Traditional Plant-Derived Compounds Inhibit Cell Migration and Induce Novel Cytoskeletal Effects in Glioblastoma Cells
Abstract
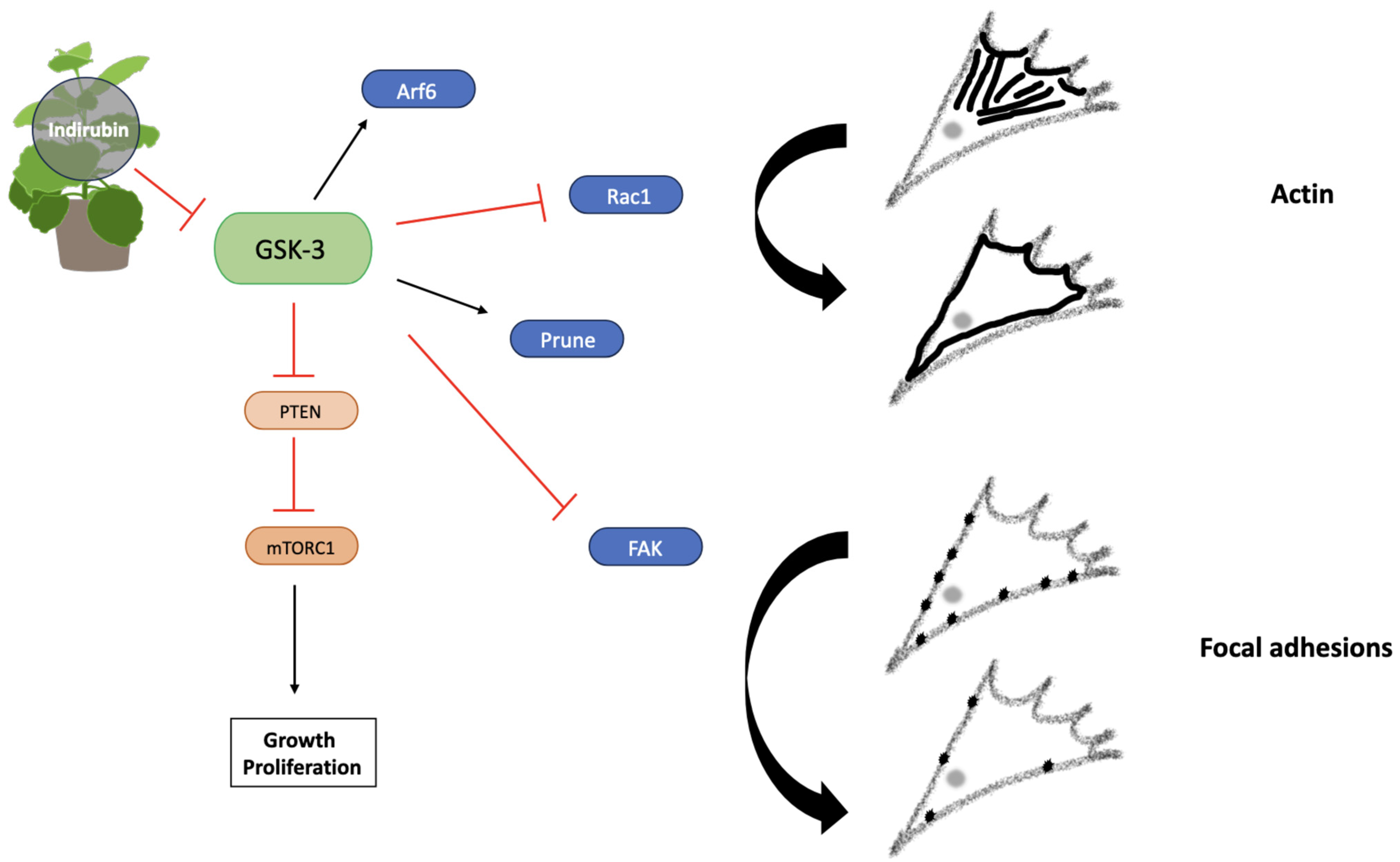
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Drug Preparation
2.3. MTT Assay
2.4. 2D Phenotypic Assay and Immunofluorescence
2.5. Scratch Assay
2.6. Spheroid Invasion Assay and Immunofluorescence
2.7. Confocal Microscopy
2.8. Focal Adhesion Analysis
2.9. Actin Localization Analysis
2.10. Statistical Analysis
3. Results
3.1. MTT Assays
3.2. Two-Dimensional Phenotypic Assay
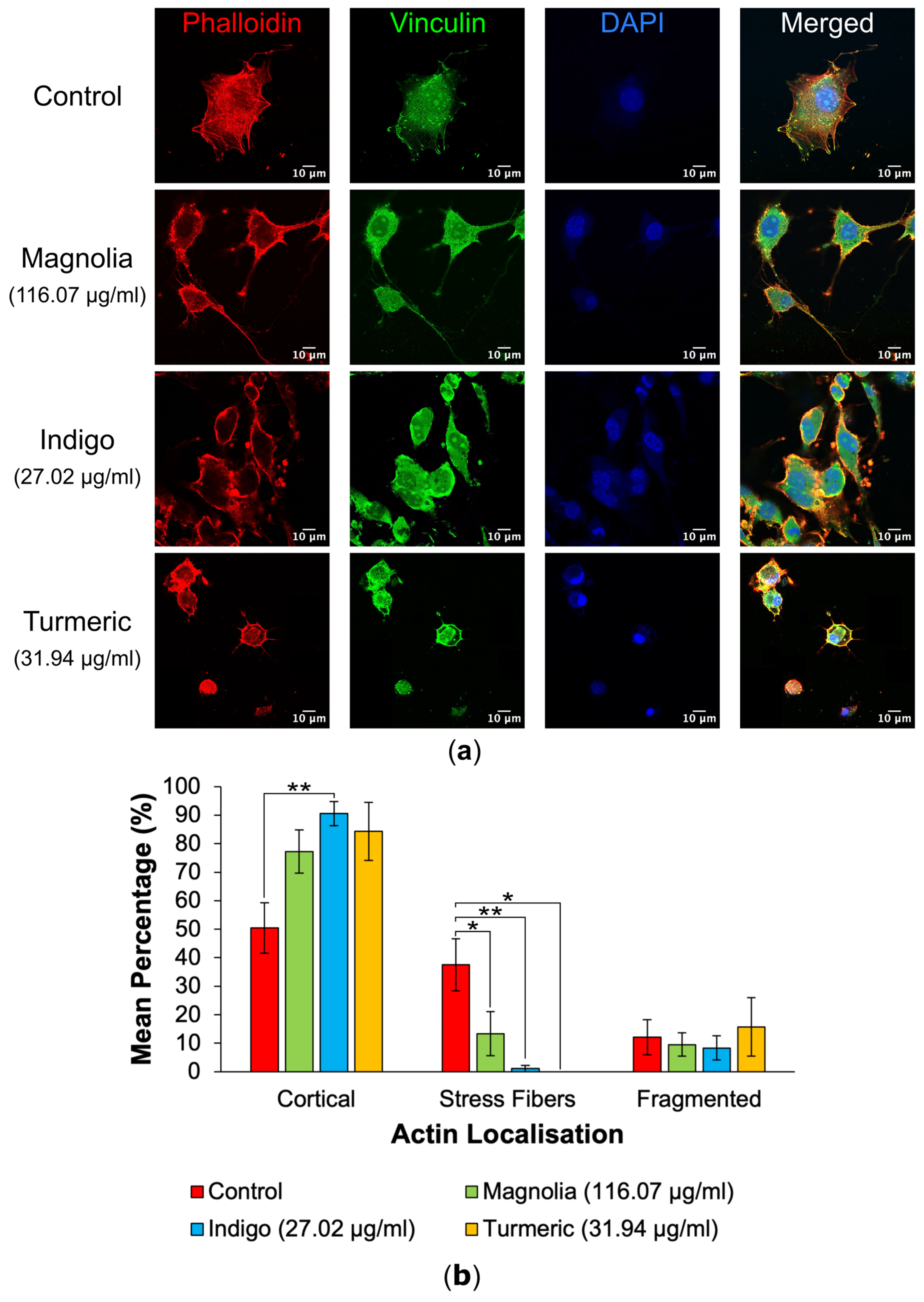
3.2.1. Actin Localization
3.2.2. FA Generation
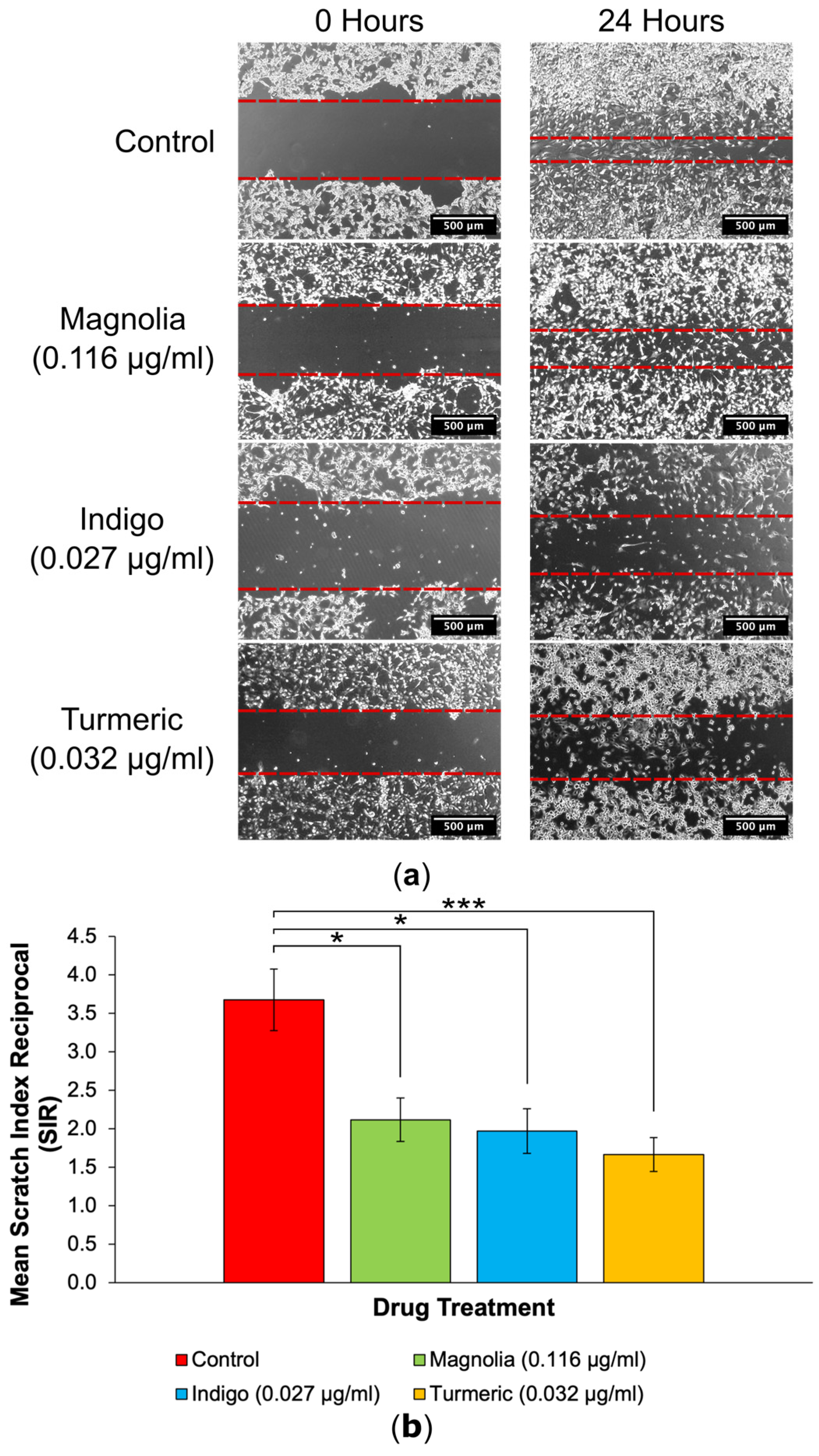
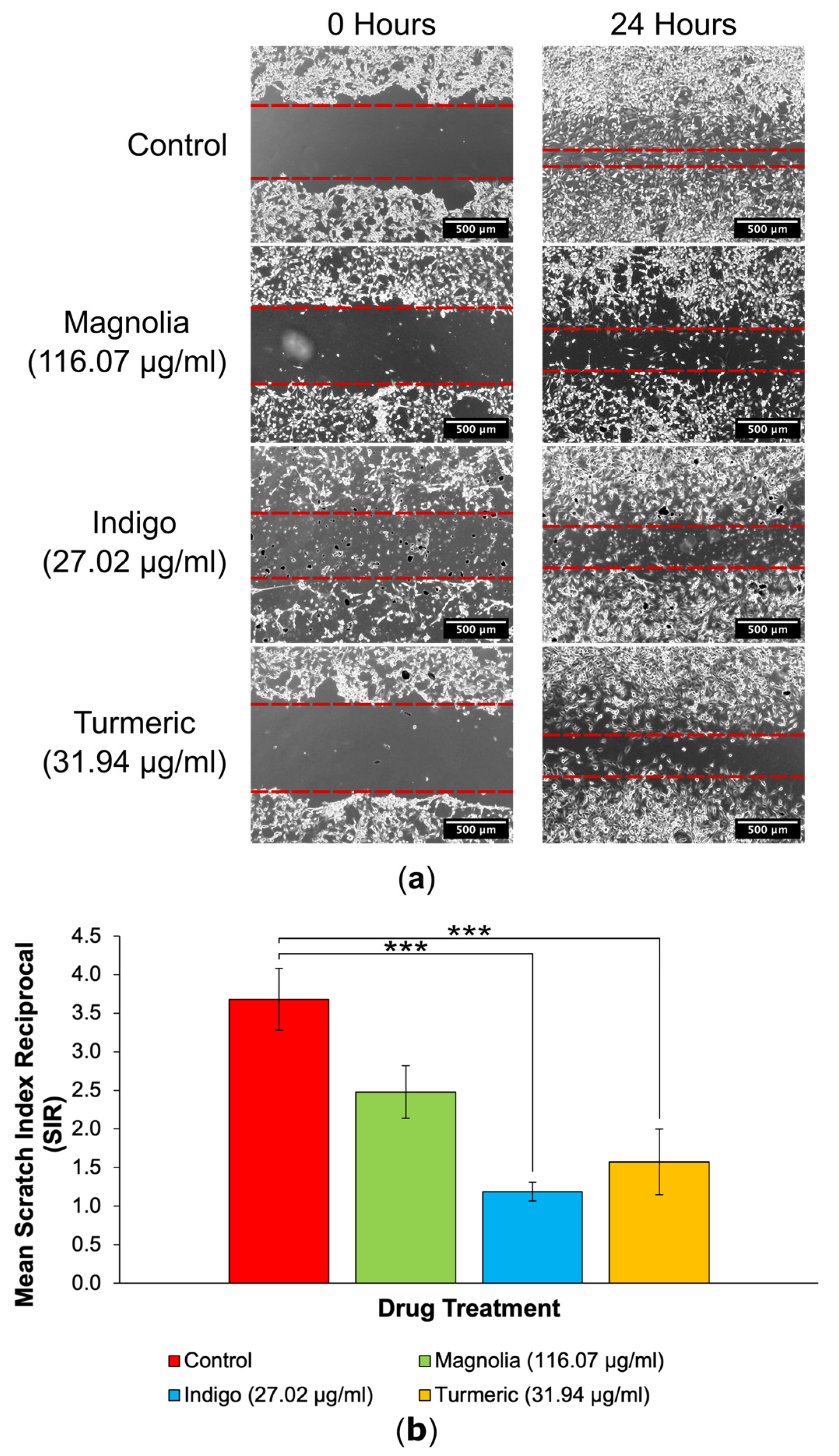
3.3. Scratch Assays
3.3.1. Drugs Administered at a Low Concentration
3.3.2. Drugs Administered at a High Concentration
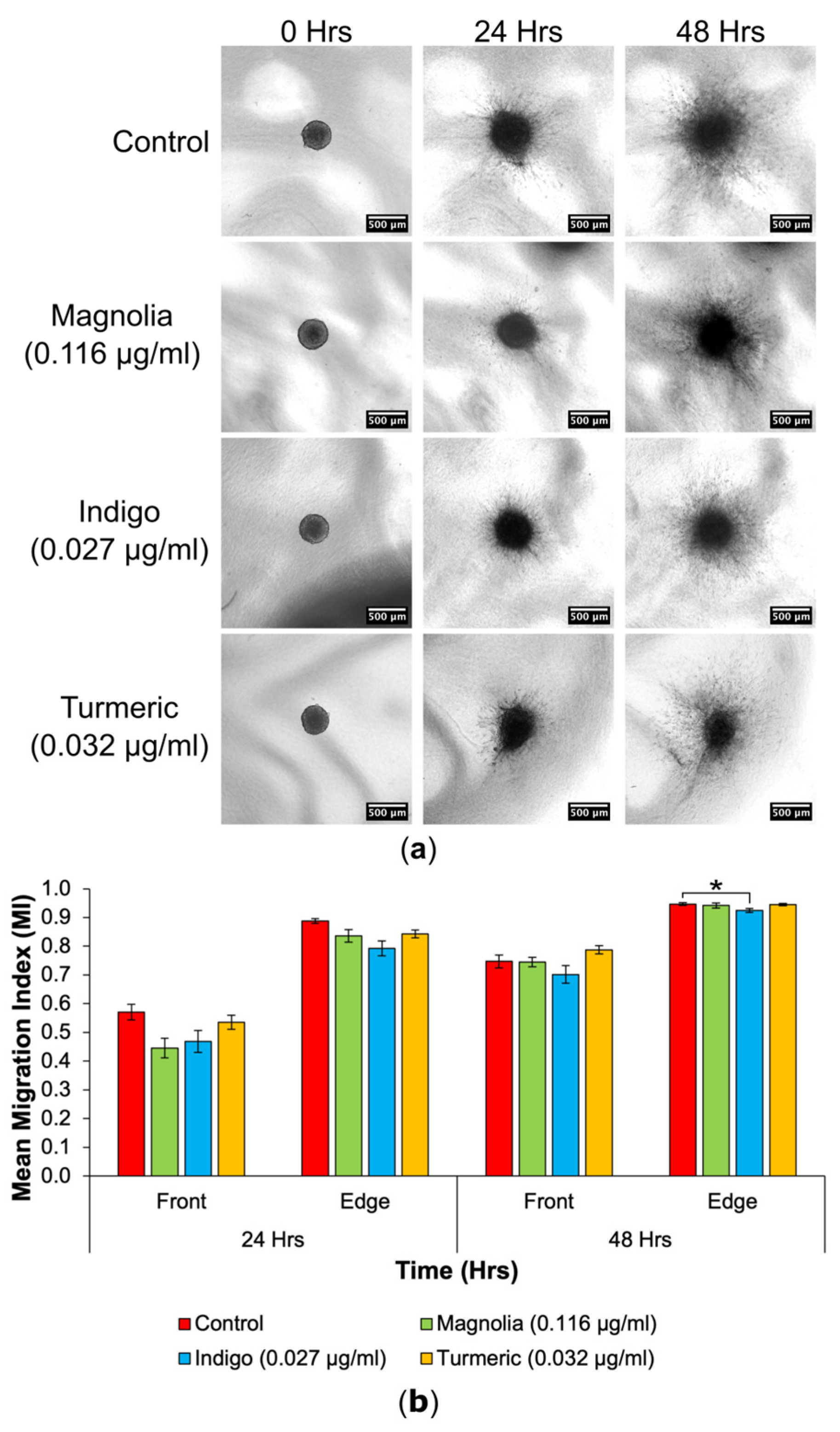
3.4. Spheroid Invasion Assays
3.4.1. Drugs Administered at a Low Concentration
3.4.2. Drugs Administered at a High Concentration
3.4.3. Spheroid Confocal Microscopy
4. Discussion
4.1. Turmeric
4.2. Indigo
4.3. Magnolia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of care for treatment of recurrent glioblastoma—Are we there yet? Neuro-Oncol. 2013, 15, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Niyazi, M.; Alongi, F.; Navarria, P.; Belka, C. Current status and recent advances in reirradiation of glioblastoma. Radiat. Oncol. 2021, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Singh, R. Nanotherapy: Targeting the tumour microenvironment. Nat. Rev. Cancer 2022, 22, 258. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. Physical view on migration modes. Cell Adhes. Migr. 2015, 9, 367–379. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, G.M. The coordination between actin filaments and adhesion in mesenchymal migration. Cell Adhes. Migr. 2009, 3, 355–357. [Google Scholar] [CrossRef]
- Wehrle-Haller, B. Structure and function of focal adhesions. Curr. Opin. Cell Biol. 2012, 24, 116–124. [Google Scholar] [CrossRef]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Biotechnol. Appl. Biodivers. 2015, 147, 59–110. [Google Scholar] [CrossRef]
- Khan, T.; Gurav, P. PhytoNanotechnology: Enhancing Delivery of Plant Based Anti-cancer Drugs. Front. Pharmacol. 2018, 8, 316410. [Google Scholar] [CrossRef] [PubMed]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and Toxicology of Magnolol and Honokiol. Planta Medica 2018, 84, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, F.; Ali, S.; Kumar, V.; Elasbali, A.M.; Alhassan, H.H.; Alharethi, S.H.; Islam, A.; Hassan, M.I. Pharmacological features, health benefits and clinical implications of honokiol. J. Biomol. Struct. Dyn. 2023, 41, 7511–7533. [Google Scholar] [CrossRef]
- Banik, K.; Ranaware, A.M.; Deshpande, V.; Nalawade, S.P.; Padmavathi, G.; Bordoloi, D.; Sailo, B.L.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; et al. Honokiol for cancer therapeutics: A traditional medicine that can modulate multiple oncogenic targets. Pharmacol. Res. 2019, 144, 192–209. [Google Scholar] [CrossRef]
- Pan, J.; Lee, Y.; Wang, Y.; You, M. Honokiol targets mitochondria to halt cancer progression and metastasis. Mol. Nutr. Food Res. 2016, 60, 1383–1395. [Google Scholar] [CrossRef]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, Y.; Li, H.-B. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef]
- Astinfeshana, M.; Rasmia, Y.; Kheradmanda, F.; Karimipourc, M.; Rahbarghazid, R.; Aramwitf, P.; Nasirzadeha, M.; Daeihassanig, B.; Shirpoorh, A.; Gholinejada, Z.; et al. Curcumin inhibits angiogenesis in endothelial cells using downregulation of the PI3K/Akt signaling pathway. Food Biosci. 2019, 29, 86–93. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Bojko, A.; Cierniak, A.; Adamczyk, A.; Ligeza, J. Modulatory Effects of Curcumin and Tyrphostins (AG494 and AG1478) on Growth Regulation and Viability of LN229 Human Brain Cancer Cells. Nutr. Cancer 2015, 67, 1170–1182. [Google Scholar] [CrossRef]
- Zanotto-Filho, A.; Braganhol, E.; Edelweiss, M.I.; Behr, G.A.; Zanin, R.; Schröder, R.; Simões-Pires, A.; Battastini, A.M.O.; Moreira, J.C.F. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J. Nutr. Biochem. 2012, 23, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Xie, W.; Hua, Z.; Cao, L.; Xiong, Z.; Tang, Y.; Yuan, Z. Recent Advancements in Natural Plant Colorants Used for Hair Dye Applications: A Review. Molecules 2022, 27, 8062. [Google Scholar] [CrossRef]
- Xie, Q.; Yu, L.; Wang, X.; Wu, Z.; Zhi, D.; Yang, J.; Guo, Z.; Wu, T.; Sun, Y.; Zhao, L.; et al. A novel realgar-indigo naturalis formula more effectively induces apoptosis in NB4 cells. Pak. J. Pharm. Sci. 2019, 32, 957–962. [Google Scholar] [PubMed]
- Williams, S.P.; Nowicki, M.O.; Liu, F.; Press, R.; Godlewski, J.; Abdel-Rasoul, M.; Kaur, B.; Fernandez, S.A.; Chiocca, E.A.; Lawler, S.E. Indirubins Decrease Glioma Invasion by Blocking Migratory Phenotypes in Both the Tumor and Stromal Endothelial Cell Compartments. Cancer Res. 2011, 71, 5374–5380. [Google Scholar] [CrossRef] [PubMed]
- Cockle, J.V.; Picton, S.; Levesley, J.; Ilett, E.; Carcaboso, A.M.; Short, S.; Steel, L.P.; Melcher, A.; Lawler, S.E.; Brüning-Richardson, A. Cell migration in paediatric glioma; characterisation and potential therapeutic targeting. Br. J. Cancer 2015, 112, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Rogers, N.J.; Allison, S.J.; Brabec, V.; Bridgewater, H.; Kostrhunova, H.; Markova, L.; Phillips, R.M.; Pinder, E.C.; Shepherd, S.L.; et al. Discovery of selective, antimetastatic and anti-cancer stem cell metallohelices via post-assembly modification. Chem. Sci. 2019, 10, 8547–8557. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- IBM Corporation. IBM SPSS Statistics for Macintosh, Version 28.0; IBM Corporation: Armonk, NY, USA, 2021. [Google Scholar]
- Revach, O.-Y.; Grosheva, I.; Geiger, B. Biomechanical regulation of focal adhesion and invadopodia formation. J. Cell Sci. 2020, 133, jcs244848. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-González, B.; Meili, R.; Firtel, R.; Bastounis, E.; Álamo, J.C.D.; Lasheras, J.C. Cytoskeletal Mechanics Regulating Amoeboid Cell Locomotion. Appl. Mech. Rev. 2014, 66, 050804. [Google Scholar] [CrossRef]
- Rösel, D.; Brábek, J.; Tolde, O.; Mierke, C.T.; Zitterbart, D.P.; Raupach, C.; Bicanová, K.; Kollmannsberger, P.; Panková, D.; Vesely, P.; et al. Up-regulation of Rho/ROCK signaling in sarcoma cells drives invasion and increased generation of protrusive forces. Mol. Cancer Res. 2008, 6, 1410–1420. [Google Scholar] [CrossRef]
- Kosla, J.; Paňková, D.; Plachý, J.; Tolde, O.; Bicanová, K.; Dvořák, M.; Rösel, D.; Brábek, J. Metastasis of aggressive amoeboid sarcoma cells is dependent on Rho/ROCK/MLC signaling. Cell Commun. Signal. 2013, 11, 51. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yashiro, M. Rho/ROCK signaling in motility and metastasis of gastric cancer. World J. Gastroenterol. 2014, 20, 13756–13766. [Google Scholar] [CrossRef] [PubMed]
- Ketchen, S.E.; Gamboa-Esteves, F.O.; Lawler, S.E.; Nowicki, M.O.; Rohwedder, A.; Knipp, S.; Prior, S.; Short, S.C.; Ladbury, J.E.; Brüning-Richardson, A. Drug Resistance in Glioma Cells Induced by a Mesenchymal-Amoeboid Migratory Switch. Biomedicines 2021, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Davoodvandi, A.; Farshadi, M.; Zare, N.; Akhlagh, S.A.; Nosrani, E.A.; Mahjoubin-Tehran, M.; Kangari, P.; Sharafi, S.M.; Khan, H.; Aschner, M.; et al. Antimetastatic Effects of Curcumin in Oral and Gastrointestinal Cancers. Front. Pharmacol. 2021, 12, 668567. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Cordero, J.J.; Magalhaes, M.A.O.; Eddy, R.J.; Hodgson, L.; Condeelis, J. Functions of cofilin in cell locomotion and invasion. Nat. Rev. Mol. Cell Biol. 2013, 14, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yue, G.G.-L.; Tsui, S.K.-W.; Fung, K.-P.; Lau, C.B.-S. Turmeric extract, with absorbable curcumin, has potent anti-metastatic effect in vitro and in vivo. Phytomedicine 2018, 46, 131–141. [Google Scholar] [CrossRef]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Yoon, S.Y.; Park, S.H.; Hwang, J.H. Anti-Migration and Anti-Invasion Effects of Curcumin via Suppression of Fascin Expression in Glioblastoma Cells. Brain Tumour Res. Treat. 2019, 7, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, T.; Liu, X.; Liu, Q. Curcumin inhibits migration and invasion of non-small cell lung cancer cells through up-regulation of miR-206 and suppression of PI3K/AKT/mTOR signaling pathway. Acta Pharm. 2020, 70, 399–409. [Google Scholar] [CrossRef]
- Bastola, S.; Pavlyukov, M.S.; Yamashita, D.; Ghosh, S.; Cho, H.; Kagaya, N.; Zhang, Z.; Minata, M.; Lee, Y.; Sadahiro, H.; et al. Glioma-initiating cells at tumor edge gain signals from tumor core cells to promote their malignancy. Nat. Commun. 2020, 11, 4660. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Q.; Lin, J. Identification of the potential oncogenes in glioblastoma based on bioinformatic analysis and elucidation of the underlying mechanisms. Oncol. Rep. 2018, 40, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hou, S.-Q.; He, J.; Gu, T.; Yin, Y.; Shen, W.H. PTEN regulates PLK1 and controls chromosomal stability during cell division. Cell Cycle 2016, 15, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Paplomata, E.; O’Regan, R. The PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex roles of cAMP-PKA-CREB signaling in cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Qin, X. miR-182 regulates trastuzumab resistance by targeting MET in breast cancer cells. Cancer Gene Ther. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Mercedes, S.A.V.; Bocci, F.; Levine, H.; Onuchic, J.N.; Jolly, M.K.; Wong, P.K. Decoding leader cells in collective cancer invasion. Nat. Rev. Cancer 2021, 21, 592–604. [Google Scholar] [CrossRef]
- Minata, M.; Audia, A.; Shi, J.; Lu, S.; Bernstock, J.; Pavlyukov, M.S.; Das, A.; Kim, S.-H.; Shin, Y.J.; Lee, Y.; et al. Phenotypic Plasticity of Invasive Edge Glioma Stem-like Cells in Response to Ionizing Radiation. Cell Rep. 2019, 26, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Rebl, H.; Sawade, M.; Hein, M.; Bergemann, C.; Wende, M.; Lalk, M.; Langer, P.; Emmert, S.; Nebe, B. Synergistic effect of plasma-activated medium and novel indirubin derivatives on human skin cancer cells by activation of the AhR pathway. Sci. Rep. 2022, 12, 2528. [Google Scholar] [CrossRef] [PubMed]
- Peat, S.; Brüning-Richardson, A.; Meijer, L.; Lawler, S.; Thiéry, V.; Morton, R. Characterisation of the anti-migratory activity of the 6-bromoindirubin-3’oxime (BIO) derivative VTIND42 in patient-derived GBM subpopulations. Neuro-Oncol. 2019, 21, iv6–iv7. [Google Scholar] [CrossRef]
- Hajka, D.; Budziak, B.; Pietras, Ł.; Duda, P.; McCubrey, J.A.; Gizak, A. GSK3 as a Regulator of Cytoskeleton Architecture: Consequences for Health and Disease. Cells 2021, 10, 2092. [Google Scholar] [CrossRef]
- Sun, T.; Rodriguez, M.; Kim, L. Glycogen synthase kinase 3 in the world of cell migration. Dev. Growth Differ. 2009, 51, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Tsao, M.-J.; Chiu, C.-Y.; Kan, P.-C.; Chen, Y. Magnolol Inhibits Human Glioblastoma Cell Migration by Regulating N-Cadherin. J. Neuropathol. Exp. Neurol. 2018, 77, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.-G.; Oh, S.-J.; Ahn, E.-J.; Kim, Y.-J.; Jung, T.-Y.; Jung, S.; Kim, K.-K.; Lee, J.-H.; Lee, K.-H.; Moon, K.-S. Prognostic significance of E-cadherin and N-cadherin expression in Gliomas. BMC Cancer 2017, 17, 583. [Google Scholar] [CrossRef]
- Yeh, P.-S.; Wang, W.; Chang, Y.-A.; Lin, C.-J.; Wang, J.-J.; Chen, R.-M. Honokiol induces autophagy of neuroblastoma cells through activating the PI3K/Akt/mTOR and endoplasmic reticular stress/ERK1/2 signaling pathways and suppressing cell migration. Cancer Lett. 2016, 370, 66–77. [Google Scholar] [CrossRef]
- Lee, J.S.; Sul, J.Y.; Park, J.B.; Lee, M.S.; Cha, E.Y.; Ko, Y.B. Honokiol induces apoptosis and suppresses migration and invasion of ovarian carcinoma cells via AMPK/mTOR signaling pathway. Int. J. Mol. Med. 2019, 43, 1969–1978. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, E.; Prior, S.; Brüning-Richardson, A. Traditional Plant-Derived Compounds Inhibit Cell Migration and Induce Novel Cytoskeletal Effects in Glioblastoma Cells. J. Xenobiot. 2024, 14, 613-633. https://doi.org/10.3390/jox14020036
Thompson E, Prior S, Brüning-Richardson A. Traditional Plant-Derived Compounds Inhibit Cell Migration and Induce Novel Cytoskeletal Effects in Glioblastoma Cells. Journal of Xenobiotics. 2024; 14(2):613-633. https://doi.org/10.3390/jox14020036
Chicago/Turabian StyleThompson, Evan, Sally Prior, and Anke Brüning-Richardson. 2024. "Traditional Plant-Derived Compounds Inhibit Cell Migration and Induce Novel Cytoskeletal Effects in Glioblastoma Cells" Journal of Xenobiotics 14, no. 2: 613-633. https://doi.org/10.3390/jox14020036
APA StyleThompson, E., Prior, S., & Brüning-Richardson, A. (2024). Traditional Plant-Derived Compounds Inhibit Cell Migration and Induce Novel Cytoskeletal Effects in Glioblastoma Cells. Journal of Xenobiotics, 14(2), 613-633. https://doi.org/10.3390/jox14020036






