Mechanistic Insights into the Neurotoxicity of 2,5-Dimethoxyphenethylamines (2C) and Corresponding N-(2-methoxybenzyl)phenethylamine (NBOMe) Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. Solvents, Reagents, and Experimental Conditions
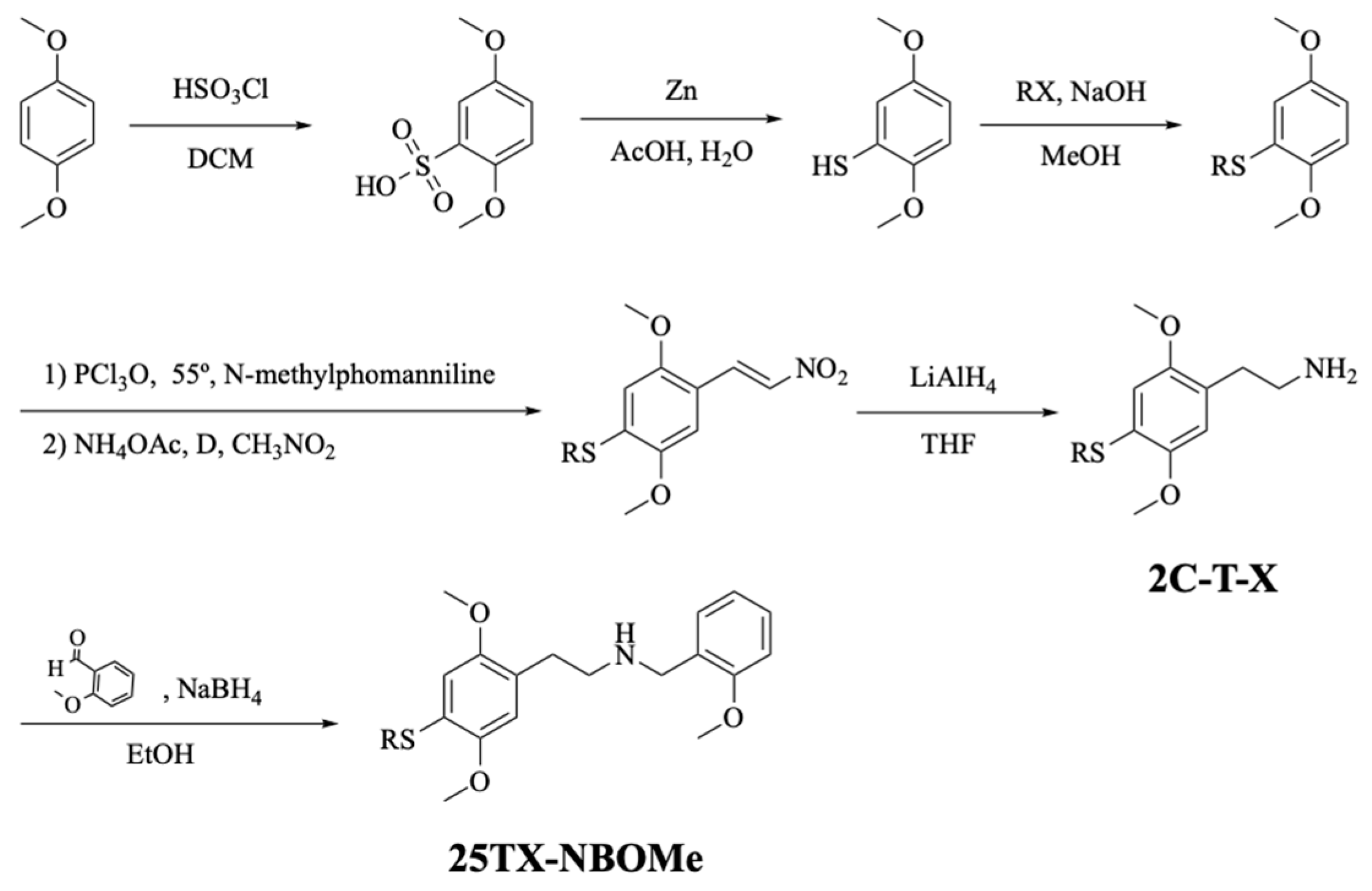
2.1.2. Synthesis and Characterization of 2C-T-X and 25TX-NBOMe Drugs
- (2,5-dimethoxyphenyl)(ethyl)sulfane. Yield: 82%. 1H NMR (400 MHz, DMSO) δ: 6.89 (1H, d, J = 8.8 Hz, H3), 6.76 (1H, d, J = 2.9 Hz, H6), 6.70 (1H, dd, J = 8.8, 3.0 Hz, H4), 3.74 (3H, s, OCH3), 3.71 (3H, s, OCH3), 2.89 (2H, q, J = 7.3 Hz, SCH2CH3), and 1.23 (3H, t, J = 7.3 Hz, SCH2CH3).
- (2,5-dimethoxyphenyl)(isopropyl)sulfane. Yield: 89%. 1H NMR (400 MHz, DMSO) δ: 6.91 (1H, d, J = 8.9 Hz, H3), 6.84 (1H, d, J = 3.0 Hz, H6), 6.76 (1H, dd, J = 8.8, 3.0 Hz, H4), 3.73 (3H, s, OCH3), 3.71 (3H, s, OCH3), 3.51 (2H, hept, J = 6.7 Hz, SCH(CH3)2), and 1.22 (6H, d, J = 6.6 Hz, SCH(CH3)2). 13C NMR (101 MHz, DMSO) δ: 153.2 (C5), 151.4 (C2), 124.7 (C1), 116.1 (C3), 112.0 (C6), 111.4 (C4), 56.1 (OCH3), 55.3 (OCH3), 34.6 (SCH(CH3)2), and 22.6 (SCH(CH3)2).
- (2,5-dimethoxyphenyl)(propyl)sulfane. Yield: 94%. 1H NMR (400 MHz, DMSO) δ: 6.88 (1H, d, J = 8.8 Hz, H3), 6.75 (1H, d, J = 2.9 Hz, H6), 6.70 (1H, dd, J = 8.8, 2.9 Hz, H4), 3.74 (3H, s, OCH3), 3.71 (3H, s, OCH3), 2.85 (2H, t, J = 7.2 Hz, SCH2CH2CH3), 1.59 (2H, h, J = 7.3 Hz, SCH2CH2CH3), and 0.98 (3H, t, J = 7.3 Hz, SCH2CH2CH3). 13C NMR (101 MHz, DMSO) δ: 153.6 (C5), 150.4 (C2), 126.2 (C1), 113.3 (C3), 111.7 (C6), 110.0 (C4), 56.1 (OCH3), 55.4 (OCH3), 32.2 (SCH2CH2CH3, 21.6 (SCH2CH2CH3), and 13.3 (SCH2CH2CH3).
- (E)-(2,5-dimethoxy-4-(2-nitrovinyl)phenyl)(ethyl)sulfane. Yield: 88%. 1H NMR (400 MHz, CDCl3) δ: 8.12 (1H, d, J = 13.5 Hz, Hα), 7.83 (1H, d, J = 13.5 Hz, Hβ), 6.84 (1H, s, H6), 6.78 (1H, s, H3), 3.93 (3H, s, OCH3), 3.89 (3H, s, OCH3), 2.99 (2H, q, J = 7.4 Hz, SCH2CH3), and 1.40 (t, J = 7.4 Hz, SCH2CH3). 13C NMR (101 MHz, CDCl3) δ: 154.4 (C2), 150.4 (C5), 137.2 (Cβ), 135.2 (Cα), 134.3 (C4), 115.6 (C1), 112.3 (C3), 109.8 (C6), 56.5 (OCH3), 56.1 (OCH3), 25.4 (SCH2CH3), and 13.6 (SCH2CH3).
- (E)-(2,5-dimethoxy-4-(2-nitrovinyl)phenyl)(isopropyl)sulfane. Yield: 52%. 1H NMR (400 MHz, DMSO) δ: 8.20 (2H, s, Hα, Hβ), 7.40 (1H, s, H6), 6.95 (1H, s, H3), 3.92 (3H, s, OCH3), 3.81 (3H, s, OCH3), 3.82–3.72 (1H, m, SCH(CH3)2), and 1.31 (6H, d, J = 6.6 Hz, SCH(CH3)2). 13C NMR (101 MHz, DMSO) δ: 153.7 (C2), 149.9 (C5), 136.8 (Cβ), 134.2 (C4), 133.6 (Cα), 114.9 (C1), 111.6 (C3), 110.7 (C6), 56.38 (OCH3), 56.37 (OCH3), 33.9 (SCH(CH3)2), and 22.4 (SCH(CH3)2).
- (E)-(2,5-dimethoxy-4-(2-nitrovinyl)phenyl)(propyl)sulfane. Yield: 47%. 1H NMR (400 MHz, CDCl3) δ: 8.12 (1H, d, J = 13.5 Hz, Hα), 7.83 (1H, d, J = 13.5 Hz, Hβ), 6.83 (1H, s, H6), 6.77 (1H, s, H3), 3.93 (3H, s, OCH3), 3.89 (3H, s, OCH3), 2.94 (2H, t, J = 7.3 Hz, SCH2CH2CH3), 1.77 (2H, h, J = 7.4 Hz, SCH2CH2CH3), and 1.09 (3H, t, J = 7.4 Hz, SCH2CH2CH3). 13C NMR (101 MHz, CDCl3) δ: 154.4 (C2), 150.4 (C5), 137.2 (Cβ), 135.2 (Cα), 134.6 (C4), 115.5 (C1), 112.3 (C3), 109.7 (C6), 56.5 (OCH3), 56.1 (OCH3), 33.3 (SCH2CH2CH3), 22.0 (SCH2CH2CH3), and 13.7 (SCH2CH2CH3).
- 2-(4-(ethylthio)-2,5-dimethoxyphenyl)ethan-1-aminium chloride (2C-T-2). Yield: 46%. 1H NMR (400 MHz, MeOD) δ: 6.92 (1H, s, H6), 6.84 (1H, s, H3), 3.83 (3H, s, OCH3), 3.82 (3H, s, OCH3), 3.17–3.09 (2H, m, Hα), 2.98–2.86 (4H, m, Hβ and SCH2CH3), and 1.26 (3H, t, J = 7.4 Hz, 1H SCH2CH3). 13C NMR (101 MHz, MeOD) δ: 151.9 (C2 and C5), 124.5 (C1), 123.0 (C4), 113.9 (C6), 112.7 (C3), 55.7 (OCH3), 55.1 (OCH3), 39.5 (Cα), 28.3 (Cβ), 25.7 (SCH2CH3), and 13.2 (SCH2CH3).
- 2-(4-(isopropylthio)-2,5-dimethoxyphenyl)ethan-1-aminium chloride (2C-T-4). Yield: 59%. 1H NMR (400 MHz, MeOD) δ: 6.99 (1H, s, H6), 6.87 (1H, s, H3), 3.82 (6H, s, 2xOCH3), 3.50 (1H, hept, J = 6.7 Hz, SCH(CH3)2), 3.14 (2H, t, J = 7.4 Hz, Hα), 2.95 (2H, t, J = 7.4 Hz, Hβ), and 1.23 (6H, d, J = 6.7 Hz, SCH(CH3)2). 13C NMR (101 MHz, MeOD) δ: 154.6 (C2), 152.9 (C5), 125.8 (C1), 124.8 (C4), 117.2 (C6), 115.5 (C3), 57.1 (OCH3), 56.5 (OCH3), 40.9 (Cα), 37.6 (SCH(CH3)2), 29.8 (Cβ), and 23.4 (SCH(CH3)2).
- 2-(2,5-dimethoxy-4-(propylthio)phenyl)ethan-1-aminium chloride (2C-T-7). Yield: 66%. 1H NMR (400 MHz, MeOD) δ: 6.92 (1H, s, H6), 6.84 (1H, s, H3), 3.83 (3H, s, OCH3), 3.82 (s, OCH3), 3.13 (2H, t, J = 7.4 Hz, Hα), 2.94 (2H, t, J = 7.4 Hz, Hβ), 2.87 (2H, t, J = 7.2 Hz, SCH2CH2CH3), 1.63 (2H, h, J = 7.3 Hz, SCH2CH2CH3), and 1.02 (3H, t, J = 7.4 Hz, SCH2CH2CH3). 13C NMR (101 MHz, MeOD) δ: 153.4 (C2), 153.2 (C5), 126.1 (C1), 124.4 (C4), 115.3 (C6), 114.1 (C3), 57.1 (OCH3), 56.5 (OCH3), 40.9 (Cα), 35.2 (SCH2CH2CH3), 29.7 (Cβ), 23.5 (SCH2CH2CH3), and 13.7 (SCH2CH2CH3).
- 2-(4-(ethylthio)-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethan-1-aminium chloride (25T2-NBOMe). Yield: 87%. 1H NMR (400 MHz, MeOD) δ: 7.46 (1H, ddd, J = 8.3, 7.4, 1.7 Hz, H4′), 7.37 (1H, dd, J = 7.5, 1.6 Hz, H6′), 7.09 (1H, d, J = 8.3 Hz, H3′), 7.02 (1H, ddd, J = 7.5, 7.5, 0.9 Hz, H5′), 6.91 (1H, s, H6), 6.84 (1H, s, H3), 4.24 (2H, s, NCH2Ph), 3.88 (3H, s, OCH3), 3.81 (3H, s, OCH3), 3.80 (3H, s, OCH3), 3.26–3.18 (2H, m, Hα), 3.07–2.96 (m, Hβ), 2.91 (q, J = 7.4 Hz, SCH2CH3), and 1.27 (3H, t, J = 7.4 Hz, SCH2CH3). 13C NMR (101 MHz, MeOD) δ: 159.4 (C2′), 153.3 (C2), 153.1 (C5), 132.8 (C6′), 132.7 (C4′), 126.3 (C1), 124.0 (C1′), 122.1 (C5′), 120.3 (C4), 115.2 (C3′), 114.0 (C6), 112.2 (C3), 57.1 (OCH3), 56.6 (OCH3), 56.2 (OCH3), 48.2 (NCH2Ph), 48.1 (Cα), 28.3 (Cβ), 27.1 (SCH2CH3), and 14.6 (SCH2CH3).
- 2-(4-(isopropylthio)-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethan-1-aminium chloride (25T4-NBOMe). Yield: 37%. 1H NMR (400 MHz, MeOD) δ: 7.46 (1H, ddd, J = 8.3, 7.5, 1.7 Hz, H4′), 7.37 (1H, dd, J = 7.5, 1.7 Hz, H6′), 7.09 (1H, dd, J = 8.3, 1.0 Hz, H3′), 7.02 (1H, ddd, J = 7.5 Hz, 1.0 Hz,, H5′), 6.99 (1H, s, H6), 6.86 (1H, s, H3), 4.24 (2H, s, NCH2Ph), 3.89 (3H, s, OCH3), 3.81 (3H, s, OCH3), 3.79 (3H, s, OCH3), 3.55–3.45 (2H, m, SCH(CH3)2), 3.27–3.18 (2H, m, Hα), 3.07–2.98 (2H, m, Hβ), and 1.23 (6H, d, J = 6.7 Hz, SCH(CH3)2).
- 2-(2,5-dimethoxy-4-(propylthio)phenyl)-N-(2-methoxybenzyl)ethan-1-aminium chloride (25T7-NBOMe). Yield: 84%. 1H NMR (400 MHz, MeOD) δ: 7.50–7.41 (1H, m, 1H4′), 7.37 (1H, dd, J = 7.5, 1.5 Hz, H6′), 7.09 (1H, d, J = 8.3 Hz, H3′), 7.02 (1H, dd, J = 7.5, 7.5 Hz, H5′), 6.91 (1H, s, H6), 6.84 (1H, s, H3), 4.24 (2H, s, NCH2Ph), 3.88 (3H, s, OCH3), 3.81 (3H, s, OCH3), 3.80 (3H, s, OCH3), 3.28–3.17 (1H, m, Hα), 3.05–2.91 (1H, m, Hβ), 2.87 (2H, t, J = 7.2 Hz, SCH2CH2CH3), 1.63 (2H, h, J = 7.3 Hz, SCH2CH2CH3), and 1.03 (3H, t, J = 7.4 Hz, SCH2CH2CH3). 13C NMR (101 MHz, MeOD) δ: 159.4 (C2′), 153.4 (C2), 153.1 (C5), 132.8 (C6′), 132.7 (C4′), 124.0 (C1′), 122.1 (C5′), 120.3 (C4), 115.2 (C2), 114.1 (C6), 112.2 (C3), 57.1 (OCH3), 56.6 (OCH3), 56.2 (OCH3), 48.2 (NCH2Ph), 48.1 (Cα), 35.2 (SCH2CH2CH3), 28.3 (Cβ), 23.6 (SCH2CH2CH3), and 13.7 (SCH2CH2CH3).
2.2. Determination of the Chromatographic Hydrophobicity Index (CHI)
2.3. Chemicals and Materials for the In Vitro Studies
2.4. Isolation of Primary Rat Cortical Neurons
2.5. Cell Culture and Differentiation
2.6. Evaluation of the Phenethylamine Derivatives Cytotoxicity
2.6.1. Neutral Red (NR) Uptake Assay
2.6.2. MTT Reduction Assay
2.7. Evaluation of Mitochondrial Integrity through the JC-1 Dye
2.8. Quantification of the Intracellular Adenosine Triphosphate (ATP) Levels
2.9. Quantification of Intracellular Calcium Levels through the Fluo-4 AM Probe
2.10. Quantification of the Intracellular Levels of Reactive Species through the DCFH-DA Probe
2.11. Quantification of the Intracellular Total Glutathione (tGSH) Levels
2.12. Impact of Glutamate-Cysteine Ligase Inhibition on Drug-Induced Cytotoxicity
2.13. Impact of Monoamine Oxidases Inhibition on Drug-Induced Cytotoxicity
2.14. Assessment of Human Monoamine Oxidase (hMAO) Inhibitory Activity
2.15. Statistical Analysis
3. Results
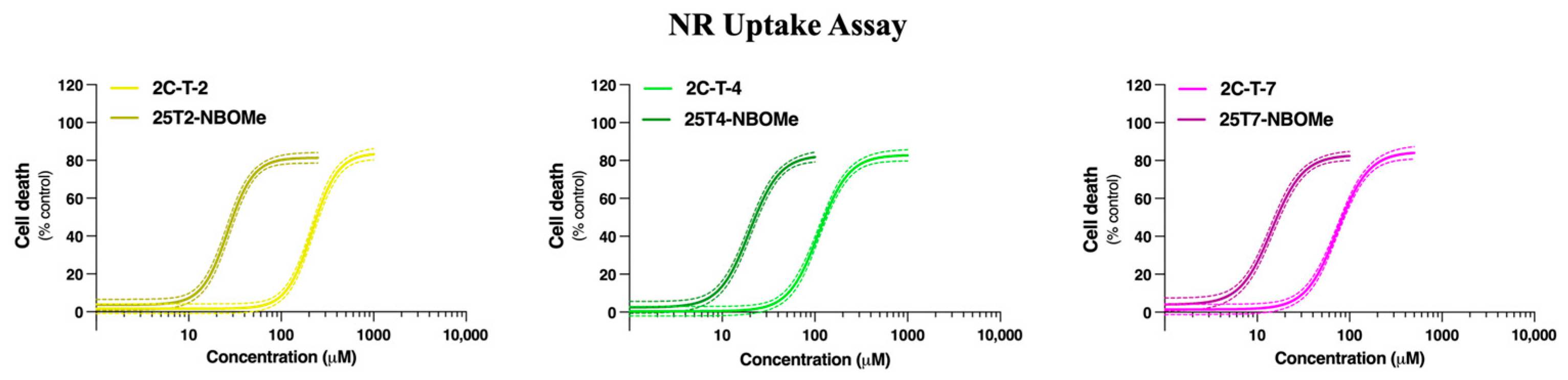
3.1. 2C-T-X and 25TX-NBOMe Drugs Induce Cell Death in a Concentration-Dependent Manner in Differentiated SH-SY5Y Cells and Primary Rat Cortical Neurons
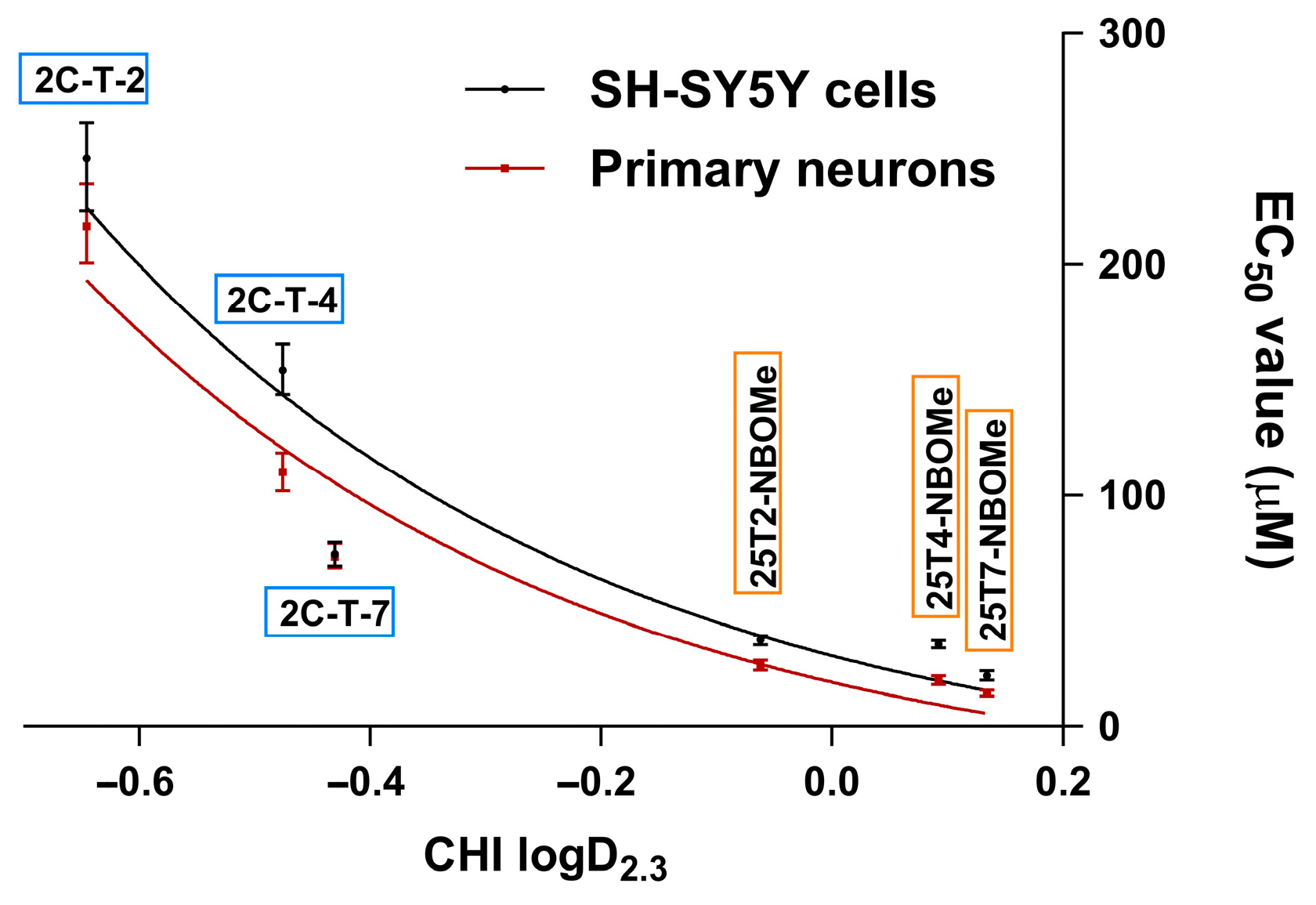
3.2. Lipophilicity of 2C-T-X and 25TX-NBOMe Drugs and Its Correlation with Their Cytotoxicity
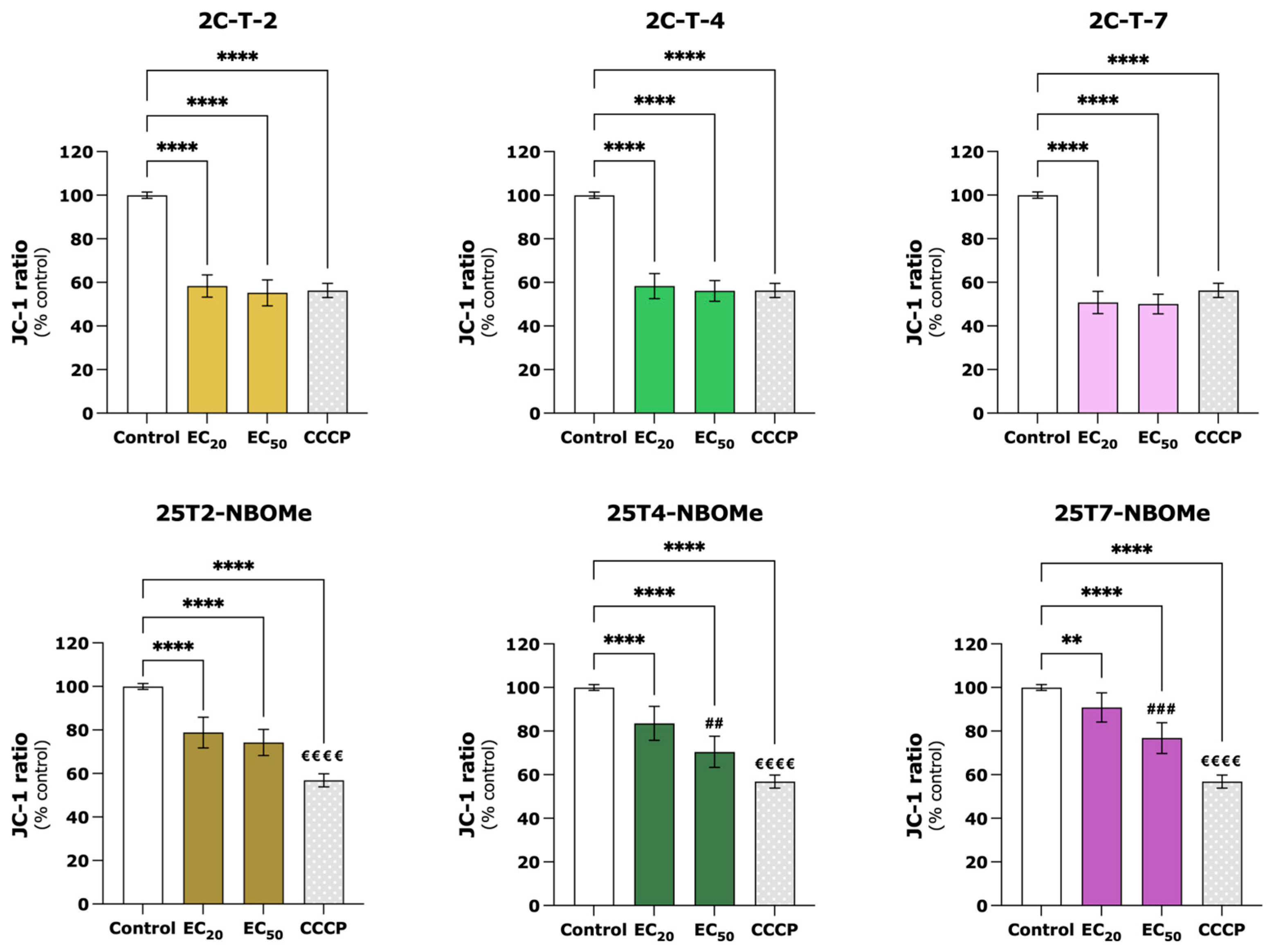
3.3. 2C-T-X and 25TX-NBOMe Drugs Significantly Induce Mitochondrial Membrane Depolarization in Differentiated SH-SY5Y Cells
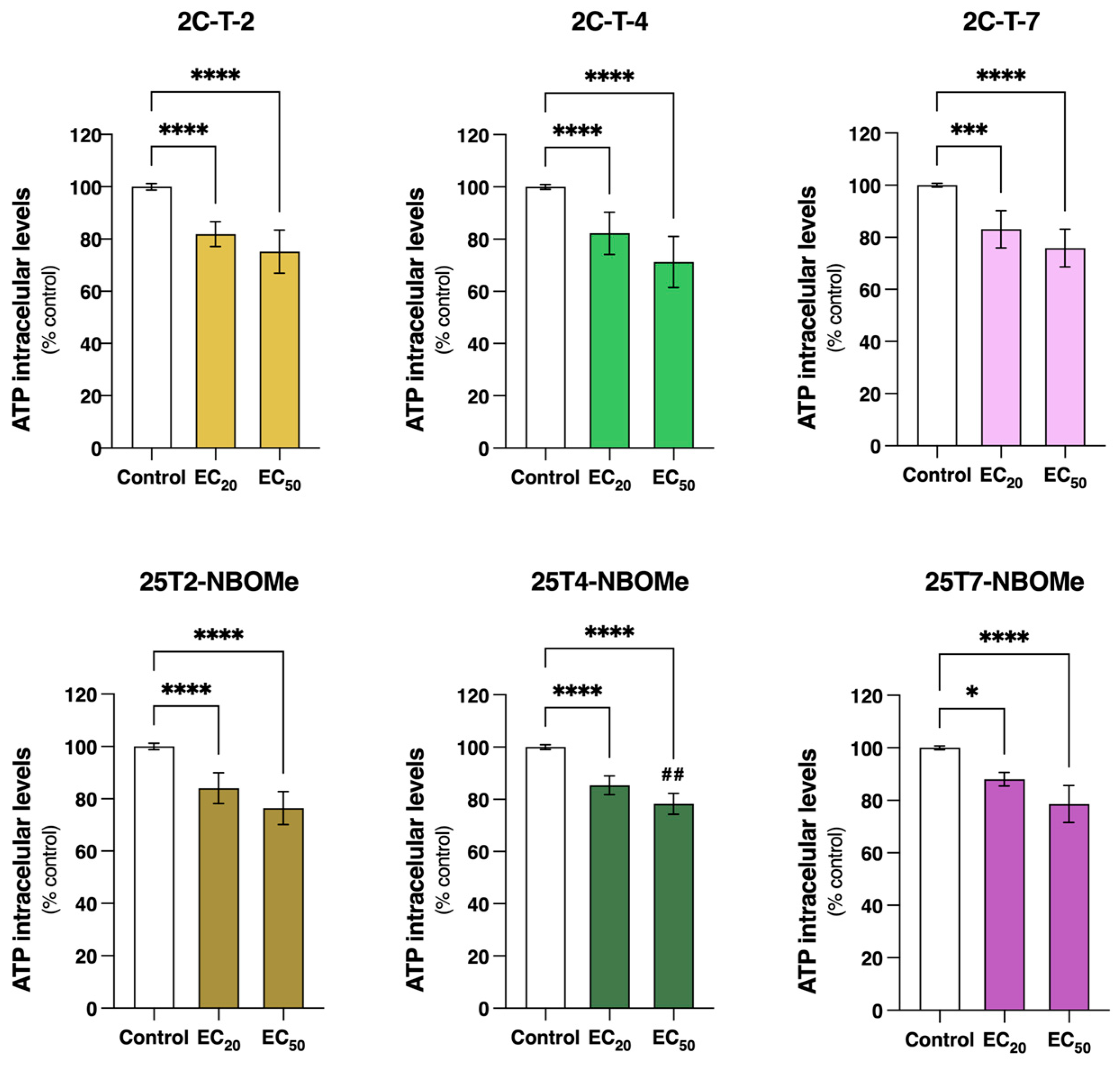
3.4. 2C-T-X and 25TX-NBOMe Drugs Significantly Reduce Intracellular ATP Levels in Differentiated SH-SY5Y Cells
3.5. 2C-T-7 and 25T7-NBOMe Significantly and “Immediately” Increase Intracellular Calcium Levels in a Concentration-Dependent Manner in Differentiated SH-SY5Y Cells
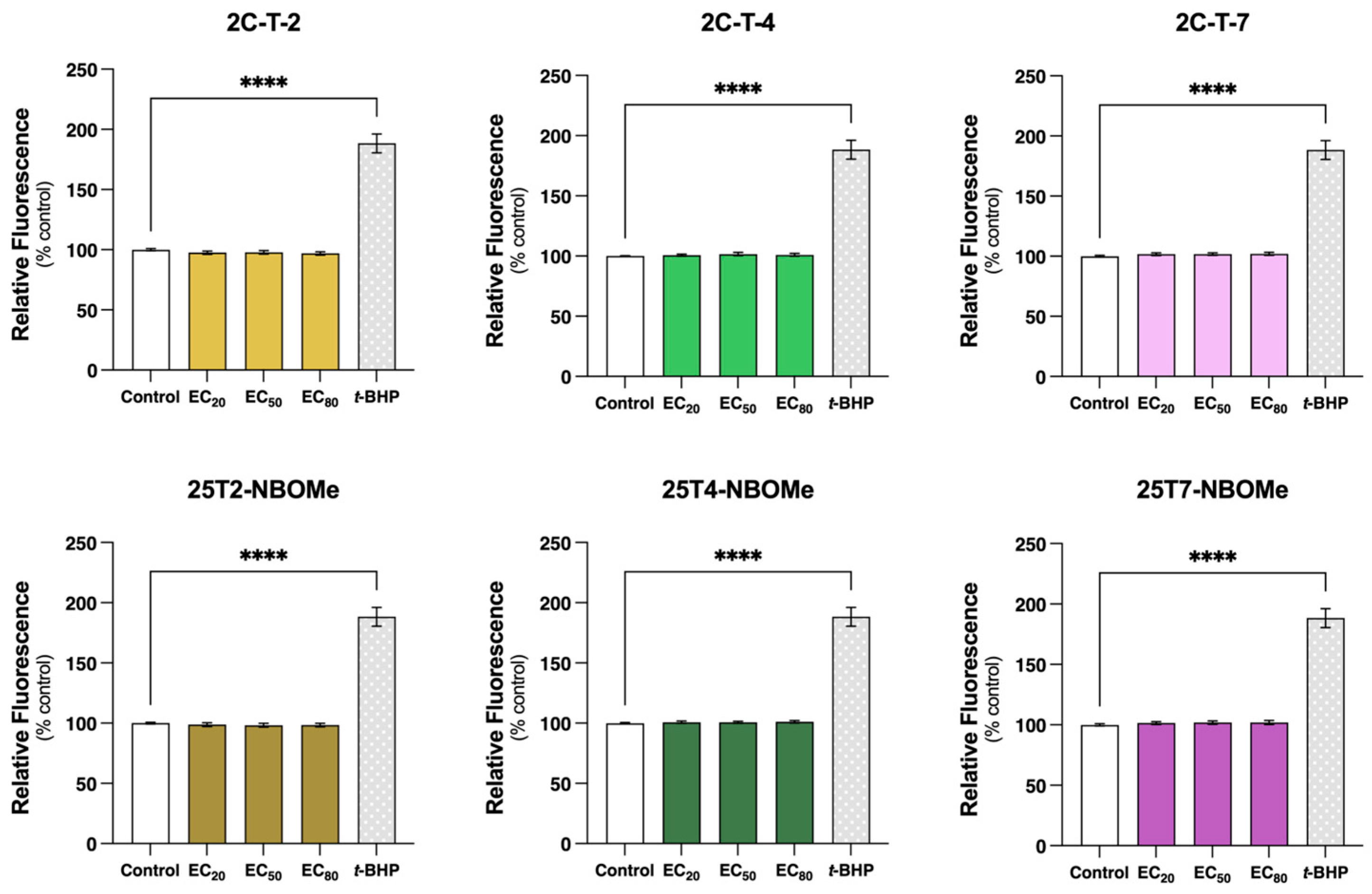
3.6. 2C-T-X and 25TX-NBOMe Drugs Do Not Change Reactive Species Intracellular Levels in SH-SY5Y Cells
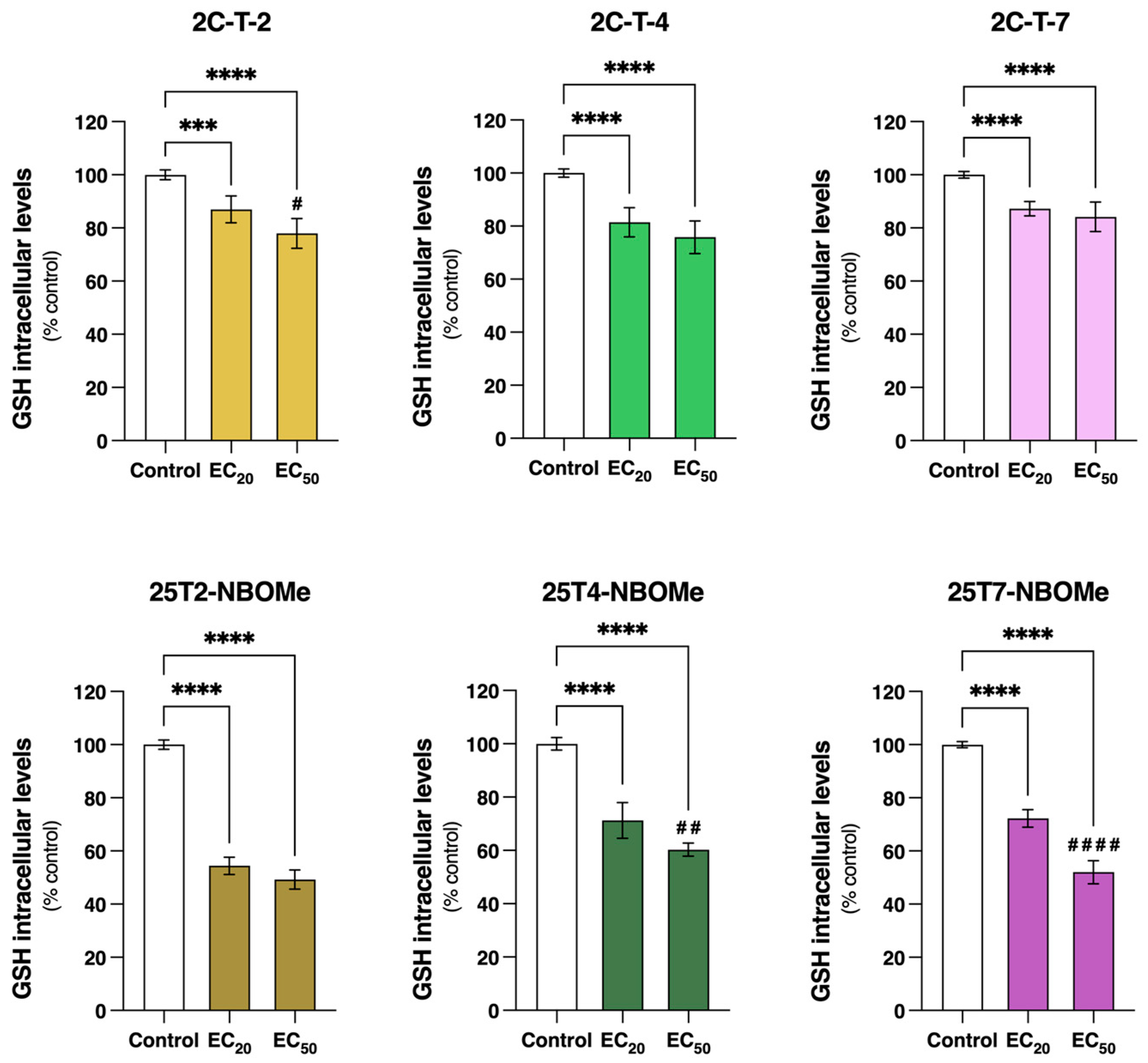
3.7. 2C-T-X and 25TX-NBOMe Drugs Significantly Deplete Intracellular Total Glutathione (tGSH) Levels in Differentiated SH-SY5Y Cells
3.8. Inhibition of Glutamate-Cysteine Ligase Does Not Change the Drugs-Induced Cytotoxicity in Differentiated SH-SY5Y Cells
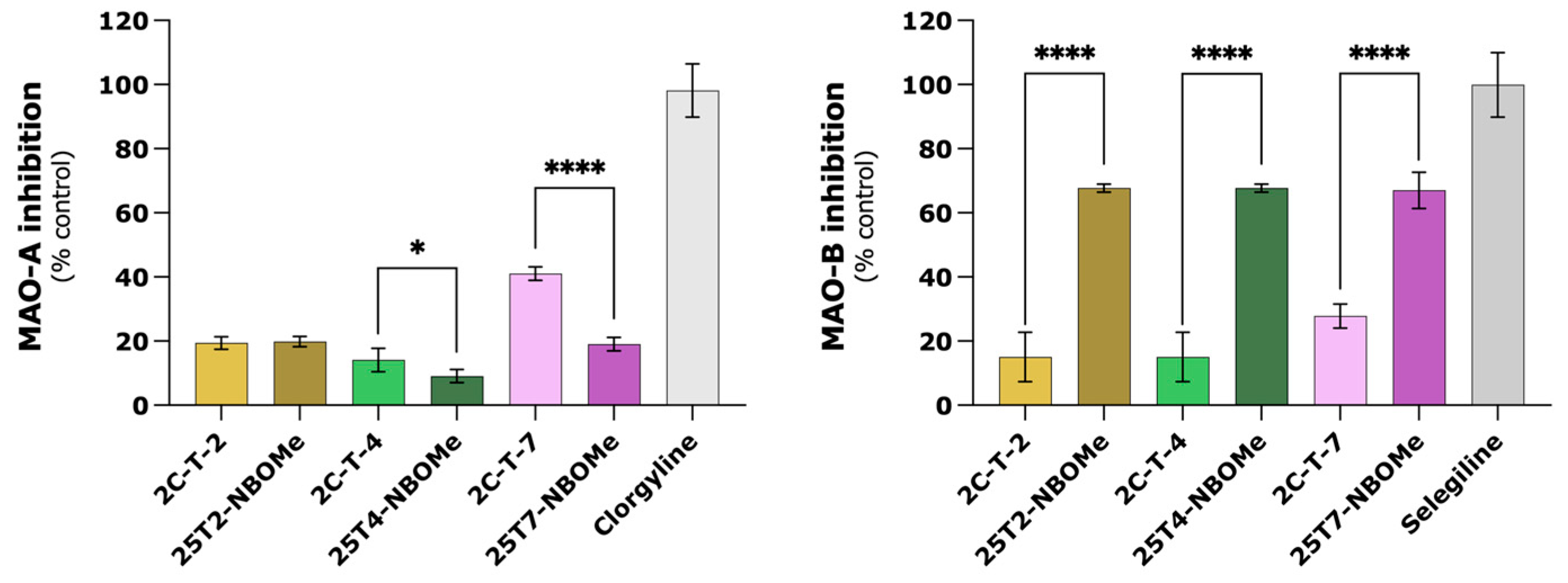
3.9. Metabolism via Monoamine Oxidases (MAO) Does Not Seem to Play a Significant Role in Drugs-Induced Cytotoxicity in Differentiated SH-SY5Y Cells
3.10. 25TX-NBOMe Drugs Seem to Have Inhibitory Activity on MAO-B
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelmendi, B.; Kaye, A.P.; Pittenger, C.; Kwan, A.C. Psychedelics. Curr. Biol. 2022, 32, R63–R67. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime. What Are NPS? United Nations Office on Drugs and Crime: Vienna, Austria, 2022. [Google Scholar]
- Shulgin, A.T.; Shulgin, A. PIHKAL: A Chemical Love Story; Transform Press: Berkeley, CA, USA, 1991; Volume 963009605. [Google Scholar]
- Heim, R. Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur: Entwicklung eines neuen Struktur-Wirkungskonzepts. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2004. [Google Scholar]
- Nichols, D.E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. [Google Scholar] [CrossRef] [PubMed]
- Rickli, A.; Luethi, D.; Reinisch, J.; Buchy, D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology 2015, 99, 546–553. [Google Scholar] [CrossRef] [PubMed]
- McClure-Begley, T.D.; Roth, B.L. The promises and perils of psychedelic pharmacology for psychiatry. Nat. Rev. Drug Discov. 2022, 21, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Saeger, H.N.; Olson, D.E. Psychedelic-inspired approaches for treating neurodegenerative disorders. J. Neurochem. 2022, 162, 109–127. [Google Scholar] [CrossRef]
- Miyajima, M.; Matsumoto, T.; Ito, S. 2C-T-4 intoxication: Acute psychosis caused by a designer drug. Psychiatry Clin. Neurosci. 2008, 62, 243. [Google Scholar] [CrossRef]
- Hermanns-Clausen, M.; Angerer, V.; Kithinji, J.; Grumann, C.; Auwärter, V. Bad trip due to 25I-NBOMe: A case report from the EU project SPICE II plus. Clin. Toxicol. 2017, 55, 922–924. [Google Scholar] [CrossRef]
- Stoller, A.; Dolder, P.C.; Bodmer, M.; Hammann, F.; Rentsch, K.M.; Exadaktylos, A.K.; Liechti, M.E.; Liakoni, E. Mistaking 2C-P for 2C-B: What a Difference a Letter Makes. J. Anal. Toxicol. 2017, 41, 77–79. [Google Scholar] [CrossRef]
- Curtis, B.; Kemp, P.; Harty, L.; Choi, C.; Christensen, D. Postmortem identification and quantitation of 2,5-dimethoxy-4-n-propylthiophenethylamine using GC-MSD and GC-NPD. J. Anal. Toxicol. 2003, 27, 493–498. [Google Scholar] [CrossRef]
- Gee, P.; Schep, L.J.; Jensen, B.P.; Moore, G.; Barrington, S. Case series: Toxicity from 25B-NBOMe—A cluster of N-bomb cases. Clin. Toxicol. 2016, 54, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kueppers, V.B.; Cooke, C.T. 25I-NBOMe related death in Australia: A case report. Forensic Sci. Int. 2015, 249, e15–e18. [Google Scholar] [CrossRef] [PubMed]
- Valko, K.L. Biomimetic chromatography-A novel application of the chromatographic principles. Anal. Sci. Adv. 2022, 3, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Benfeito, S.; Fernandes, C.; Chavarria, D.; Barreiro, S.; Cagide, F.; Sequeira, L.; Teixeira, J.; Silva, R.; Remiao, F.; Oliveira, P.J.; et al. Modulating Cytotoxicity with Lego-like Chemistry: Upgrading Mitochondriotropic Antioxidants with Prototypical Cationic Carrier Bricks. J. Med. Chem. 2023, 66, 1835–1851. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Huertas, C.; Rico, B. CREB-Dependent Regulation of GAD65 Transcription by BDNF/TrkB in Cortical Interneurons. Cereb. Cortex 2011, 21, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, D.J.; Capela, J.P.; Silva, R.; Vilas-Boas, V.; Ferreira, L.M.; Branco, P.S.; Fernandes, E.; de Lourdes Bastos, M.; Carvalho, F. The mixture of “ecstasy” and its metabolites is toxic to human SH-SY5Y differentiated cells at in vivo relevant concentrations. Arch. Toxicol. 2014, 88, 455–473. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Gil-Martins, E.; Cagide, F.; da Fonseca, C.; Benfeito, S.; Fernandes, C.; Chavarria, D.; Remião, F.; Silva, R.; Borges, F. Unraveling the In Vitro Toxicity Profile of Psychedelic 2C Phenethylamines and Their N-Benzylphenethylamine (NBOMe) Analogues. Pharmaceuticals 2023, 16, 1158. [Google Scholar] [CrossRef] [PubMed]
- Borenfreund, E.; Babich, H.; Martin-Alguacil, N. Comparisons of two in vitro cytotoxicity assays-The neutral red (NR) and tetrazolium MTT tests. Toxicol. Vitr. 1988, 2, 1–6. [Google Scholar] [CrossRef]
- Cossarizza, A.; Baccarani-Contri, M.; Kalashnikova, G.; Franceschi, C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem. Biophys. Res. Commun. 1993, 197, 40–45. [Google Scholar] [CrossRef]
- Dunn, J.; Grider, M.H. Physiology, Adenosine Triphosphate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Green, A.; McElroy, W.D. Function of adenosine triphosphate in the activation of luciferin. Arch. Biochem. Biophys. 1956, 64, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [PubMed]
- Gee, K.R.; Brown, K.A.; Chen, W.N.; Bishop-Stewart, J.; Gray, D.; Johnson, I. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium 2000, 27, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.J. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, MS, USA, 2015. [Google Scholar]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta BBA-Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Drew, R.; Miners, J.O. The effects of buthionine sulphoximine (BSO) on glutathione depletion and xenobiotic biotransformation. Biochem. Pharmacol. 1984, 33, 2989–2994. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.E.; Binda, C. Monoamine Oxidases. In Membrane Protein Complexes: Structure and Function; Harris, J.R., Boekema, E.J., Eds.; Springer: Singapore, 2018; pp. 117–139. [Google Scholar]
- Hagenow, S.; Stasiak, A.; Ramsay, R.R.; Stark, H. Ciproxifan, a histamine H(3) receptor antagonist, reversibly inhibits monoamine oxidase A and B. Sci. Rep. 2017, 7, 40541. [Google Scholar] [CrossRef]
- Chavarria, D.; Cagide, F.; Pinto, M.; Gomes, L.R.; Low, J.N.; Borges, F. Development of piperic acid-based monoamine oxidase inhibitors: Synthesis, structural characterization and biological evaluation. J. Mol. Struct. 2019, 1182, 298–307. [Google Scholar] [CrossRef]
- King, L.A.; Kicman, A.T. A brief history of ‘new psychoactive substances’. Drug Test. Anal. 2011, 3, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E.; Fantegrossi, W.E. Emerging designer drugs. In The Effects of Drug Abuse on the human Nervous System; Elsevier: Amsterdam, The Netherlands, 2014; pp. 575–596. [Google Scholar]
- Drug Enforcement Administration. Controlled Substances. Available online: https://www.deadiversion.usdoj.gov/schedules/orangebook/e_cs_sched.pdf (accessed on 16 May 2024).
- Lo Faro, A.F.; Berardinelli, D.; Cassano, T.; Dendramis, G.; Montanari, E.; Montana, A.; Berretta, P.; Zaami, S.; Busardo, F.P.; Huestis, M.A. New Psychoactive Substances Intoxications and Fatalities during the COVID-19 Epidemic. Biology 2023, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Van Buskirk, J.; Naicker, S.; Roxburgh, A.; Bruno, R.; Burns, L. Who sells what? Country specific differences in substance availability on the Agora cryptomarket. Int. J. Drug Policy 2016, 35, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, M.; Miyazaki, I.; Funada, M. The neurotoxicity of psychoactive phenethylamines “2C series” in cultured monoaminergic neuronal cell lines. Forensic Toxicol. 2020, 38, 394–408. [Google Scholar] [CrossRef]
- Chan, S.; Wu, J.; Lee, B. Fatalities related to new psychoactive substances in Singapore-A case series. Forensic Sci. Int. 2019, 304, 109892. [Google Scholar] [CrossRef] [PubMed]
- Palamar, J.J.; Le, A. Prevalence of self-reported adverse effects associated with drug use among nightclub and festival attendees, 2019–2022. Drug Alcohol Depend. Rep. 2023, 7, 100149. [Google Scholar] [CrossRef] [PubMed]
- Rugarli, E.I.; Langer, T. Mitochondrial quality control: A matter of life and death for neurons. EMBO J. 2012, 31, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Trigo, D.; Avelar, C.; Fernandes, M.; Sa, J.; da Cruz, E.S.O. Mitochondria, energy, and metabolism in neuronal health and disease. FEBS Lett. 2022, 596, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Soares, J.; Costa, V.M.; Gaspar, H.; Santos, S.; Bastos, M.L.; Carvalho, F.; Capela, J.P. Adverse outcome pathways induced by 3,4-dimethylmethcathinone and 4-methylmethcathinone in differentiated human SH-SY5Y neuronal cells. Arch. Toxicol. 2020, 94, 2481–2503. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.J.; Bastos, M.L.; Fernandes, E.; Carvalho, F.; Guedes de Pinho, P.; Carvalho, M. Neurotoxicity of beta-Keto Amphetamines: Deathly Mechanisms Elicited by Methylone and MDPV in Human Dopaminergic SH-SY5Y Cells. ACS Chem. Neurosci. 2017, 8, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Cali, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 2018, 69, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J. The role of calcium in apoptosis. Biometals 1998, 11, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Barbosa, D.J.; Serrat, R.; Mirra, S.; Quevedo, M.; de Barreda, E.G.; Àvila, J.; Ferreira, L.M.; Branco, P.S.; Fernandes, E.; Bastos, M.L.; et al. The Mixture of “Ecstasy” and Its Metabolites Impairs Mitochondrial Fusion/Fission Equilibrium and Trafficking in Hippocampal Neurons, at In Vivo Relevant Concentrations. Toxicol. Sci. 2014, 139, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, V.; Gasperini, S.; Hrelia, P.; Tirri, M.; Marti, M.; Lenzi, M. Novel Psychoactive Phenethylamines: Impact on Genetic Material. Int. J. Mol. Sci. 2020, 21, 9616. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Arbo, M.D.; Silva, R.; Barbosa, D.J.; Dias da Silva, D.; Silva, S.P.; Teixeira, J.P.; Bastos, M.L.; Carmo, H. In vitro neurotoxicity evaluation of piperazine designer drugs in differentiated human neuroblastoma SH-SY5Y cells. J. Appl. Toxicol. 2016, 36, 121–130. [Google Scholar] [CrossRef]
- Theobald, D.S.; Maurer, H.H. Identification of monoamine oxidase and cytochrome P450 isoenzymes involved in the deamination of phenethylamine-derived designer drugs (2C-series). Biochem. Pharmacol. 2007, 73, 287–297. [Google Scholar] [CrossRef]



| SH-SY5Y Cells | Primary Rat Cortical Cultures | ||
|---|---|---|---|
| NR EC50 Values (μM) | MTT EC50 Values (μM) | NR EC50 Values (μM) | |
| 2C-T-2 | 245.9 (223.2 to 261.3) | 305.5 (294.5 to 317.3) | 216.4 (200.6 to 234.9) |
| 25T2-NBOMe | 37.4 **** (35.4 to 39.5) | 46.8 **** (45.2 to 48.4) | 26.5 **** (24.4 to 28.7) |
| 2C-T-4 | 154.1 (143.7 to 165.5) | 190.7 (182.9 to 199.5) | 110.2 (102.1 to 118.5) |
| 25T4-NBOMe | 35.7 **** (34.0 to 37.2) | 36.0 **** (34.5 to 37.6) | 19.9 **** (18.2 to 21.8) |
| 2C-T-7 | 74.5 (69.4 to 79.8) | 92.3 (75.3 to 100.8) | 73.6 (68.6 to 79.1) |
| 25T7-NBOMe | 21.8 **** (21.4 to 22.3) | 23.3 **** (22.7 to 23.9) | 14.4 **** (13.1 to 15.8) |
| SH-SY5Y Cells NR EC Values (μM) | |||
|---|---|---|---|
| EC20 | EC50 | EC80 | |
| 2C-T-2 | 123.2 (107.1 to 138.8) | 245.9 (223.2 to 261.3) | 491.0 (413.7 to 514.4) |
| 25T2-NBOMe | 22.2 **** (20.3 to 24.4) | 37.4 **** (35.4 to 39.5) | 62.9 **** (58.8 to 67.7) |
| 2C-T-4 | 81.5 (79.9 to 92.1) | 154.1 (143.7 to 165.5) | 291.4 (255.9 to 342.3) |
| 25T4-NBOMe | 22.1 **** (20.1 to 23.8) | 35.7 **** (34.0 to 37.2) | 57.8 **** (54.4 to 59.6) |
| 2C-T-7 | 39.5 (34.4 to 44.4) | 74.5 (69.4 to 79.8) | 140.5 (125.4 to 161.2) |
| 25T7-NBOMe | 16.4 **** (15.6 to 17.2) | 21.8 **** (21.4 to 22.3) | 28.9 **** (27.5 to 30.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Martins, E.; Cagide-Fagín, F.; Martins, D.; Borer, A.; Barbosa, D.J.; Fernandes, C.; Chavarria, D.; Remião, F.; Borges, F.; Silva, R. Mechanistic Insights into the Neurotoxicity of 2,5-Dimethoxyphenethylamines (2C) and Corresponding N-(2-methoxybenzyl)phenethylamine (NBOMe) Drugs. J. Xenobiot. 2024, 14, 772-797. https://doi.org/10.3390/jox14020044
Gil-Martins E, Cagide-Fagín F, Martins D, Borer A, Barbosa DJ, Fernandes C, Chavarria D, Remião F, Borges F, Silva R. Mechanistic Insights into the Neurotoxicity of 2,5-Dimethoxyphenethylamines (2C) and Corresponding N-(2-methoxybenzyl)phenethylamine (NBOMe) Drugs. Journal of Xenobiotics. 2024; 14(2):772-797. https://doi.org/10.3390/jox14020044
Chicago/Turabian StyleGil-Martins, Eva, Fernando Cagide-Fagín, Daniel Martins, Ana Borer, Daniel José Barbosa, Carlos Fernandes, Daniel Chavarria, Fernando Remião, Fernanda Borges, and Renata Silva. 2024. "Mechanistic Insights into the Neurotoxicity of 2,5-Dimethoxyphenethylamines (2C) and Corresponding N-(2-methoxybenzyl)phenethylamine (NBOMe) Drugs" Journal of Xenobiotics 14, no. 2: 772-797. https://doi.org/10.3390/jox14020044
APA StyleGil-Martins, E., Cagide-Fagín, F., Martins, D., Borer, A., Barbosa, D. J., Fernandes, C., Chavarria, D., Remião, F., Borges, F., & Silva, R. (2024). Mechanistic Insights into the Neurotoxicity of 2,5-Dimethoxyphenethylamines (2C) and Corresponding N-(2-methoxybenzyl)phenethylamine (NBOMe) Drugs. Journal of Xenobiotics, 14(2), 772-797. https://doi.org/10.3390/jox14020044












