Phenolic Compounds of Therapeutic Interest in Neuroprotection
Abstract
1. Introduction
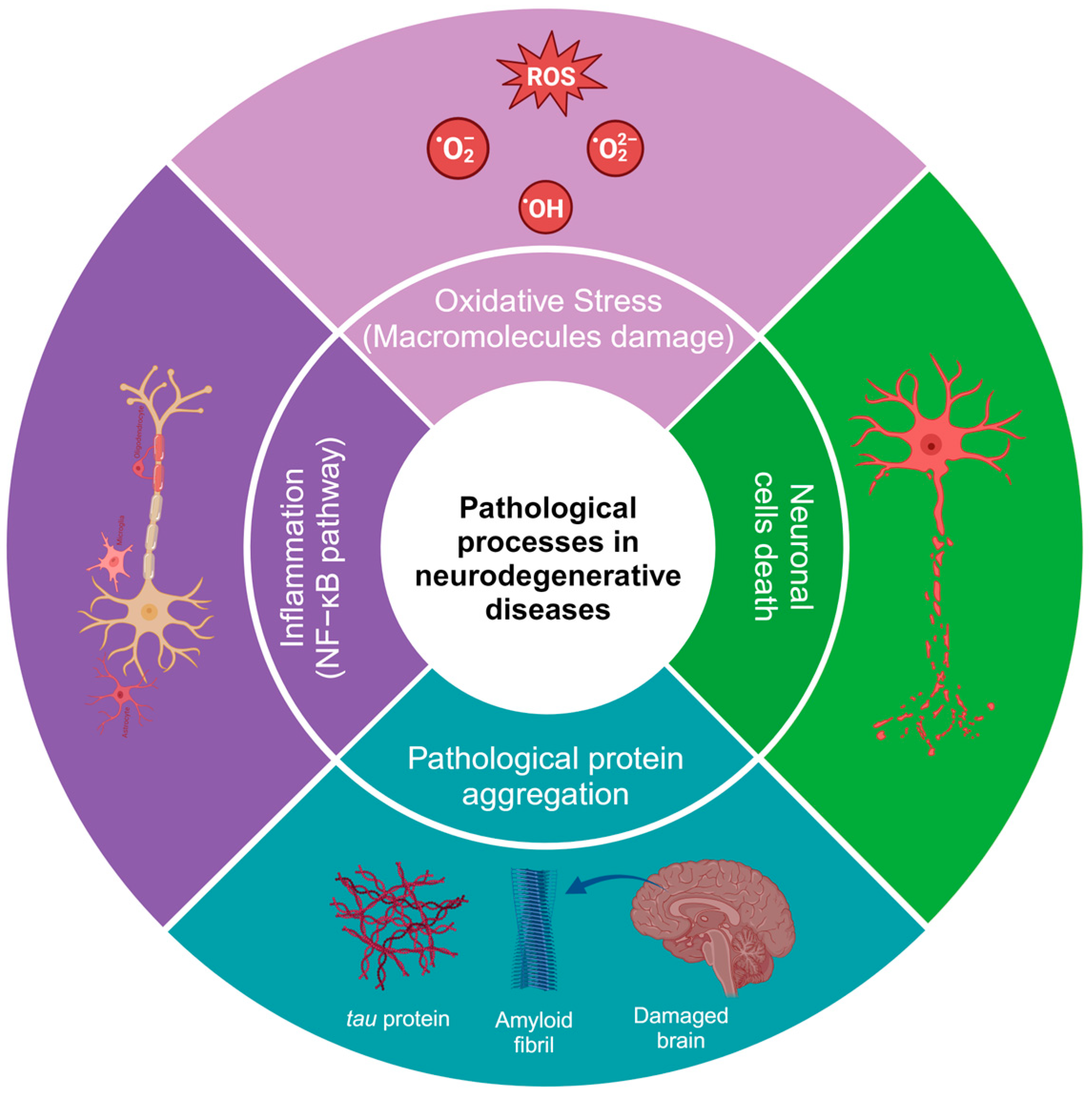
2. Physiopathology of Neurodegenerative Diseases
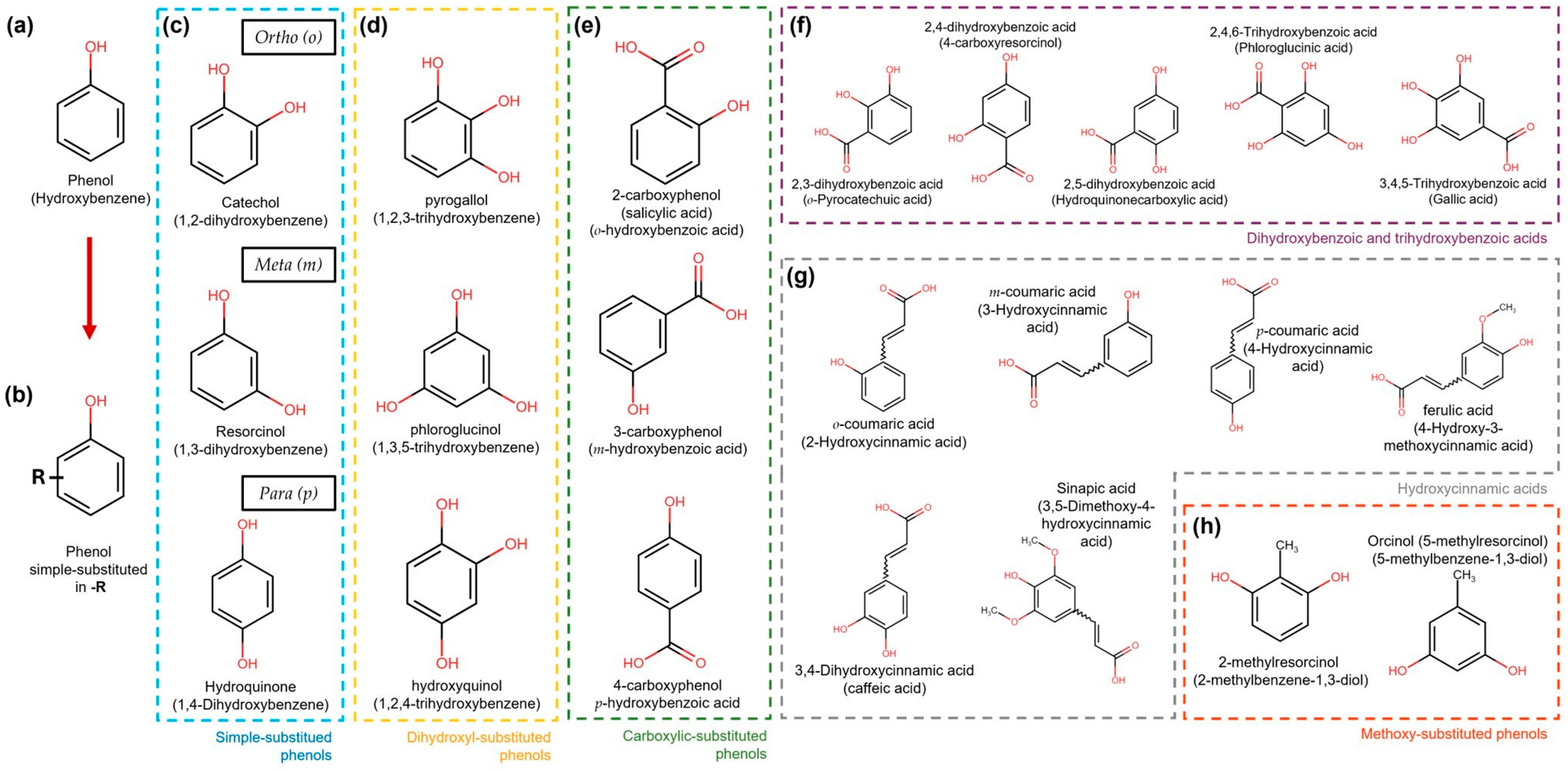
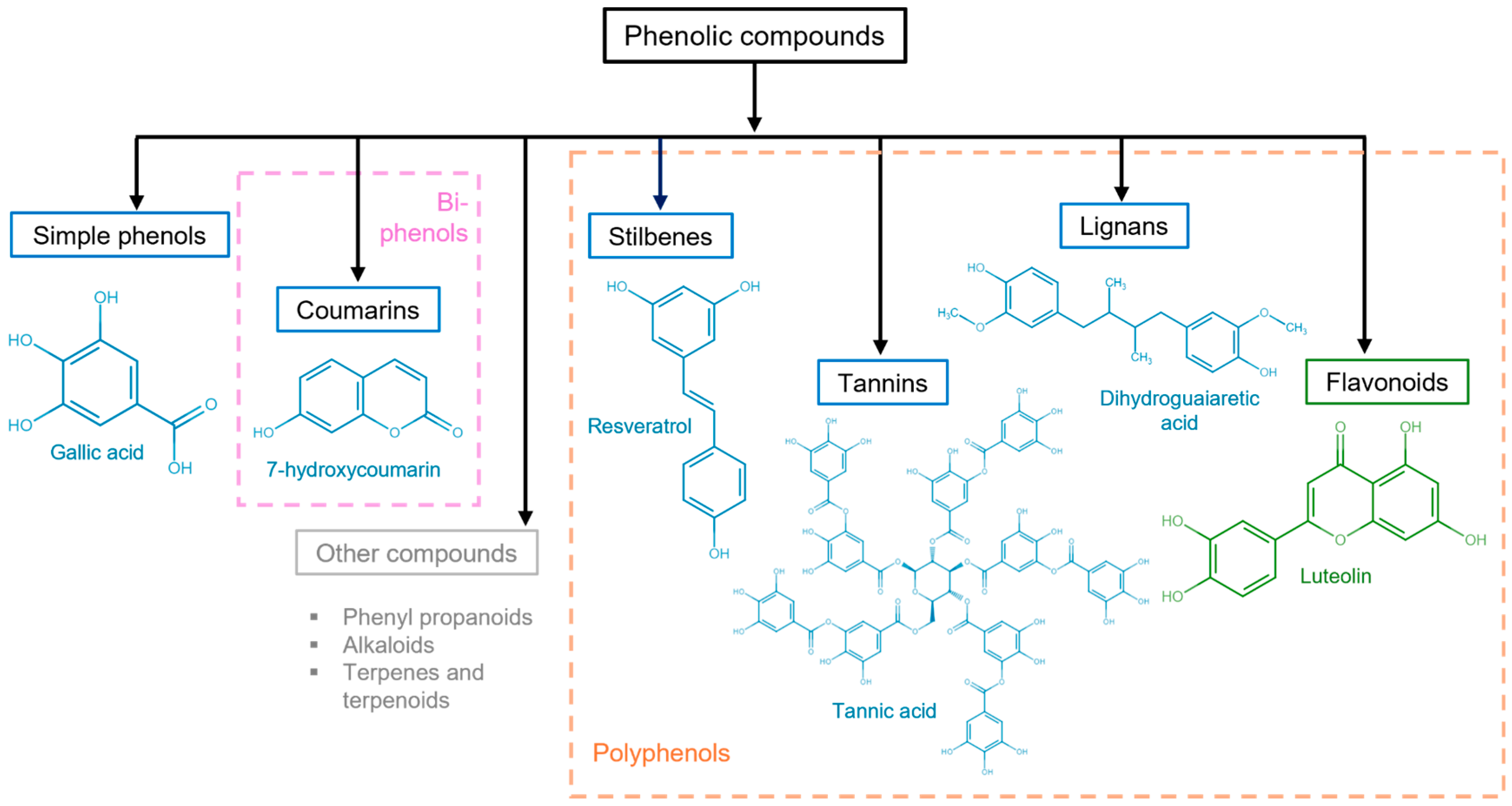
3. General Aspects of Phenolic Compounds
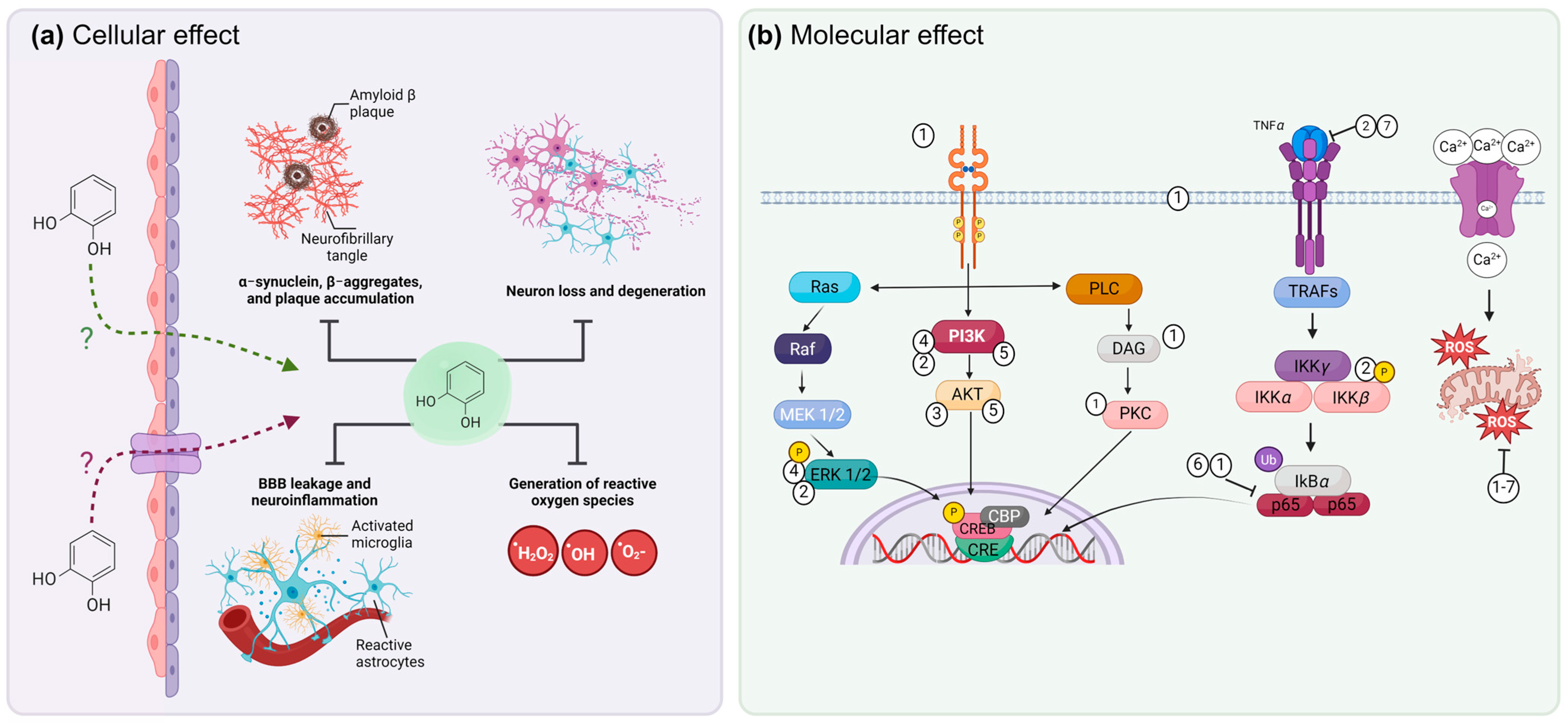
4. Molecular Mechanisms of Phenols to Prevent Neurodegeneration
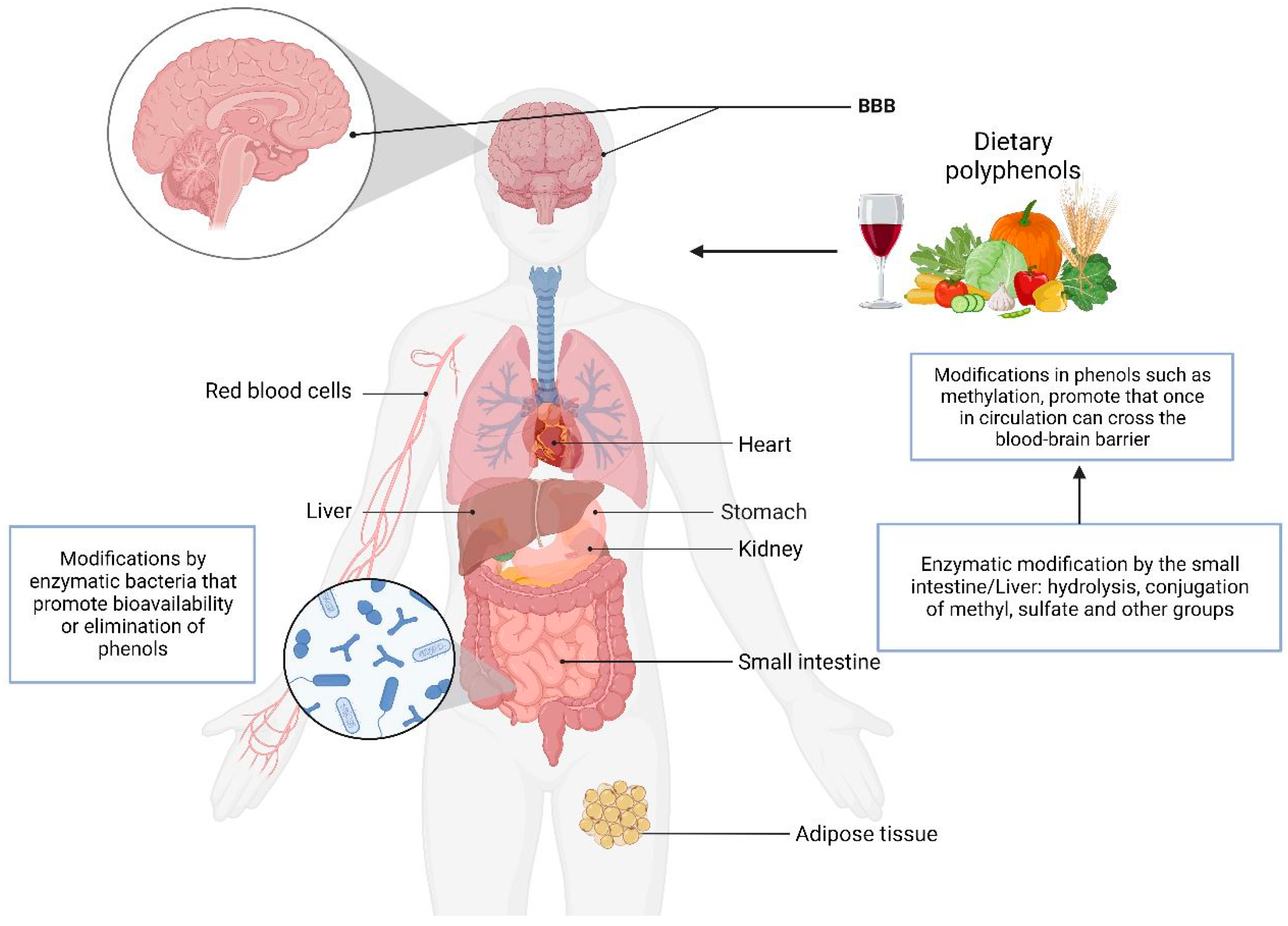
5. Metabolism of Phenol Compounds
6. Effect of Phenolic Compounds In Vitro in Models of Neurodegeneration
7. Effect of Phenolic Compounds in Models of Neurodegeneration In Vivo
8. Effect of Phenolic Compounds on Neurodegeneration in Clinical Trials
9. Mechanisms of Damage Modulation by Phenolic Compounds
10. Conclusions and Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wyss-Coray, T. Ageing, Neurodegeneration and Brain Rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Meng, L.; Zhang, Z. What Is Strain in Neurodegenerative Diseases? Cell. Mol. Life Sci. 2020, 77, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Katz Sand, I. Classification, Diagnosis, and Differential Diagnosis of Multiple Sclerosis. Curr. Opin. Neurol. 2015, 28, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Ferini-Strambi, L.; Salsone, M. COVID-19 and Neurological Disorders: Are Neurodegenerative or Neuroimmunological Diseases More Vulnerable? J. Neurol. 2021, 268, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; McLean, A.S. The Role of Mitochondria in the Immune Response in Critical Illness. Crit. Care 2022, 26, 80. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Phenolic Compounds, Antioxidant Activity and in Vitro Inhibitory Potential against Key Enzymes Relevant for Hyperglycemia and Hypertension of Commonly Used Medicinal Plants, Herbs and Spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Gomes, E.; Barbosa, M.; Bernardo, J.; Valentão, P. Camel Grass Phenolic Compounds: Targeting Inflammation and Neurologically Related Conditions. Molecules 2022, 27, 7707. [Google Scholar] [CrossRef]
- Lee, B.K.; Hyun, S.-W.; Jung, Y.-S. Yuzu Hesperidin Ameliorate Blood-Brain Barrier Disruption during Hypoxia via Antioxidant Activity. Antioxidants 2020, 9, 843. [Google Scholar] [CrossRef]
- Roda, A.R.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-Beta Peptide and Tau Protein Crosstalk in Alzheimer’s Disease. Neural Regen. Res. 2022, 17, 1666–1674. [Google Scholar] [CrossRef]
- Ribeiro, F.M.; Vieira, L.B.; Pires, R.G.W.; Olmo, R.P.; Ferguson, S.S.G. Metabotropic Glutamate Receptors and Neurodegenerative Diseases. Pharmacol. Res. 2017, 115, 179–191. [Google Scholar] [CrossRef]
- Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front. Mol. Neurosci. 2019, 12, 20. [Google Scholar] [CrossRef]
- Jakaria, M.; Park, S.-Y.; Haque, M.E.; Karthivashan, G.; Kim, I.-S.; Ganesan, P.; Choi, D.-K. Neurotoxic Agent-Induced Injury in Neurodegenerative Disease Model: Focus on Involvement of Glutamate Receptors. Front. Mol. Neurosci. 2018, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Park, J.Y.; Kang, K.S.; Hwang, G.S. Neuroprotective Effect of Gallocatechin Gallate on Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells. Molecules 2021, 26, 1387. [Google Scholar] [CrossRef] [PubMed]
- Atlante, A.; Calissano, P.; Bobba, A.; Giannattasio, S.; Marra, E.; Passarella, S. Glutamate Neurotoxicity, Oxidative Stress and Mitochondria. FEBS Lett. 2001, 497, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.-I.; Jou, M.-J. Oxidative Stress Caused by Mitochondrial Calcium Overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chowdhury, N.S. Novel Anti-Amyloid-Beta (Aβ) Monoclonal Antibody Lecanemab for Alzheimer’s Disease: A Systematic Review. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320231209839. [Google Scholar] [CrossRef]
- Fox, J.H.; Connor, T.; Stiles, M.; Kama, J.; Lu, Z.; Dorsey, K.; Lieberman, G.; Sapp, E.; Cherny, R.A.; Banks, M.; et al. Cysteine Oxidation within N-Terminal Mutant Huntingtin Promotes Oligomerization and Delays Clearance of Soluble Protein. J. Biol. Chem. 2011, 286, 18320–18330. [Google Scholar] [CrossRef] [PubMed]
- Bartzokis, G.; Lu, P.H.; Tishler, T.A.; Fong, S.M.; Oluwadara, B.; Finn, J.P.; Huang, D.; Bordelon, Y.; Mintz, J.; Perlman, S. Myelin Breakdown and Iron Changes in Huntington’s Disease: Pathogenesis and Treatment Implications. Neurochem. Res. 2007, 32, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Yamout, B.I.; Alroughani, R. Multiple Sclerosis. Semin. Neurol. 2018, 38, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS Neurodegenerative Diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Foss, K.; Przybyłowicz, K.E.; Sawicki, T. Antioxidant Activity and Profile of Phenolic Compounds in Selected Herbal Plants. Plant Foods Hum. Nutr. 2022, 77, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar]
- Mamari, H.H. Al Phenolic Compounds: Classification, Chemistry, and Updated Techniques of Analysis and Synthesis. In Phenolic Compounds; Badria, F.A., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods. In Phenolic Compounds; Soto-Hernandez, M., Palma-Tenango, M., del Rosario Garcia-Mateos, M., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. ISBN 978-0-12-814774-0. [Google Scholar]
- Sharma, C.; Suhalka, P.; Bhatnagar, M. Curcumin and Resveratrol Rescue Cortical-Hippocampal System from Chronic Fluoride-Induced Neurodegeneration and Enhance Memory Retrieval. Int. J. Neurosci. 2018, 128, 1007–1021. [Google Scholar] [CrossRef]
- Rahimi, M.; Kordrostami, M.; Mohamadhasani, F.; Chaeikar, S.S. Antioxidant Gene Expression Analysis and Evaluation of Total Phenol Content and Oxygen-Scavenging System in Tea Accessions under Normal and Drought Stress Conditions. BMC Plant Biol. 2021, 21, 494. [Google Scholar] [CrossRef]
- Bopardikar, M.; Koti Ainavarapu, S.R.; Hosur, R.V. Pyrogallol, Corilagin and Chebulagic Acid Target the “Fuzzy Coat” of Alpha-Synuclein to Inhibit the Fibrillization of the Protein. RSC Adv. 2022, 12, 35770–35777. [Google Scholar] [CrossRef]
- Garcia-Moreno, J.C.; de la Riva, M.P.; Martínez-Lara, E.; Siles, E.; Cañuelo, A. Tyrosol, a Simple Phenol from EVOO, Targets Multiple Pathogenic Mechanisms of Neurodegeneration in a C. Elegans Model of Parkinson’s Disease. Neurobiol. Aging 2019, 82, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lu, J.; Yang, T.; Chen, C.; Bao, Y.; Jiang, L.; Wei, H.; Wu, X.; Zhao, L.; He, S.; et al. Phloroglucinol, a Clinical-Used Antispasmodic, Inhibits Amyloid Aggregation and Degrades the Pre-Formed Amyloid Proteins. Int. J. Biol. Macromol. 2022, 213, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi Akbar Boojar, M. An Overview of the Cellular Mechanisms of Flavonoids Radioprotective Effects. Adv. Pharm. Bull. 2020, 10, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C.E. Chapter 3—Phenolic Compounds: A Good Choice Against Chronic Degenerative Diseases. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 79–108. [Google Scholar]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Rabadi, G.J.; Tranchant, C.C.; Almajwal, A.; Kubow, S.; Alli, I. Occurrence, Types, Properties, and Interactions of Phenolic Compounds with Other Food Constituents in Oil-Bearing Plants. Crit. Rev. Food Sci. Nutr. 2018, 58, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Hidalgo, F.J. The Triple Defensive Barrier of Phenolic Compounds against the Lipid Oxidation-Induced Damage in Food Products. Trends Food Sci. Technol. 2016, 54, 165–174. [Google Scholar] [CrossRef]
- Li, Y.; Jongberg, S.; Andersen, M.L.; Davies, M.J.; Lund, M.N. Quinone-Induced Protein Modifications: Kinetic Preference for Reaction of 1,2-Benzoquinones with Thiol Groups in Proteins. Free Radic. Biol. Med. 2016, 97, 148–157. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, N.E.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic-Protein Interactions: Effects on Food Properties and Health Benefits. J. Med. Food 2018, 21, 188–198. [Google Scholar] [CrossRef]
- Borges, G.; Lean, M.E.J.; Roberts, S.A.; Crozier, A. Bioavailability of Dietary (Poly)Phenols: A Study with Ileostomists to Discriminate between Absorption in Small and Large Intestine. Food Funct. 2013, 4, 754–762. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, Diet and Stress as Modulators of Gut Microbiota: Implications for Neurodegenerative Diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, A. Blood-Brain Barrier Overview: Structural and Functional Correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols Journey through Blood-Brain Barrier towards Neuronal Protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid Permeability across an in Situ Model of the Blood-Brain Barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Chaturvedi, M.; Mishra, S.; Kumar, P.; Somvanshi, P.; Chaturvedi, R. Reductive Metabolites of Curcumin and Their Therapeutic Effects. Heliyon 2020, 6, e05469. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral Bioavailability of Curcumin: Problems and Advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.; Zhang, L.; Wang, L.; Peng, Z.; Chen, Y. The Pharmacokinetics and Tissue Distribution of Curcumin and Its Metabolites in Mice. Biomed. Chromatogr. 2018, 32, e4267. [Google Scholar] [CrossRef]
- Hosannas, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the Curcumin Metabolic Pathway Involving a Unique Enzyme in an Intestinal Microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef]
- Szymusiak, M.; Hu, X.; Leon Plata, P.A.; Ciupinski, P.; Wang, Z.J.; Liu, Y. Bioavailability of Curcumin and Curcumin Glucuronide in the Central Nervous System of Mice after Oral Delivery of Nano-Curcumin. Int. J. Pharm. 2016, 511, 415–423. [Google Scholar] [CrossRef]
- Makhouri, F.R.; Ghasemi, J.B. In Silico Studies in Drug Research Against Neurodegenerative Diseases. Curr. Neuropharmacol. 2018, 16, 664–725. [Google Scholar] [CrossRef]
- Gioia, D.; Bertazzo, M.; Recanatini, M.; Masetti, M.; Cavalli, A. Dynamic Docking: A Paradigm Shift in Computational Drug Discovery. Molecules 2017, 22, 2029. [Google Scholar] [CrossRef] [PubMed]
- Venko, K.; Novič, M. An In Silico Approach for Assessment of the Membrane Transporter Activities of Phenols: A Case Study Based on Computational Models of Transport Activity for the Transporter Bilitranslocase. Molecules 2019, 24, 837. [Google Scholar] [CrossRef] [PubMed]
- Ezaouine, A.; Salam, M.R.; Nouadi, B.; Anachad, O.; El Messal, M.; Chegdani, F.; Bennis, F. In Silico Prediction of the Bioactive Profile and Metabolites of Satureja Nepeta in Diseases Related to the Excessive Production of Interleukin-6. Bioinform. Biol. Insights 2022, 16, 11779322221115664. [Google Scholar] [CrossRef]
- Mandel, S.; Grünblatt, E.; Youdim, M. CDNA Microarray to Study Gene Expression of Dopaminergic Neurodegeneration and Neuroprotection in MPTP and 6-Hydroxydopamine Models: Implications for Idiopathic Parkinson’s Disease. J. Neural Transm. Suppl. 2000, 60, 117–124. [Google Scholar] [CrossRef]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Descat, A.; Drittij-Reijnders, M.-J.; Weseler, A.R.; Bast, A.; Stahl, W.; Heyman, E.; Meeusen, R. Acute Cocoa Flavanols Intake Has Minimal Effects on Exercise-Induced Oxidative Stress and Nitric Oxide Production in Healthy Cyclists: A Randomized Controlled Trial. J. Int. Soc. Sports Nutr. 2017, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; DaSilva, N.A.; Liu, W.; Nahar, P.P.; Wei, Z.; Liu, Y.; Pham, P.T.; Crews, R.; Vattem, D.A.; Slitt, A.L.; et al. Effects of a Standardized Phenolic-Enriched Maple Syrup Extract on β-Amyloid Aggregation, Neuroinflammation in Microglial and Neuronal Cells, and β-Amyloid Induced Neurotoxicity in Caenorhabditis Elegans. Neurochem. Res. 2016, 41, 2836–2847. [Google Scholar] [CrossRef] [PubMed]
- Carecho, R.; Figueira, I.; Terrasso, A.P.; Godinho-Pereira, J.; de Oliveira Sequeira, C.; Pereira, S.A.; Milenkovic, D.; Leist, M.; Brito, C.; Dos Santos, C.N. Circulating (Poly)Phenol Metabolites: Neuroprotection in a 3D Cell Model of Parkinson’s Disease. Mol. Nutr. Food Res. 2021, 66, e2100959. [Google Scholar] [CrossRef]
- Campos-Esparza, M.R.; Sánchez-Gómez, M.V.; Matute, C. Molecular Mechanisms of Neuroprotection by Two Natural Antioxidant Polyphenols. Cell Calcium 2009, 45, 358–368. [Google Scholar] [CrossRef]
- Ibarretxe, G.; Sánchez-Gómez, M.V.; Campos-Esparza, M.R.; Alberdi, E.; Matute, C. Differential Oxidative Stress in Oligodendrocytes and Neurons after Excitotoxic Insults and Protection by Natural Polyphenols. Glia 2006, 53, 201–211. [Google Scholar] [CrossRef]
- Svajger, U.; Obermajer, N.; Jeras, M. Dendritic Cells Treated with Resveratrol during Differentiation from Monocytes Gain Substantial Tolerogenic Properties upon Activation. Immunology 2010, 129, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin Inhibits LPS-Induced Neuroinflammation by Promoting Microglial M2 Polarization via TREM2/TLR4/NF-ΚB Pathways in BV2 Cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Park, S.-H.; Park, S.J.; Oh, S.W.; Yoo, J.A.; Kwon, K.; Kim, J.; Yu, E.; Cho, J.Y.; Lee, J. P44/42 MAPK Signaling Is a Prime Target Activated by Phenylethyl Resorcinol in Its Anti-Melanogenic Action. Phytomedicine 2019, 58, 152877. [Google Scholar] [CrossRef] [PubMed]
- López, P.; Ferraro, G.; Anesini, C. Comparative Antioxidant Activity of an Extract of Lithraea molleoides and an Isolated 5-Alkyl Resorcinol Derivative. Effects on the Proliferation of Normal and Tumoral Lymphocytes. Phytother. Res. 2011, 25, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-C.; Chang, H.-H.; Chan, C.-P.; Chou, H.-Y.; Chang, B.-E.; Yeung, S.-Y.; Wang, T.-M.; Jeng, J.-H. Antiplatelet Effect of Phloroglucinol Is Related to Inhibition of Cyclooxygenase, Reactive Oxygen Species, ERK/P38 Signaling and Thromboxane A2 Production. Toxicol. Appl. Pharmacol. 2012, 263, 287–295. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-Q.; Zhang, W.-T.; Shi, C.-H.; Wang, F.-M.; Tian, X.-J.; Ma, L.-L. Phloroglucinol Protects the Urinary Bladder via Inhibition of Oxidative Stress and Inflammation in a Rat Model of Cyclophosphamide-Induced Interstitial Cystitis. Chin. Med. J. 2015, 128, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Arai, S.; Iwamoto, T.; Takasaki, M.; Tomoda, A. Metabolism of Pyrogallol to Purpurogallin by Human Erythrocytic Hemoglobin. Tohoku J. Exp. Med. 2004, 203, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Kim, S.Z.; Kim, S.H.; Park, W.H. Apoptosis in Pyrogallol-Treated Calu-6 Cells Is Correlated with the Changes of Intracellular GSH Levels Rather than ROS Levels. Lung Cancer 2008, 59, 301–314. [Google Scholar] [CrossRef]
- Johnson, J.; Mercado-Ayon, E.; Mercado-Ayon, Y.; Dong, Y.N.; Halawani, S.; Ngaba, L.; Lynch, D.R. Mitochondrial Dysfunction in the Development and Progression of Neurodegenerative Diseases. Arch. Biochem. Biophys. 2021, 702, 108698. [Google Scholar] [CrossRef]
- Duong, T.T.H.; Chami, B.; McMahon, A.C.; Fong, G.M.; Dennis, J.M.; Freedman, S.B.; Witting, P.K. Pre-Treatment with the Synthetic Antioxidant T-Butyl Bisphenol Protects Cerebral Tissues from Experimental Ischemia Reperfusion Injury. J. Neurochem. 2014, 130, 733–747. [Google Scholar] [CrossRef]
- Devi, L.; Ohno, M. 7,8-Dihydroxyflavone, a Small-Molecule TrkB Agonist, Reverses Memory Deficits and BACE1 Elevation in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2012, 37, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Do, J.M.; Yoo, J.-N.; Lee, H.W.; Kim, N.K.; Yoo, H.-S.; Gee, M.S.; Kim, J.-H.; Seong, J.H.; Inn, K.-S.; et al. Identification of Ortho Catechol-Containing Isoflavone as a Privileged Scaffold That Directly Prevents the Aggregation of Both Amyloid β Plaques and Tau-Mediated Neurofibrillary Tangles and Its In Vivo Evaluation. Bioorg. Chem. 2021, 113, 105022. [Google Scholar] [CrossRef] [PubMed]
- Chesworth, R.; Gamage, R.; Ullah, F.; Sonego, S.; Millington, C.; Fernandez, A.; Liang, H.; Karl, T.; Münch, G.; Niedermayer, G.; et al. Spatial Memory and Microglia Activation in a Mouse Model of Chronic Neuroinflammation and the Anti-Inflammatory Effects of Apigenin. Front. Neurosci. 2021, 15, 699329. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Selvaraj, K.; Khare, S.K.; Chaudhary, N. Superoxide Dismutase as Multipotent Therapeutic Antioxidant Enzyme: Role in Human Diseases. Biotechnol. Lett. 2021, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. Antioxidant Properties of Phenols. J. Pharm. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.M.; Chan, P.H. Role of Superoxide Dismutases in Oxidative Damage and Neurodegenerative Disorders. Neuroscientist 2002, 8, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Botanical Phenolics and Brain Health. Neuromol. Med. 2008, 10, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Rice-Evans, C. Polyphenolic Flavanols as Scavengers of Aqueous Phase Radicals and as Chain-Breaking Antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef]
- Mandel, S.; Youdim, M.B.H. Catechin Polyphenols: Neurodegeneration and Neuroprotection in Neurodegenerative Diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef]
- Rice-Evans, C. Implications of the Mechanisms of Action of Tea Polyphenols as Antioxidants In Vitro for Chemoprevention in Humans. Proc. Soc. Exp. Biol. Med. 1999, 220, 262–266. [Google Scholar] [CrossRef]
- Chico, L.; Ienco, E.C.; Bisordi, C.; Lo Gerfo, A.; Petrozzi, L.; Petrucci, A.; Mancuso, M.; Siciliano, G. Amyotrophic Lateral Sclerosis and Oxidative Stress: A Double-Blind Therapeutic Trial after Curcumin Supplementation. CNS Neurol. Disord. Drug Targets 2018, 17, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Dangles, O. Flavonoid-Serum Albumin Complexation: Determination of Binding Constants and Binding Sites by Fluorescence Spectroscopy. Biochim. Biophys. Acta 2005, 1721, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Kitson, T.M. Spectrophotometric and Kinetic Studies on the Binding of the Bioflavonoid Quercetin to Bovine Serum Albumin. Biosci. Biotechnol. Biochem. 2004, 68, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Fezeu, L.; Andreeva, V.A.; Touvier, M.; Scalbert, A.; Hercberg, S.; Galan, P. Total and Specific Polyphenol Intakes in Midlife Are Associated with Cognitive Function Measured 13 Years Later. J. Nutr. 2012, 142, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.F. Intake of Flavonoids and Risk of Dementia. Eur. J. Epidemiol. 2000, 16, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, D.; Wu, H.; Jia, H.; Yang, C.; Zhang, L. Fisetin Prolongs Therapy Window of Brain Ischemic Stroke Using Tissue Plasminogen Activator: A Double-Blind Randomized Placebo-Controlled Clinical Trial. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619871359. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s Disease: A Target for Neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol Regulates Neuro-Inflammation and Induces Adaptive Immunity in Alzheimer’s Disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Widera, D.; Kaltschmidt, C. Signaling via NF-KappaB in the Nervous System. Biochim. Biophys. Acta 2005, 1745, 287–299. [Google Scholar] [CrossRef]
- Hong Byun, E.; Fujimura, Y.; Yamada, K.; Tachibana, H. TLR4 Signaling Inhibitory Pathway Induced by Green Tea Polyphenol Epigallocatechin-3-Gallate through 67-KDa Laminin Receptor. J. Immunol. 2010, 185, 33–45. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 Signaling Pathway by Polyphenols: A Novel Therapeutic Strategy for Neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ganapathy, S.; Singh, K.P.; Shankar, S.; Srivastava, R.K. Resveratrol Induces Growth Arrest and Apoptosis through Activation of FOXO Transcription Factors in Prostate Cancer Cells. PLoS ONE 2010, 5, e15288. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Wang, S.Y.; Wang, X.D.; Jiang, H.Q.; Yang, Y.Q.; Wang, Y.; Cheng, J.L.; Zhang, C.T.; Liang, W.W.; Feng, H.L. Fisetin Exerts Antioxidant and Neuroprotective Effects in Multiple Mutant HSOD Models of Amyotrophic Lateral Sclerosis by Activating ERK. Neuroscience 2018, 379, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Jo, M.H.; Ikram, M.; Khan, A.; Kim, M.O. Deciphering the Potential Neuroprotective Effects of Luteolin against Aβ(1)-(42)-Induced Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9583. [Google Scholar] [CrossRef]
- Dasgupta, B.; Milbrandt, J. Resveratrol Stimulates AMP Kinase Activity in Neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-J.; Li, F.; Yu, P.H.-F.; Chan, S.-W. Neuroprotective Effects of Luteolin against Apoptosis Induced by 6-Hydroxydopamine on Rat Pheochromocytoma PC12 Cells. Pharm. Biol. 2013, 51, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Javed, H.; Azimullah, S.; Abul Khair, S.B.; Haque, M.E. Neuroprotective Potential of Ferulic Acid in the Rotenone Model of Parkinson’s Disease. Drug Des. Dev. Ther. 2015, 9, 5499–5510. [Google Scholar] [CrossRef]
- Teixeira, M.D.A.; Souza, C.M.; Menezes, A.P.F.; Carmo, M.R.S.; Fonteles, A.A.; Gurgel, J.P.; Lima, F.A.V.; Viana, G.S.B.; Andrade, G.M. Catechin Attenuates Behavioral Neurotoxicity Induced by 6-OHDA in Rats. Pharmacol. Biochem. Behav. 2013, 110, 1–7. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, T.; Park, H.Y.; Badshah, H.; Rehman, S.U.; Kim, M.O. Neuroprotective Effect of Fisetin Against Amyloid-Beta-Induced Cognitive/Synaptic Dysfunction, Neuroinflammation, and Neurodegeneration in Adult Mice. Mol. Neurobiol. 2017, 54, 2269–2285. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Sun, M.-F.; Jia, X.-B.; Cheng, K.; Xu, Y.-D.; Zhou, Z.-L.; Zhang, P.-H.; Qiao, C.-M.; Cui, C.; Chen, X.; et al. Neuroprotective Effects of Astilbin on MPTP-Induced Parkinson’s Disease Mice: Glial Reaction, α-Synuclein Expression and Oxidative Stress. Int. Immunopharmacol. 2019, 66, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic Acid Exerts Neuroprotective Effects against Cerebral Ischemia/Reperfusion-Induced Injury via Antioxidant and Anti-Apoptotic Mechanisms In Vitro and In Vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef]
- Dai, W.; Wang, H.; Fang, J.; Zhu, Y.; Zhou, J.; Wang, X.; Zhou, Y.; Zhou, M. Curcumin Provides Neuroprotection in Model of Traumatic Brain Injury via the Nrf2-ARE Signaling Pathway. Brain Res. Bull. 2018, 140, 65–71. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Wang, X.; Yu, S.; Li, L.; Wu, X.; Chen, Y.; Zhao, J.; Zhao, Y. Neuroprotection by Curcumin in Ischemic Brain Injury Involves the Akt/Nrf2 Pathway. PLoS ONE 2013, 8, e59843. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Wei, H.; Gu, T.; Wang, J.; Wu, Z.; Yang, Q. Genistein Attenuates Acute Cerebral Ischemic Damage by Inhibiting the NLRP3 Inflammasome in Reproductively Senescent Mice. Front. Aging Neurosci. 2020, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Escudero, J.; Baquero, M.; Cebrián, M.; Carbonell-Asíns, J.A.; Muñoz, J.E.; Satorres, E.; Meléndez, J.C.; Ferrer-Rebolleda, J.; del Puig Cózar-Santiago, M.; et al. Genistein Effect on Cognition in Prodromal Alzheimer’s Disease Patients. The GENIAL Clinical Trial. Alzheimer’s Res. Ther. 2022, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, H.; Rahimi, H.R.; Aghili, S.M.; Saberi, A.; Shoeibi, A. Evaluation of Curcumin as Add-on Therapy in Patients with Parkinson’s Disease: A Pilot Randomized, Triple-Blind, Placebo-Controlled Trial. Clin. Neurol. Neurosurg. 2022, 218, 107300. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Rosato, J.I.; Dias, C.B.; Burrows, T.L.; Martins, R.N.; Garg, M.L. Dietary Supplementation with Curcumin Reduce Circulating Levels of Glycogen Synthase Kinase-3β and Islet Amyloid Polypeptide in Adults with High Risk of Type 2 Diabetes and Alzheimer’s Disease. Nutrients 2020, 12, 1032. [Google Scholar] [CrossRef]
- Philpott, K.L.; Facci, L. MAP Kinase Pathways in Neuronal Cell Death. CNS Neurol. Disord. Drug Targets 2008, 7, 83–97. [Google Scholar] [CrossRef]
- Takeda, K.; Ichijo, H. Neuronal P38 MAPK Signalling: An Emerging Regulator of Cell Fate and Function in the Nervous System. Genes Cells 2002, 7, 1099–1111. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Zhang, J.; Tian, B.-B.; Wang, L.; Liu, G.-K.; Liu, Y. A Relationship between MAPK/ERK Pathway Expression and Neuronal Apoptosis in Rats with White Matter Lesions. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4412–4419. [Google Scholar] [CrossRef]
- Leow, S.-S.; Sekaran, S.D.; Tan, Y.; Sundram, K.; Sambanthamurthi, R. Oil Palm Phenolics Confer Neuroprotective Effects Involving Cognitive and Motor Functions in Mice. Nutr. Neurosci. 2013, 16, 207–217. [Google Scholar] [CrossRef]
- Watanabe, C.M.; Wolffram, S.; Ader, P.; Rimbach, G.; Packer, L.; Maguire, J.J.; Schultz, P.G.; Gohil, K. The In Vivo Neuromodulatory Effects of the Herbal Medicine Ginkgo Biloba. Proc. Natl. Acad. Sci. USA 2001, 98, 6577–6580. [Google Scholar] [CrossRef]
| Assay | Disease | Model | Treatment | Phenolic Compound | Finds | Ref. |
|---|---|---|---|---|---|---|
| In vitro | ALS | Mouse motor neuron (NSC-34) mutant hSOD1G93A gene | 10 µM 4 h before the damage with H2O2 | Fisetin | Fisetin reduced ROS damage, increased cell survival and the expression of phosphorylated ERK, and upregulated antioxidant factors, which were reversed by MAPK/ERK inhibition. | [99] |
| AD | HT-22 Mouse Hippocampal Neuronal Cell Line | 20 μM (No time specified at the same time with Aβ) | Luteolin | Decrease of inflammatory markers p-NF-kB, TNF-α, and IL-1β. Decreased proapoptotic proteins Bax, Bcl-2, Caspase-3, and Cox-2. Decrease in BACE-1 enzyme and subsequent decrease in Aβ. | [100] | |
| Caloric restriction associated with neurodegenerative diseases in general | Neuro2a neuroblastoma cells | 10 μM (2–72 h) cells grown under serum starvation conditions | Resveratrol | Resveratrol increases AMP activation (AMPK) during caloric restriction, promoting energy conservation, cell survival, and neurite outgrowth. | [101] | |
| PD | PC-12 cells (6-hydroxy-dopamine) (6-OHDA) | 3.1, 12.5, 50 µM or without luteolin for 2 h | Luteolin | Increased cell viability and decreased expression of proapoptotic proteins Bax and Bcl-2. | [102] | |
| In vivo | PD | Male Wistar rats—using rotenone (ROT)-induced rat model of PD | 4 weeks at the dose of 50 mg/kg, 30 min before damage is induced. | Ferulic acid | Protection of dopaminergic neurons, lipid peroxidation, and reduced levels of inflammatory markers IL-1β, IL-6, and TNF-α | [103] |
| PD | (6-OHDA lesioned rats). | 10 and 30 mg/kg injection 2 h before surgery and for 14 days afterward | Catechin | After treatments, they significantly reversed this abnormal motor behavior and memory deficits and protected animals from the observed decrease in dopamine and noradrenaline. | [104] | |
| AD | Male wildtype C57BL/6N mice | 20 mg/kg/day for 2 weeks, starting 24 h after Aβ injection | Fisetin | Decreased the expression of BACE-1 and the formation of Aβ aggregates. Decreased synaptic dysfunction (promoted the expression of postsynaptic proteins PSD-95, SNAP-23, p-GluR1). Promoted activation of p-PI3K, p-AKT, and p-GSK3β and subsequent cell survival. Decreased proinflammatory proteins p-IKKβ, p-NFKB, TNFα, and IL-1β. | [105] | |
| PD | Male C57BL/6 mice (Parkinson’s disease model) | 50 mg/kg/day for 7 days after damage with MPTP | Astilbin | Increased cell survival by promoting phosphorylation of p-PI3K/p-AKT. Decreased oxidative stress by increasing GSH and SOD activity. Reduced the loss of dopaminergic neurons and the activation of microglia (Iba-1) and astrocytes in the substantia nigra. | [106] | |
| Hypoxia/ischemia | Male Sprague–Dawley rats and pheochromocytoma (PC-12) cells | 28, 56, and 112 mg/kg/day after ischemia for 5 consecutive days | Ferulic acid | Attenuated memory impairment reduced hippocampal neuronal apoptosis and oxidative stress in a dose-dependent manner and inactivated the Toll-like receptor TLR4 and MyD88. Increased levels of Bcl-2 and decreased levels of caspase-3 and Bax. | [107] | |
| TBI | Adult male ICR mice, traumatic brain injury model | 50 and 100 mg/kg 30 min after TBI | Curcumin | Reduced proapoptotic proteins Bcl-2 and cleaved caspase 3. Promoted activation of the Nrf2–ARE pathway and subsequent enhancement of SOD, GPx, and MDA expression. | [108] | |
| Ischemic | Nine-week-old Sprague–Dawley male rats/culture primary cortical neurons | 300 mg/kg 1 h after Ischemic brain injury | Curcumin | Attenuated infarction volumes, upregulated NAD(P)H, NQO1 levels. Promoted p-AKT/Nrf2 activation. | [109] | |
| Ischemic | Adult male C57/BL6J mice | 10 mg/kg for 2 weeks before cerebral ischemia | Genistein | It decreased infarct volume, improved neurological scores, attenuated cleaved caspase-1 apoptosis, decreased the release of inflammatory factors TNF-α, IL-1β, IL-18, and IL-6, and negatively regulated inflammasome activation assessed with the NLRP3 marker. | [110] | |
| Clinical trial/Randomized Controlled Trial | AD | Alzheimer’s disease patients | 120 mg for 12 months | Genistein | The cognitive ability of the treated patients improved, and there was no increase in Aβ deposition compared to the placebo control. | [111] |
| PD | Idiopathic PD patients aged ≥30 | 30, 80 mg/day for nine months | Curcumin | This trial was unsuccessful in showing its efficacy in quality of life and clinical symptoms of PD patients | [112] | |
| AD | Patients at high risk of developing Alzheimer’s disease | 180 mg/day for 12 weeks | Curcumin | Reduction of circulating levels of Amyloid Polypeptide compared to the placebo control. | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nájera-Maldonado, J.M.; Salazar, R.; Alvarez-Fitz, P.; Acevedo-Quiroz, M.; Flores-Alfaro, E.; Hernández-Sotelo, D.; Espinoza-Rojo, M.; Ramírez, M. Phenolic Compounds of Therapeutic Interest in Neuroprotection. J. Xenobiot. 2024, 14, 227-246. https://doi.org/10.3390/jox14010014
Nájera-Maldonado JM, Salazar R, Alvarez-Fitz P, Acevedo-Quiroz M, Flores-Alfaro E, Hernández-Sotelo D, Espinoza-Rojo M, Ramírez M. Phenolic Compounds of Therapeutic Interest in Neuroprotection. Journal of Xenobiotics. 2024; 14(1):227-246. https://doi.org/10.3390/jox14010014
Chicago/Turabian StyleNájera-Maldonado, José Manuel, Ricardo Salazar, Patricia Alvarez-Fitz, Macdiel Acevedo-Quiroz, Eugenia Flores-Alfaro, Daniel Hernández-Sotelo, Mónica Espinoza-Rojo, and Mónica Ramírez. 2024. "Phenolic Compounds of Therapeutic Interest in Neuroprotection" Journal of Xenobiotics 14, no. 1: 227-246. https://doi.org/10.3390/jox14010014
APA StyleNájera-Maldonado, J. M., Salazar, R., Alvarez-Fitz, P., Acevedo-Quiroz, M., Flores-Alfaro, E., Hernández-Sotelo, D., Espinoza-Rojo, M., & Ramírez, M. (2024). Phenolic Compounds of Therapeutic Interest in Neuroprotection. Journal of Xenobiotics, 14(1), 227-246. https://doi.org/10.3390/jox14010014






