; Damsker, J.M.; Albani, S.; Afsar, N.; Kamimura, S.; Pratt, D.; Kleiner, D.E.; Quezado, M.; Gordish-Dressman, H.; Quezado, Z.M.N. The corticosteroid compounds prednisolone and vamorolone do not alter the nociception phenotype and exacerbate liver injury in sickle cell mice. Sci. Rep. 2018, 8, 6081. [Google Scholar] [CrossRef]
- Lopez-Sanroman, A.; Clofent, J.; Garcia-Planella, E.; Menchen, L.; Nos, P.; Rodriguez-Lago, I.; Domenech, E. Reviewing the therapeutic role of budesonide in Crohn’s disease. Gastroenterol. Hepatol. 2018, 41, 458–471. [Google Scholar] [CrossRef]
- Komkova, I.I.; Tkachenko, P.E.; Maevskaya, M.V.; Ivashkin, V.T. Budesonide in Severe Alcoholic Hepatitis: Results of the Original Research. Am. J. Clin. Med. Res. 2016, 4, 7–10. [Google Scholar]
- Yao, T.C.; Huang, Y.W.; Chang, S.M.; Tsai, S.Y.; Wu, A.C.; Tsai, H.J. Association Between Oral Corticosteroid Bursts and Severe Adverse Events: A Nationwide Population-Based Cohort Study. Ann. Intern. Med. 2020, 173, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.K.; Meena, A.S.; Pierre, J.F.; Rao, R. Central role of intestinal epithelial glucocorticoid receptor in alcohol- and corticosterone-induced gut permeability and systemic response. FASEB J. 2022, 36, e22061. [Google Scholar] [CrossRef] [PubMed]
- Vergis, N.; Atkinson, S.R.; Knapp, S.; Maurice, J.; Allison, M.; Austin, A.; Forrest, E.H.; Masson, S.; McCune, A.; Patch, D.; et al. In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated With High Circulating Levels of Bacterial DNA. Gastroenterology 2017, 152, 1068–1077.e4. [Google Scholar] [CrossRef] [PubMed]
- Affo, S.; Dominguez, M.; Lozano, J.J.; Sancho-Bru, P.; Rodrigo-Torres, D.; Morales-Ibanez, O.; Moreno, M.; Millan, C.; Loaeza-del-Castillo, A.; Altamirano, J.; et al. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut 2013, 62, 452–460. [Google Scholar] [CrossRef]
- Menke, A.; Arloth, J.; Putz, B.; Weber, P.; Klengel, T.; Mehta, D.; Gonik, M.; Rex-Haffner, M.; Rubel, J.; Uhr, M.; et al. Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology 2012, 37, 1455–1464. [Google Scholar] [CrossRef]
- Rulcova, A.; Krausova, L.; Smutny, T.; Vrzal, R.; Dvorak, Z.; Jover, R.; Pavek, P. Glucocorticoid receptor regulates organic cation transporter 1 (OCT1, SLC22A1) expression via HNF4alpha upregulation in primary human hepatocytes. Pharmacol. Rep. 2013, 65, 1322–1335. [Google Scholar] [CrossRef]
- Han, S.; Ray, J.W.; Pathak, P.; Sweet, D.R.; Zhang, R.; Gao, H.; Jain, N.; Koritzinsky, E.H.; Matoba, K.; Xu, W.; et al. KLF15 regulates endobiotic and xenobiotic metabolism. Nat. Metab. 2019, 1, 422–430. [Google Scholar] [CrossRef]
- Bougarne, N.; Paumelle, R.; Caron, S.; Hennuyer, N.; Mansouri, R.; Gervois, P.; Staels, B.; Haegeman, G.; De Bosscher, K. PPARalpha blocks glucocorticoid receptor alpha-mediated transactivation but cooperates with the activated glucocorticoid receptor alpha for transrepression on NF-kappaB. Proc. Natl. Acad. Sci. USA 2009, 106, 7397–7402. [Google Scholar] [CrossRef]
- Vandevyver, S.; Dejager, L.; Van Bogaert, T.; Kleyman, A.; Liu, Y.; Tuckermann, J.; Libert, C. Glucocorticoid receptor dimerization induces MKP1 to protect against TNF-induced inflammation. J. Clin. Investig. 2012, 122, 2130–2140. [Google Scholar] [CrossRef]
- Murtagh, J.; McArdle, E.; Gilligan, E.; Thornton, L.; Furlong, F.; Martin, F. Organization of mammary epithelial cells into 3D acinar structures requires glucocorticoid and JNK signaling. J. Cell Biol. 2004, 166, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Rog-Zielinska, E.A.; Craig, M.A.; Manning, J.R.; Richardson, R.V.; Gowans, G.J.; Dunbar, D.R.; Gharbi, K.; Kenyon, C.J.; Holmes, M.C.; Hardie, D.G.; et al. Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: A role for PGC-1alpha. Cell Death Differ. 2015, 22, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; McQueen, A.; Chen, T.C.; Wang, J.C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar] [PubMed]
- Connaughton, S.; Chowdhury, F.; Attia, R.R.; Song, S.; Zhang, Y.; Elam, M.B.; Cook, G.A.; Park, E.A. Regulation of pyruvate dehydrogenase kinase isoform 4 (PDK4) gene expression by glucocorticoids and insulin. Mol. Cell Endocrinol. 2010, 315, 159–167. [Google Scholar] [CrossRef]
- Rowling, M.J.; Schalinske, K.L. Retinoic acid and glucocorticoid treatment induce hepatic glycine N-methyltransferase and lower plasma homocysteine concentrations in rats and rat hepatoma cells. J. Nutr. 2003, 133, 3392–3398. [Google Scholar] [CrossRef]
- Martinho, A.; Goncalves, I.; Santos, C.R. Glucocorticoids regulate metallothionein-1/2 expression in rat choroid plexus: Effects on apoptosis. Mol. Cell Biochem. 2013, 376, 41–51. [Google Scholar] [CrossRef]
- Lu, H. Crosstalk of HNF4alpha with extracellular and intracellular signaling pathways in the regulation of hepatic metabolism of drugs and lipids. Acta Pharm. Sin. B 2016, 6, 393–408. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Yahagi, N.; Aita, Y.; Murayama, Y.; Sawada, Y.; Piao, X.; Toya, N.; Oya, Y.; Shikama, A.; Takarada, A.; et al. KLF15 Enables Rapid Switching between Lipogenesis and Gluconeogenesis during Fasting. Cell Rep. 2016, 16, 2373–2386. [Google Scholar] [CrossRef]
- Fan, H.; Morand, E.F. Targeting the side effects of steroid therapy in autoimmune diseases: The role of GILZ. Discov. Med. 2012, 13, 123–133. [Google Scholar]
- De Smaele, E.; Zazzeroni, F.; Papa, S.; Nguyen, D.U.; Jin, R.; Jones, J.; Cong, R.; Franzoso, G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature 2001, 414, 308–313. [Google Scholar] [CrossRef]
- Papa, S.; Zazzeroni, F.; Fu, Y.X.; Bubici, C.; Alvarez, K.; Dean, K.; Christiansen, P.A.; Anders, R.A.; Franzoso, G. Gadd45beta promotes hepatocyte survival during liver regeneration in mice by modulating JNK signaling. J. Clin. Investig. 2008, 118, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Brenner, D.A.; Karin, M. A liver full of JNK: Signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 2012, 143, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, E.; Villani, G.; Moschetta, A. Metabolic aspects in NAFLD, NASH and hepatocellular carcinoma: The role of PGC1 coactivators. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.C.; Puigserver, P.; Chen, G.; Donovan, J.; Wu, Z.; Rhee, J.; Adelmant, G.; Stafford, J.; Kahn, C.R.; Granner, D.K.; et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 2001, 413, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Monteillet, L.; Gjorgjieva, M.; Silva, M.; Verzieux, V.; Imikirene, L.; Duchampt, A.; Guillou, H.; Mithieux, G.; Rajas, F. Intracellular lipids are an independent cause of liver injury and chronic kidney disease in non alcoholic fatty liver disease-like context. Mol. Metab. 2018, 16, 100–115. [Google Scholar] [CrossRef]
- Mutel, E.; Abdul-Wahed, A.; Ramamonjisoa, N.; Stefanutti, A.; Houberdon, I.; Cavassila, S.; Pilleul, F.; Beuf, O.; Gautier-Stein, A.; Penhoat, A.; et al. Targeted deletion of liver glucose-6 phosphatase mimics glycogen storage disease type 1a including development of multiple adenomas. J. Hepatol. 2011, 54, 529–537. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, G.Y.; Pan, C.J.; Anduaga, J.; Choi, E.J.; Mansfield, B.C.; Chou, J.Y. Downregulation of SIRT1 signaling underlies hepatic autophagy impairment in glycogen storage disease type Ia. PLoS Genet. 2017, 13, e1006819. [Google Scholar] [CrossRef]
- Park, B.Y.; Jeon, J.H.; Go, Y.; Ham, H.J.; Kim, J.E.; Yoo, E.K.; Kwon, W.H.; Jeoung, N.H.; Jeon, Y.H.; Koo, S.H.; et al. PDK4 Deficiency Suppresses Hepatic Glucagon Signaling by Decreasing cAMP Levels. Diabetes 2018, 67, 2054–2068. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Park, Y.K.; Lee, J.Y.; Gao, L.; Zhao, J.; Wang, L. Loss of PDK4 switches the hepatic NF-kappaB/TNF pathway from pro-survival to pro-apoptosis. Hepatology 2018, 68, 1111–1124. [Google Scholar] [CrossRef]
- Lu, S.C.; Mato, J.M. S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 2012, 92, 1515–1542. [Google Scholar] [CrossRef]
- Sato, M.; Kawakami, T.; Kondoh, M.; Takiguchi, M.; Kadota, Y.; Himeno, S.; Suzuki, S. Development of high-fat-diet-induced obesity in female metallothionein-null mice. FASEB J. 2010, 24, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, X.; James Kang, Y. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp. Biol. Med. 2002, 227, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, Q.; Wu, L.; Gao, F.; Xie, H.; Zhou, L.; Zheng, S.; Xu, X. Metallothionein 1 family profiling identifies MT1X as a tumor suppressor involved in the progression and metastastatic capacity of hepatocellular carcinoma. Mol. Carcinog. 2018, 57, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Lauriola, M.; Enuka, Y.; Zeisel, A.; D’Uva, G.; Roth, L.; Sharon-Sevilla, M.; Lindzen, M.; Sharma, K.; Nevo, N.; Feldman, M.; et al. Diurnal suppression of EGFR signalling by glucocorticoids and implications for tumour progression and treatment. Nat. Commun. 2014, 5, 5073. [Google Scholar] [CrossRef] [PubMed]
- Quattrocelli, M.; Wintzinger, M.; Miz, K.; Levine, D.C.; Peek, C.B.; Bass, J.; McNally, E.M. Muscle mitochondrial remodeling by intermittent glucocorticoid drugs requires an intact circadian clock and muscle PGC1alpha. Sci. Adv. 2022, 8, eabm1189. [Google Scholar] [CrossRef]
- Kim, K.; Boo, K.; Yu, Y.S.; Oh, S.K.; Kim, H.; Jeon, Y.; Bhin, J.; Hwang, D.; Kim, K.I.; Lee, J.S.; et al. RORalpha controls hepatic lipid homeostasis via negative regulation of PPARgamma transcriptional network. Nat. Commun. 2017, 8, 162. [Google Scholar] [CrossRef]
- Han, Y.H.; Shin, K.O.; Kim, J.Y.; Khadka, D.B.; Kim, H.J.; Lee, Y.M.; Cho, W.J.; Cha, J.Y.; Lee, B.J.; Lee, M.O. A maresin 1/RORalpha/12-lipoxygenase autoregulatory circuit prevents inflammation and progression of nonalcoholic steatohepatitis. J. Clin. Investig. 2019, 129, 1684–1698. [Google Scholar] [CrossRef]
- Chai, C.; Cox, B.; Yaish, D.; Gross, D.; Rosenberg, N.; Amblard, F.; Shemuelian, Z.; Gefen, M.; Korach, A.; Tirosh, O.; et al. Agonist of RORA Attenuates Nonalcoholic Fatty Liver Progression in Mice via Up-regulation of MicroRNA 122. Gastroenterology 2020, 159, 999–1014. [Google Scholar] [CrossRef]
- Park, B.K.; Lee, E.A.; Kim, H.Y.; Lee, J.C.; Kim, K.S.; Jeong, W.H.; Kim, K.Y.; Ku, B.J.; Rhee, S.D. Fatty Liver and Insulin Resistance in the Liver-Specific Knockout Mice of Mitogen Inducible Gene-6. J. Diabetes Res. 2016, 2016, 1632061. [Google Scholar] [CrossRef]
- Ku, B.J.; Kim, T.H.; Lee, J.H.; Buras, E.D.; White, L.D.; Stevens, R.D.; Ilkayeva, O.R.; Bain, J.R.; Newgard, C.B.; DeMayo, F.J.; et al. Mig-6 plays a critical role in the regulation of cholesterol homeostasis and bile acid synthesis. PLoS ONE 2012, 7, e42915. [Google Scholar] [CrossRef]
- Nishikawa, T.; Bellance, N.; Damm, A.; Bing, H.; Zhu, Z.; Handa, K.; Yovchev, M.I.; Sehgal, V.; Moss, T.J.; Oertel, M.; et al. A switch in the source of ATP production and a loss in capacity to perform glycolysis are hallmarks of hepatocyte failure in advance liver disease. J. Hepatol. 2014, 60, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Kang, J.E.; Peng, L.J.; Li, H.; Khan, S.A.; Hillard, C.J.; Okar, D.A.; Lange, A.J. Enhancing hepatic glycolysis reduces obesity: Differential effects on lipogenesis depend on site of glycolytic modulation. Cell Metab. 2005, 2, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Domenech, E.; Maestre, C.; Esteban-Martinez, L.; Partida, D.; Pascual, R.; Fernandez-Miranda, G.; Seco, E.; Campos-Olivas, R.; Perez, M.; Megias, D.; et al. AMPK and PFKFB3 mediate glycolysis and survival in response to mitophagy during mitotic arrest. Nat. Cell Biol. 2015, 17, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, X.; Wang, T.; Chen, B.; Xing, J. HAO2 inhibits malignancy of clear cell renal cell carcinoma by promoting lipid catabolic process. J. Cell Physiol. 2019, 234, 23005–23016. [Google Scholar] [CrossRef] [PubMed]
- Um, J.H.; Pendergast, J.S.; Springer, D.A.; Foretz, M.; Viollet, B.; Brown, A.; Kim, M.K.; Yamazaki, S.; Chung, J.H. AMPK regulates circadian rhythms in a tissue- and isoform-specific manner. PLoS ONE 2011, 6, e18450. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, J.; Fan, R.; Zhang, C.; Xu, L.; Sun, X.; Huang, Y.; Wang, Q.; Ruan, H.B.; Qian, X. NAMPT overexpression alleviates alcohol-induced hepatic steatosis in mice. PLoS ONE 2019, 14, e0212523. [Google Scholar] [CrossRef]
- Zhang, X.; Heckmann, B.L.; Campbell, L.E.; Liu, J. G0S2: A small giant controller of lipolysis and adipose-liver fatty acid flux. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1146–1154. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, D.; Winkler, R.; Wang, X.; Luo, J.; Lu, H. Development of novel liver-targeting glucocortiocid prodrugs for the treatment of inflammatory liver diseases and sepsis. SUNY Upstate Med. Univ. Syracuse N. Y. USA 2022. to be submitted. [Google Scholar]
- Xue, R.; Meng, Q. The Management of Glucocorticoid Therapy in Liver Failure. Front. Immunol. 2019, 10, 2490. [Google Scholar] [CrossRef]
- Vandewalle, J.; Libert, C. Glucocorticoids in Sepsis: To Be or Not to Be. Front. Immunol. 2020, 11, 1318. [Google Scholar] [CrossRef]
- Gustot, T.; Fernandez, J.; Szabo, G.; Albillos, A.; Louvet, A.; Jalan, R.; Moreau, R.; Moreno, C. Sepsis in alcohol-related liver disease. J. Hepatol. 2017, 67, 1031–1050. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, O.; Gonzalez-Reimers, E.; Quintero-Platt, G.; Abreu-Gonzalez, P.; Vega-Prieto, M.J.; Sanchez-Perez, M.J.; Martin-Gonzalez, C.; Martinez-Riera, A.; Santolaria-Fernandez, F. Malondialdehyde as a Prognostic Factor in Alcoholic Hepatitis. Alcohol Alcohol. 2017, 52, 305–310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ray, K. Alcoholic liver disease: Alcoholic hepatitis: A warning for prednisolone and infection risk? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 68. [Google Scholar] [PubMed]
- Yeligar, S.M.; Chen, M.M.; Kovacs, E.J.; Sisson, J.H.; Burnham, E.L.; Brown, L.A. Alcohol and lung injury and immunity. Alcohol 2016, 55, 51–59. [Google Scholar] [CrossRef]
- Louvet, A.; Wartel, F.; Castel, H.; Dharancy, S.; Hollebecque, A.; Canva-Delcambre, V.; Deltenre, P.; Mathurin, P. Infection in patients with severe alcoholic hepatitis treated with steroids: Early response to therapy is the key factor. Gastroenterology 2009, 137, 541–548. [Google Scholar] [CrossRef]
- Jenniskens, M.; Weckx, R.; Dufour, T.; Vander Perre, S.; Pauwels, L.; Derde, S.; Teblick, A.; Guiza, F.; Van den Berghe, G.; Langouche, L. The Hepatic Glucocorticoid Receptor Is Crucial for Cortisol Homeostasis and Sepsis Survival in Humans and Male Mice. Endocrinology 2018, 159, 2790–2802. [Google Scholar] [CrossRef]
- Mookerjee, R.P.; Stadlbauer, V.; Lidder, S.; Wright, G.A.; Hodges, S.J.; Davies, N.A.; Jalan, R. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology 2007, 46, 831–840. [Google Scholar] [CrossRef]
- Takeuchi, M.; Vidigal, P.T.; Guerra, M.T.; Hundt, M.A.; Robert, M.E.; Olave-Martinez, M.; Aoki, S.; Khamphaya, T.; Kersten, R.; Kruglov, E.; et al. Neutrophils interact with cholangiocytes to cause cholestatic changes in alcoholic hepatitis. Gut 2021, 70, 342–356. [Google Scholar] [CrossRef]
- Ma, J.; Guillot, A.; Yang, Z.; Mackowiak, B.; Hwang, S.; Park, O.; Peiffer, B.J.; Ahmadi, A.R.; Melo, L.; Kusumanchi, P.; et al. Distinct histopathological phenotypes of severe alcoholic hepatitis suggest different mechanisms driving liver injury and failure. J. Clin. Investig. 2022, 132, e157780. [Google Scholar] [CrossRef]
- Ku, N.O.; Strnad, P.; Bantel, H.; Omary, M.B. Keratins: Biomarkers and modulators of apoptotic and necrotic cell death in the liver. Hepatology 2016, 64, 966–976. [Google Scholar] [CrossRef]
- Atkinson, S.R.; Grove, J.I.; Liebig, S.; Astbury, S.; Vergis, N.; Goldin, R.; Quaglia, A.; Bantel, H.; Guha, I.N.; Thursz, M.R.; et al. In Severe Alcoholic Hepatitis, Serum Keratin-18 Fragments Are Diagnostic, Prognostic, and Theragnostic Biomarkers. Am. J. Gastroenterol. 2020, 115, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, J.; Miquel, R.; Katoonizadeh, A.; Abraldes, J.G.; Duarte-Rojo, A.; Louvet, A.; Augustin, S.; Mookerjee, R.P.; Michelena, J.; Smyrk, T.C.; et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 2014, 146, 1231–1239.e6. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, T.; Goulis, I.; Soulaidopoulos, S.; Karasmani, A.; Doumtsis, P.; Tsioni, K.; Mandala, E.; Akriviadis, E.; Cholongitas, E. High serum ferritin is associated with worse outcome of patients with decompensated cirrhosis. Ann. Gastroenterol. 2017, 30, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Sahlman, P.; Nissinen, M.; Simonen, P.; Farkkila, M. Noncholesterol Sterols as Surrogate Markers in Patients with Severe Alcoholic Hepatitis. Lipids 2018, 53, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Cots, M.; Argemi, J.; Jones, P.D.; Lackner, C.; El Hag, M.; Abraldes, J.G.; Alvarado, E.; Clemente, A.; Ravi, S.; Alves, A.; et al. Clinical, histological and molecular profiling of different stages of alcohol-related liver disease. Gut 2022, 71, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Baweja, S.; Maras, J.S.; Shasthry, S.M.; Moreau, R.; Sarin, S.K. Differential blood transcriptome modules predict response to corticosteroid therapy in alcoholic hepatitis. JHEP Rep. 2021, 3, 100283. [Google Scholar] [CrossRef]
- Maras, J.S.; Das, S.; Sharma, S.; Shasthry, S.M.; Colsch, B.; Junot, C.; Moreau, R.; Sarin, S.K. Baseline urine metabolic phenotype in patients with severe alcoholic hepatitis and its association with outcome. Hepatol. Commun. 2018, 2, 628–643. [Google Scholar] [CrossRef]
- Chalmers, R.A.; Roe, C.R.; Stacey, T.E.; Hoppel, C.L. Urinary excretion of l-carnitine and acylcarnitines by patients with disorders of organic acid metabolism: Evidence for secondary insufficiency of l-carnitine. Pediatr. Res. 1984, 18, 1325–1328. [Google Scholar] [CrossRef]
- Manoli, I.; De Martino, M.U.; Kino, T.; Alesci, S. Modulatory effects of L-carnitine on glucocorticoid receptor activity. Ann. N. Y. Acad. Sci. 2004, 1033, 147–157. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H. Liver-specific knockout of glucocorticoid receptor worsens steatohepatitis induced by high-fat-diet-plus binge alcohol in mice. SUNY Upstate Med. Univ. Syracuse N. Y. USA 2022. to be submitted. [Google Scholar]
- Gomez, M.; Raju, S.V.; Viswanathan, A.; Painter, R.G.; Bonvillain, R.; Byrne, P.; Nguyen, D.H.; Bagby, G.J.; Kolls, J.K.; Nelson, S.; et al. Ethanol upregulates glucocorticoid-induced leucine zipper expression and modulates cellular inflammatory responses in lung epithelial cells. J. Immunol. 2010, 184, 5715–5722. [Google Scholar] [CrossRef] [PubMed]
- Vandevyver, S.; Dejager, L.; Libert, C. Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocr. Rev. 2014, 35, 671–693. [Google Scholar] [PubMed]
- Gasic, V.; Zukic, B.; Stankovic, B.; Janic, D.; Dokmanovic, L.; Lazic, J.; Krstovski, N.; Dolzan, V.; Jazbec, J.; Pavlovic, S.; et al. Pharmacogenomic markers of glucocorticoid response in the initial phase of remission induction therapy in childhood acute lymphoblastic leukemia. Radiol. Oncol. 2018, 52, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Kok, L.; Hillegers, M.H.; Veldhuijzen, D.S.; Boks, M.P.; Dieleman, J.M.; van Dijk, D.; Joels, M.; Vinkers, C.H. Genetic variation in the glucocorticoid receptor and psychopathology after dexamethasone administration in cardiac surgery patients. J. Psychiatr. Res. 2018, 103, 167–172. [Google Scholar] [CrossRef]
- De Iudicibus, S.; Stocco, G.; Martelossi, S.; Drigo, I.; Norbedo, S.; Lionetti, P.; Pozzi, E.; Barabino, A.; Decorti, G.; Bartoli, F.; et al. Association of BclI polymorphism of the glucocorticoid receptor gene locus with response to glucocorticoids in inflammatory bowel disease. Gut 2007, 56, 1319–1320. [Google Scholar] [CrossRef]
- Sharma, S.; Maras, J.S.; Das, S.; Hussain, S.; Mishra, A.K.; Shasthry, S.M.; Sharma, C.B.; Weiss, E.; Elkrief, L.; Rautou, P.E.; et al. Pre-therapy liver transcriptome landscape in Indian and French patients with severe alcoholic hepatitis and steroid responsiveness. Sci. Rep. 2017, 7, 6816. [Google Scholar] [CrossRef]
- Vandewalle, J.; Timmermans, S.; Paakinaho, V.; Vancraeynest, L.; Dewyse, L.; Vanderhaeghen, T.; Wallaeys, C.; Van Wyngene, L.; Van Looveren, K.; Nuyttens, L.; et al. Combined glucocorticoid resistance and hyperlactatemia contributes to lethal shock in sepsis. Cell Metab. 2021, 33, 1763–1776.e5. [Google Scholar] [CrossRef]
- Krebs, H.A.; Freedland, R.A.; Hems, R.; Stubbs, M. Inhibition of hepatic gluconeogenesis by ethanol. Biochem. J. 1969, 112, 117–124. [Google Scholar] [CrossRef]
- Schnabl, B.; Brenner, D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef]
- Neuman, M.G.; Maor, Y.; Nanau, R.M.; Melzer, E.; Mell, H.; Opris, M.; Cohen, L.; Malnick, S. Alcoholic Liver Disease: Role of Cytokines. Biomolecules 2015, 5, 2023–2034. [Google Scholar] [CrossRef]
- Michelena, J.; Altamirano, J.; Abraldes, J.G.; Affo, S.; Morales-Ibanez, O.; Sancho-Bru, P.; Dominguez, M.; Garcia-Pagan, J.C.; Fernandez, J.; Arroyo, V.; et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology 2015, 62, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Van Bogaert, T.; Vandevyver, S.; Dejager, L.; Van Hauwermeiren, F.; Pinheiro, I.; Petta, I.; Engblom, D.; Kleyman, A.; Schutz, G.; Tuckermann, J.; et al. Tumor necrosis factor inhibits glucocorticoid receptor function in mice: A strong signal toward lethal shock. J. Biol. Chem. 2011, 286, 26555–26567. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.; Banerjee, A.; Ramaiah, S.K. Calpain inhibition attenuates iNOS production and midzonal hepatic necrosis in a repeat dose model of endotoxemia in rats. Toxicol. Pathol. 2006, 34, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Su, L.; Liu, Z. Critical role of calpain in inflammation. Biomed. Rep. 2016, 5, 647–652. [Google Scholar] [CrossRef]
- Flores-Toro, J.; Chun, S.K.; Shin, J.K.; Campbell, J.; Lichtenberger, M.; Chapman, W.; Zendejas, I.; Behrns, K.; Leeuwenburgh, C.; Kim, J.S. Critical Roles of Calpastatin in Ischemia/Reperfusion Injury in Aged Livers. Cells 2021, 10, 1863. [Google Scholar] [CrossRef]
- Mehendale, H.M.; Limaye, P.B. Calpain: A death protein that mediates progression of liver injury. Trends Pharmacol. Sci. 2005, 26, 232–236. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Xu, D.; Deng, W.; Yang, W.; Tang, F.; Da, M. Knockout of calpain-1 protects against high-fat diet-induced liver dysfunction in mouse through inhibiting oxidative stress and inflammation. Food Sci. Nutr. 2021, 9, 367–374. [Google Scholar] [CrossRef]
- Sun, L.N.; Zhou, D.F.; Zhou, J.Y.; Zhao, C.Y.; Zhen, Z. Role of endoplasmic reticulum stress in alcoholic liver disease-related hepatocyte apoptosis. Zhonghua Gan. Zang Bing Za Zhi 2012, 20, 35–39. [Google Scholar]
- Kim, Y.S.; Kim, J.; Kim, Y.; Lee, Y.H.; Kim, J.H.; Lee, S.J.; Shin, S.Y.; Ko, J. The role of calpains in ligand-induced degradation of the glucocorticoid receptor. Biochem. Biophys. Res. Commun. 2008, 374, 373–377. [Google Scholar] [CrossRef]
- Bellocq, A.; Doublier, S.; Suberville, S.; Perez, J.; Escoubet, B.; Fouqueray, B.; Puyol, D.R.; Baud, L. Somatostatin increases glucocorticoid binding and signaling in macrophages by blocking the calpain-specific cleavage of Hsp 90. J. Biol. Chem. 1999, 274, 36891–36896. [Google Scholar] [CrossRef]
- Gordon, R.; Anantharam, V.; Kanthasamy, A.G.; Kanthasamy, A. Proteolytic activation of proapoptotic kinase protein kinase Cdelta by tumor necrosis factor alpha death receptor signaling in dopaminergic neurons during neuroinflammation. J. Neuroinflamm. 2012, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kang, J.H.; Choi, S.Y.; Suk, K.T.; Kim, D.J.; Kwon, O.S. PKCdelta as a regulator for TGFbeta1-induced alpha-SMA production in a murine nonalcoholic steatohepatitis model. PLoS ONE 2013, 8, e55979. [Google Scholar]
- Gong, J.; Park, M.; Steinberg, S.F. Cleavage Alters the Molecular Determinants of Protein Kinase C-delta Catalytic Activity. Mol. Cell Biol. 2017, 37, e00324-17. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Banno, Y.; Nakashima, S.; Yoshimura, S.; Sawada, M.; Nishimura, Y.; Nozawa, Y.; Sakai, N. Crucial role of calpain in hypoxic PC12 cell death: Calpain, but not caspases, mediates degradation of cytoskeletal proteins and protein kinase C-alpha and -delta. Neurol. Res. 2001, 23, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, H.W.; Ozdemir, M.; Yoshihara, T.; Nguyen, B.L.; Deminice, R.; Powers, S.K. Calpains play an essential role in mechanical ventilation-induced diaphragmatic weakness and mitochondrial dysfunction. Redox. Biol. 2021, 38, 101802. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Yeh, J.Y.; Forsberg, N.E.; Ou, B.R. Involvement of mu- and m-calpains and protein kinase C isoforms in L8 myoblast differentiation. Int. J. Biochem. Cell Biol. 2006, 38, 662–670. [Google Scholar] [CrossRef]
- Matassa, A.A.; Carpenter, L.; Biden, T.J.; Humphries, M.J.; Reyland, M.E. PKCdelta is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J. Biol Chem. 2001, 276, 29719–29728. [Google Scholar] [CrossRef]
- Lee, S.U.; Oh, E.S.; Ryu, H.W.; Kim, M.O.; Kang, M.J.; Song, Y.N.; Lee, R.W.; Kim, D.Y.; Ro, H.; Jung, S.; et al. Longifolioside A inhibits TLR4-mediated inflammatory responses by blocking PKCdelta activation in LPS-stimulated THP-1 macrophages. Cytokine 2020, 131, 155116. [Google Scholar] [CrossRef]
- Venugopal, S.K.; Chen, J.; Zhang, Y.; Clemens, D.; Follenzi, A.; Zern, M.A. Role of MAPK phosphatase-1 in sustained activation of JNK during ethanol-induced apoptosis in hepatocyte-like VL-17A cells. J. Biol. Chem. 2007, 282, 31900–31908. [Google Scholar] [CrossRef]
- Yu, D.; Chen, G.; Pan, M.; Zhang, J.; He, W.; Liu, Y.; Nian, X.; Sheng, L.; Xu, B. High fat diet-induced oxidative stress blocks hepatocyte nuclear factor 4alpha and leads to hepatic steatosis in mice. J. Cell. Physiol. 2018, 233, 4770–4782. [Google Scholar] [CrossRef]
- Koh, E.H.; Yoon, J.E.; Ko, M.S.; Leem, J.; Yun, J.Y.; Hong, C.H.; Cho, Y.K.; Lee, S.E.; Jang, J.E.; Baek, J.Y.; et al. Sphingomyelin synthase 1 mediates hepatocyte pyroptosis to trigger non-alcoholic steatohepatitis. Gut 2021, 70, 1954–1964. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, S.J.; Lee, H.S.; Kwon, O.S. PKCdelta Mediates NF-kappaB Inflammatory Response and Downregulates SIRT1 Expression in Liver Fibrosis. Int. J. Mol. Sci. 2019, 20, 4607. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Adachi, M.; Yasui, H.; Takekawa, M.; Tanaka, H.; Imai, K. Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol. Endocrinol. 2002, 16, 2382–2392. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, D.; Wang, X.; Cederbaum, A.I. Cytochrome P4502E1, oxidative stress, JNK, and autophagy in acute alcohol-induced fatty liver. Free Radic. Biol. Med. 2012, 53, 1170–1180. [Google Scholar] [CrossRef]
- Qian, H.; Chao, X.; Williams, J.; Fulte, S.; Li, T.; Yang, L.; Ding, W.X. Autophagy in liver diseases: A review. Mol. Asp. Med. 2021, 82, 100973. [Google Scholar] [CrossRef] [PubMed]
- Ila, R.; Solem, M. Chronic-alcohol exposure alters IGF1 signaling in H9c2 cells via changes in PKC delta. Alcohol 2006, 39, 169–178. [Google Scholar] [CrossRef][Green Version]
- Rao, Y.P.; Stravitz, R.T.; Vlahcevic, Z.R.; Gurley, E.C.; Sando, J.J.; Hylemon, P.B. Activation of protein kinase C alpha and delta by bile acids: Correlation with bile acid structure and diacylglycerol formation. J. Lipid Res. 1997, 38, 2446–2454. [Google Scholar] [CrossRef]
- Argemi, J.; Latasa, M.U.; Atkinson, S.R.; Blokhin, I.O.; Massey, V.; Gue, J.P.; Cabezas, J.; Lozano, J.J.; Van Booven, D.; Bell, A.; et al. Defective HNF4alpha-dependent gene expression as a driver of hepatocellular failure in alcoholic hepatitis. Nat. Commun. 2019, 10, 3126. [Google Scholar]
- Fang, W.; Chen, Q.; Cui, K.; Chen, Q.; Li, X.; Xu, N.; Mai, K.; Ai, Q. Lipid overload impairs hepatic VLDL secretion via oxidative stress-mediated PKCdelta-HNF4alpha-MTP pathway in large yellow croaker (Larimichthys crocea). Free Radic. Biol. Med. 2021, 172, 213–225. [Google Scholar] [CrossRef]
- Suzuki, S.; Iben, J.R.; Coon, S.L.; Kino, T. SIRT1 is a transcriptional enhancer of the glucocorticoid receptor acting independently to its deacetylase activity. Mol. Cell Endocrinol. 2018, 461, 178–187. [Google Scholar] [CrossRef]
- Wang, S.; Wan, T.; Ye, M.; Qiu, Y.; Pei, L.; Jiang, R.; Pang, N.; Huang, Y.; Liang, B.; Ling, W.; et al. Nicotinamide riboside attenuates alcohol induced liver injuries via activation of SirT1/PGC-1alpha/mitochondrial biosynthesis pathway. Redox. Biol. 2018, 17, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, T.; Li, Y.M.; Yin, S.; Xu, M.J.; Feng, D.; Zhou, Z.; Zang, M.; Mukhopadhyay, P.; Varga, Z.V.; Pacher, P.; et al. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J. Hepatol. 2017, 66, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.; Lu, H. Cell-specific regulation of inflammatory cytokines and acute phase proteins by glucocorticoid receptor. SUNY Upstate Med. Univ. Syracuse N. Y. USA 2022. in preparation. [Google Scholar]
- Katakura, Y.; Udono, M.; Katsuki, K.; Nishide, H.; Tabira, Y.; Ikei, T.; Yamashita, M.; Fujiki, T.; Shirahata, S. Protein kinase C delta plays a key role in cellular senescence programs of human normal diploid cells. J. Biochem. 2009, 146, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, K.; Riabowol, K. Protein kinase C delta blocks immediate-early gene expression in senescent cells by inactivating serum response factor. Mol. Cell Biol. 2004, 24, 7298–7311. [Google Scholar] [CrossRef][Green Version]
- Yamashita, S.; Fujii, K.; Zhao, C.; Takagi, H.; Katakura, Y. Involvement of the NFX1-repressor complex in PKC-delta-induced repression of hTERT transcription. J. Biochem. 2016, 160, 309–313. [Google Scholar] [CrossRef]
- Byun, H.O.; Jung, H.J.; Kim, M.J.; Yoon, G. PKCdelta phosphorylation is an upstream event of GSK3 inactivation-mediated ROS generation in TGF-beta1-induced senescence. Free Radic. Res. 2014, 48, 1100–1108. [Google Scholar] [CrossRef]
- Varecza, Z.; Kvell, K.; Talaber, G.; Miskei, G.; Csongei, V.; Bartis, D.; Anderson, G.; Jenkinson, E.J.; Pongracz, J.E. Multiple suppression pathways of canonical Wnt signalling control thymic epithelial senescence. Mech. Ageing Dev. 2011, 132, 249–256. [Google Scholar] [CrossRef]
- Huck, I.; Gunewardena, S.; Espanol-Suner, R.; Willenbring, H.; Apte, U. Hepatocyte Nuclear Factor 4 Alpha Activation Is Essential for Termination of Liver Regeneration in Mice. Hepatology 2019, 70, 666–681. [Google Scholar] [CrossRef]
- Shteyer, E.; Liao, Y.; Muglia, L.J.; Hruz, P.W.; Rudnick, D.A. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology 2004, 40, 1322–1332. [Google Scholar] [CrossRef]
- Bellet, M.M.; Masri, S.; Astarita, G.; Sassone-Corsi, P.; Della Fazia, M.A.; Servillo, G. Histone Deacetylase SIRT1 Controls Proliferation, Circadian Rhythm, and Lipid Metabolism during Liver Regeneration in Mice. J. Biol. Chem. 2016, 291, 23318–23329. [Google Scholar] [CrossRef] [PubMed]
- Mathurin, P.; Duchatelle, V.; Ramond, M.J.; Degott, C.; Bedossa, P.; Erlinger, S.; Benhamou, J.P.; Chaput, J.C.; Rueff, B.; Poynard, T. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology 1996, 110, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef]
- Peiseler, M.; Kubes, P. More friend than foe: The emerging role of neutrophils in tissue repair. J. Clin. Investig. 2019, 129, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Saiman, Y.; Friedman, S.L. The role of chemokines in acute liver injury. Front. Physiol. 2012, 3, 213. [Google Scholar] [CrossRef] [PubMed]
- Proost, P.; De Wolf-Peeters, C.; Conings, R.; Opdenakker, G.; Billiau, A.; Van Damme, J. Identification of a novel granulocyte chemotactic protein (GCP-2) from human tumor cells. In vitro and in vivo comparison with natural forms of GRO, IP-10, and IL-8. J. Immunol. 1993, 150, 1000–1010. [Google Scholar] [PubMed]
- Zhou, Z.; Xu, M.J.; Gao, B. Hepatocytes: A key cell type for innate immunity. Cell Mol. Immunol. 2016, 13, 301–315. [Google Scholar]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Liu, M.; Cao, S.; He, L.; Gao, J.; Arab, J.P.; Cui, H.; Xuan, W.; Gao, Y.; Sehrawat, T.S.; Hamdan, F.H.; et al. Super enhancer regulation of cytokine-induced chemokine production in alcoholic hepatitis. Nat. Commun. 2021, 12, 4560. [Google Scholar] [CrossRef]
- Huang, Y.S.; Chan, C.Y.; Wu, J.C.; Pai, C.H.; Chao, Y.; Lee, S.D. Serum levels of interleukin-8 in alcoholic liver disease: Relationship with disease stage, biochemical parameters and survival. J. Hepatol. 1996, 24, 377–384. [Google Scholar] [CrossRef]
- Hill, D.B.; Marsano, L.S.; McClain, C.J. Increased plasma interleukin-8 concentrations in alcoholic hepatitis. Hepatology 1993, 18, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Maltby, J.; Wright, S.; Bird, G.; Sheron, N. Chemokine levels in human liver homogenates: Associations between GRO alpha and histopathological evidence of alcoholic hepatitis. Hepatology 1996, 24, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.; Miquel, R.; Colmenero, J.; Moreno, M.; Garcia-Pagan, J.C.; Bosch, J.; Arroyo, V.; Gines, P.; Caballeria, J.; Bataller, R. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology 2009, 136, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, Y.; Takada, H.; Hikiba, Y.; Nakata, R.; Okano, K.; Komatsu, Y.; Niwa, Y.; Matsumura, M.; Shiina, S.; Omata, M.; et al. Production of chemotactic factor, interleukin-8, from hepatocytes exposed to ethanol. Hepatology 1993, 18, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, B.; Jiang, H.; Ma, Y.; Bao, Y.; Zhu, X.; Xia, H.; Jin, Y. IL-8 exacerbates alcohol-induced fatty liver disease via the Akt/HIF-1alpha pathway in human IL-8-expressing mice. Cytokine 2021, 138, 155402. [Google Scholar] [CrossRef]
- Taieb, J.; Mathurin, P.; Elbim, C.; Cluzel, P.; Arce-Vicioso, M.; Bernard, B.; Opolon, P.; Gougerot-Pocidalo, M.A.; Poynard, T.; Chollet-Martin, S. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: Effect of corticosteroids. J. Hepatol. 2000, 32, 579–586. [Google Scholar] [CrossRef]
- Hoggatt, J.; Singh, P.; Tate, T.A.; Chou, B.K.; Datari, S.R.; Fukuda, S.; Liu, L.; Kharchenko, P.V.; Schajnovitz, A.; Baryawno, N.; et al. Rapid Mobilization Reveals a Highly Engraftable Hematopoietic Stem Cell. Cell 2018, 172, 191–204.e10. [Google Scholar] [CrossRef]
- Fukuda, S.; Bian, H.; King, A.G.; Pelus, L.M. The chemokine GRObeta mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftment. Blood 2007, 110, 860–869. [Google Scholar] [CrossRef][Green Version]
- Ren, X.; Carpenter, A.; Hogaboam, C.; Colletti, L. Mitogenic properties of endogenous and pharmacological doses of macrophage inflammatory protein-2 after 70% hepatectomy in the mouse. Am. J. Pathol. 2003, 163, 563–570. [Google Scholar] [CrossRef]
- Hogaboam, C.M.; Simpson, K.J.; Chensue, S.W.; Steinhauser, M.L.; Lukacs, N.W.; Gauldie, J.; Strieter, R.M.; Kunkel, S.L. Macrophage inflammatory protein-2 gene therapy attenuates adenovirus- and acetaminophen-mediated hepatic injury. Gene Ther. 1999, 6, 573–584. [Google Scholar] [CrossRef]
- Kuboki, S.; Shin, T.; Huber, N.; Eismann, T.; Galloway, E.; Schuster, R.; Blanchard, J.; Edwards, M.J.; Lentsch, A.B. Hepatocyte signaling through CXC chemokine receptor-2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology 2008, 48, 1213–1223. [Google Scholar] [PubMed]
- Pelus, L.M.; Fukuda, S. Peripheral blood stem cell mobilization: The CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. Exp. Hematol. 2006, 34, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Keisham, A.; Bhalla, A.; Sharma, N.; Agarwal, R.; Sharma, R.; Singh, A. Efficacy of Granulocyte Colony-Stimulating Factor and N-Acetylcysteine Therapies in Patients With Severe Alcoholic Hepatitis. Clin. Gastroenterol. Hepatol. 2018, 16, 1650–1656.e2. [Google Scholar] [CrossRef] [PubMed]
- Marot, A.; Singal, A.K.; Moreno, C.; Deltenre, P. Granulocyte colony-stimulating factor for alcoholic hepatitis: A systematic review and meta-analysis of randomised controlled trials. JHEP Rep. 2020, 2, 100139. [Google Scholar] [CrossRef]
- Adrover, J.M.; Del Fresno, C.; Crainiciuc, G.; Cuartero, M.I.; Casanova-Acebes, M.; Weiss, L.A.; Huerga-Encabo, H.; Silvestre-Roig, C.; Rossaint, J.; Cossio, I.; et al. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity 2019, 50, 390–402.e10. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; Aroca-Crevillen, A.; Crainiciuc, G.; Ostos, F.; Rojas-Vega, Y.; Rubio-Ponce, A.; Cilloniz, C.; Bonzon-Kulichenko, E.; Calvo, E.; Rico, D.; et al. Programmed ‘disarming’ of the neutrophil proteome reduces the magnitude of inflammation. Nat. Immunol. 2020, 21, 135–144. [Google Scholar] [CrossRef]
- Flores, C.; Maca-Meyer, N.; Perez-Mendez, L.; Sanguesa, R.; Espinosa, E.; Muriel, A.; Blanco, J.; Villar, J.; GRECIA group; GEN-SEP group. A CXCL2 tandem repeat promoter polymorphism is associated with susceptibility to severe sepsis in the Spanish population. Genes Immun. 2006, 7, 141–149. [Google Scholar] [CrossRef]
- Niu, B.; Kim, B.; Limketkai, B.N.; Sun, J.; Li, Z.; Woreta, T.; Chen, P.H. Mortality from Spontaneous Bacterial Peritonitis Among Hospitalized Patients in the USA. Dig. Dis. Sci. 2018, 63, 1327–1333. [Google Scholar] [CrossRef]
- Lee, D.U.; Fan, G.H.; Hastie, D.J.; Addonizio, E.A.; Prakasam, V.N.; Ahern, R.R.; Seog, K.J.; Karagozian, R. The Impact of Malnutrition on the Hospital and Infectious Outcomes of Patients Admitted With Alcoholic Hepatitis: 2011 to 2017 Analysis of US Hospitals. J. Clin. Gastroenterol. 2021, 56, 349–359. [Google Scholar] [CrossRef]
- Craciun, F.L.; Schuller, E.R.; Remick, D.G. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J. Immunol. 2010, 185, 6930–6938. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Bukong, T.N.; Cho, Y.; Iracheta-Vellve, A.; Saha, B.; Lowe, P.; Adejumo, A.; Furi, I.; Ambade, A.; Gyongyosi, B.; Catalano, D.; et al. Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J. Hepatol. 2018, 69, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, F.S.; Xu, R. Neutrophils in liver diseases: Pathogenesis and therapeutic targets. Cell Mol. Immunol. 2021, 18, 38–44. [Google Scholar] [CrossRef]
- Tranah, T.H.; Vijay, G.K.M.; Ryan, J.M.; Abeles, R.D.; Middleton, P.K.; Shawcross, D.L. Dysfunctional neutrophil effector organelle mobilization and microbicidal protein release in alcohol-related cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G203–G211. [Google Scholar] [CrossRef]
- Dalakas, E.; Newsome, P.N.; Boyle, S.; Brown, R.; Pryde, A.; McCall, S.; Hayes, P.C.; Bickmore, W.A.; Harrison, D.J.; Plevris, J.N. Bone marrow stem cells contribute to alcohol liver fibrosis in humans. Stem Cells Dev. 2010, 19, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Q.; Liu, W.; Liu, F.; Ji, A.; Li, Y. The Orphan Nuclear Receptor 4A1: A Potential New Therapeutic Target for Metabolic Diseases. J. Diabetes Res. 2018, 2018, 9363461. [Google Scholar] [CrossRef]
- Martens, C.; Bilodeau, S.; Maira, M.; Gauthier, Y.; Drouin, J. Protein-protein interactions and transcriptional antagonism between the subfamily of NGFI-B/Nur77 orphan nuclear receptors and glucocorticoid receptor. Mol. Endocrinol. 2005, 19, 885–897. [Google Scholar]
- Carpentier, R.; Sacchetti, P.; Segard, P.; Staels, B.; Lefebvre, P. The glucocorticoid receptor is a co-regulator of the orphan nuclear receptor Nurr1. J. Neurochem. 2008, 104, 777–789. [Google Scholar] [CrossRef]
- Deng, J.W.; Yang, Q.; Cai, X.P.; Zhou, J.M.; An, Y.D.; Zheng, Q.X.; Hong, M.; Ren, Y.L.; Guan, J.; Wang, G.; et al. Early use of dexamethasone increases Nr4a1 in Kupffer cells ameliorating acute liver failure in mice in a glucocorticoid receptor-dependent manner. J. Zhejiang Univ. Sci. B 2020, 21, 727–739. [Google Scholar] [CrossRef]
- Jimenez, C.; Ventura-Cots, M.; Sala, M.; Calafat, M.; Garcia-Retortillo, M.; Cirera, I.; Canete, N.; Soriano, G.; Poca, M.; Simon-Talero, M.; et al. Effect of rifaximin on infections, acute-on-chronic liver failure and mortality in alcoholic hepatitis: A pilot study (RIFA-AH). Liver Int. 2022, 42, 1109–1120. [Google Scholar] [CrossRef]
- Aoun, E.G.; Jimenez, V.A.; Vendruscolo, L.F.; Walter, N.A.R.; Barbier, E.; Ferrulli, A.; Haass-Koffler, C.L.; Darakjian, P.; Lee, M.R.; Addolorato, G.; et al. A relationship between the aldosterone-mineralocorticoid receptor pathway and alcohol drinking: Preliminary translational findings across rats, monkeys and humans. Mol. Psychiatry 2018, 23, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Makhijani, V.H.; Irukulapati, P.; Van Voorhies, K.; Fortino, B.; Besheer, J. Central amygdala mineralocorticoid receptors modulate alcohol self-administration. Neuropharmacology 2020, 181, 108337. [Google Scholar] [PubMed]
- Choi, D.S.; Wei, W.; Deitchman, J.K.; Kharazia, V.N.; Lesscher, H.M.; McMahon, T.; Wang, D.; Qi, Z.H.; Sieghart, W.; Zhang, C.; et al. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J. Neurosci. 2008, 28, 11890–11899. [Google Scholar] [CrossRef] [PubMed]
- Gerstin, E.H., Jr.; McMahon, T.; Dadgar, J.; Messing, R.O. Protein kinase Cdelta mediates ethanol-induced up-regulation of L-type calcium channels. J. Biol. Chem. 1998, 273, 16409–16414. [Google Scholar] [CrossRef]
- Domi, E.; Xu, L.; Toivainen, S.; Nordeman, A.; Gobbo, F.; Venniro, M.; Shaham, Y.; Messing, R.O.; Visser, E.; van den Oever, M.C.; et al. A neural substrate of compulsive alcohol use. Sci. Adv. 2021, 7, eabg9045. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Davel, A.P.; McGraw, A.P.; Rao, S.P.; Newfell, B.G.; Jaffe, I.Z. PKCdelta Mediates Mineralocorticoid Receptor Activation by Angiotensin II to Modulate Smooth Muscle Cell Function. Endocrinology 2019, 160, 2101–2114. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H. Narrative Review: Glucocorticoids in Alcoholic Hepatitis—Benefits, Side Effects, and Mechanisms. J. Xenobiot. 2022, 12, 266-288. https://doi.org/10.3390/jox12040019
Lu H. Narrative Review: Glucocorticoids in Alcoholic Hepatitis—Benefits, Side Effects, and Mechanisms. Journal of Xenobiotics. 2022; 12(4):266-288. https://doi.org/10.3390/jox12040019
Chicago/Turabian StyleLu, Hong. 2022. "Narrative Review: Glucocorticoids in Alcoholic Hepatitis—Benefits, Side Effects, and Mechanisms" Journal of Xenobiotics 12, no. 4: 266-288. https://doi.org/10.3390/jox12040019
APA StyleLu, H. (2022). Narrative Review: Glucocorticoids in Alcoholic Hepatitis—Benefits, Side Effects, and Mechanisms. Journal of Xenobiotics, 12(4), 266-288. https://doi.org/10.3390/jox12040019





