Toxic Effects of Carbaryl Exposure on Juvenile Asian Seabass (Lates calcarifer)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Preparation
2.2. Liver Enzyme Activity Determination and Serum Biochemical Index Analysis
2.3. Histological Analysis
2.4. Validation of RNA-Seq Results by Quantitative Real-Time PCR (qRT-PCR)
2.5. Calculations and Statistical Analysis
3. Results
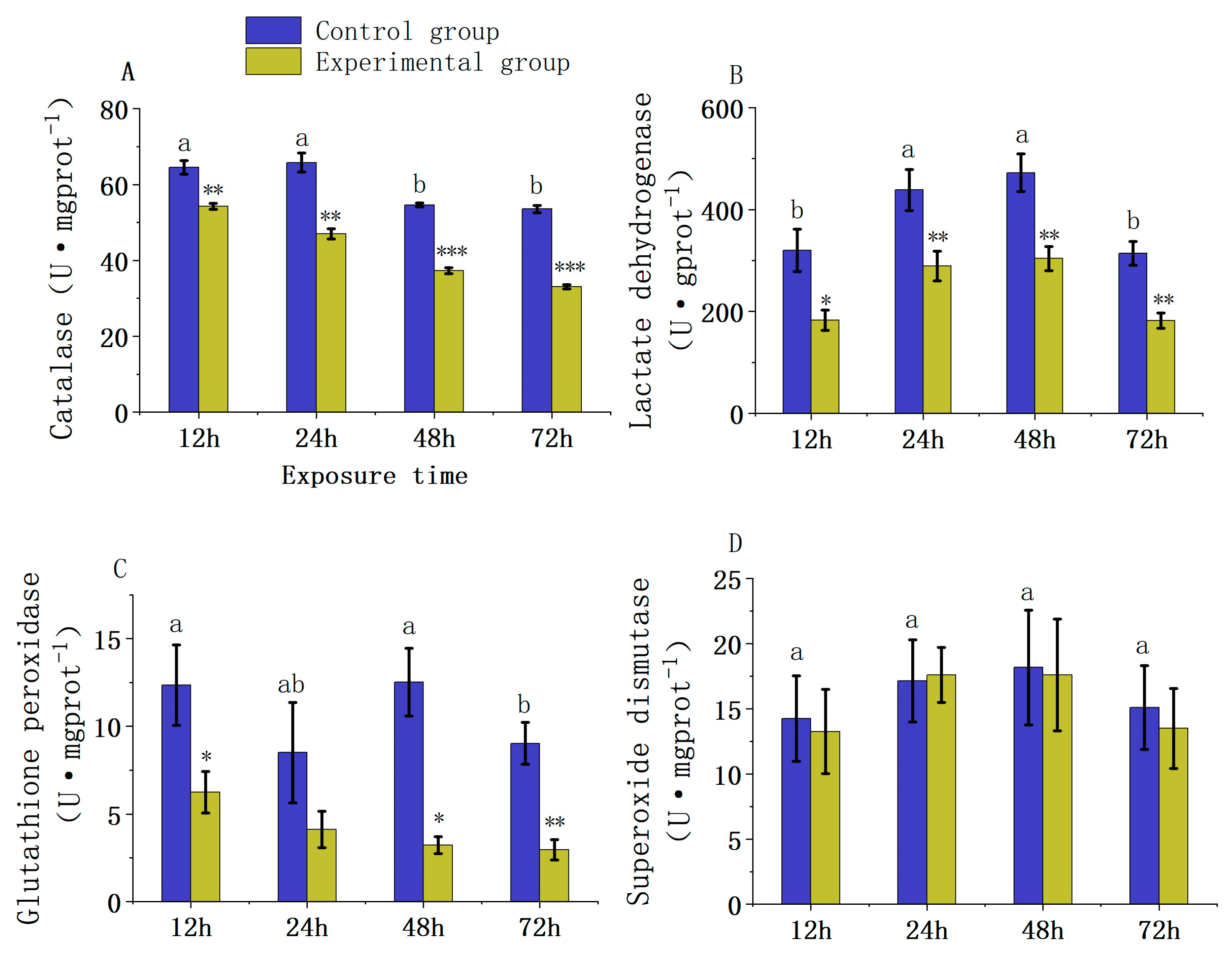
3.1. The Impact of Carbaryl Exposure on the Liver of L. calcarifer
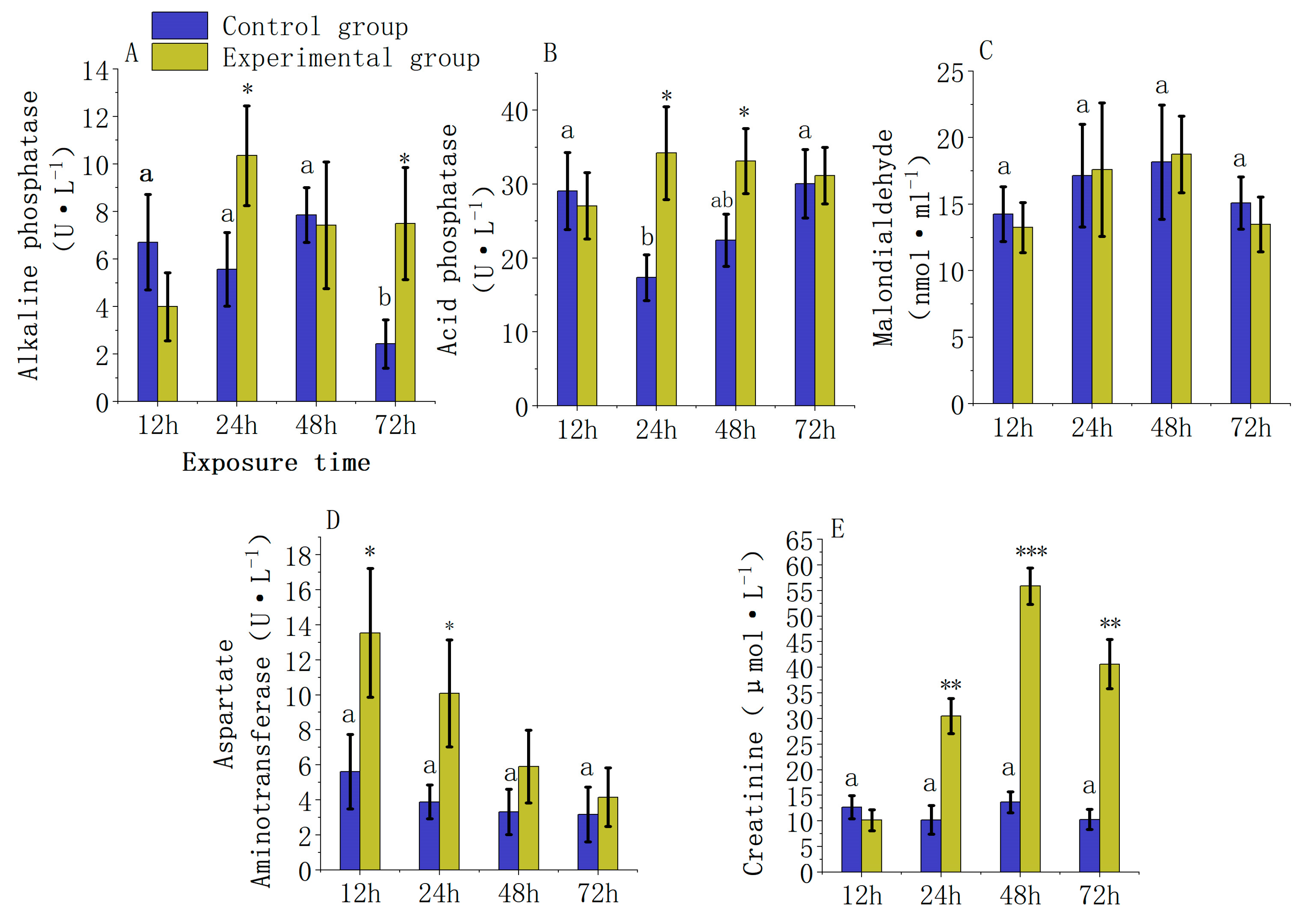
3.2. The Effects of Carbaryl Exposure on Serum Biochemical Indices in L. calcarifer
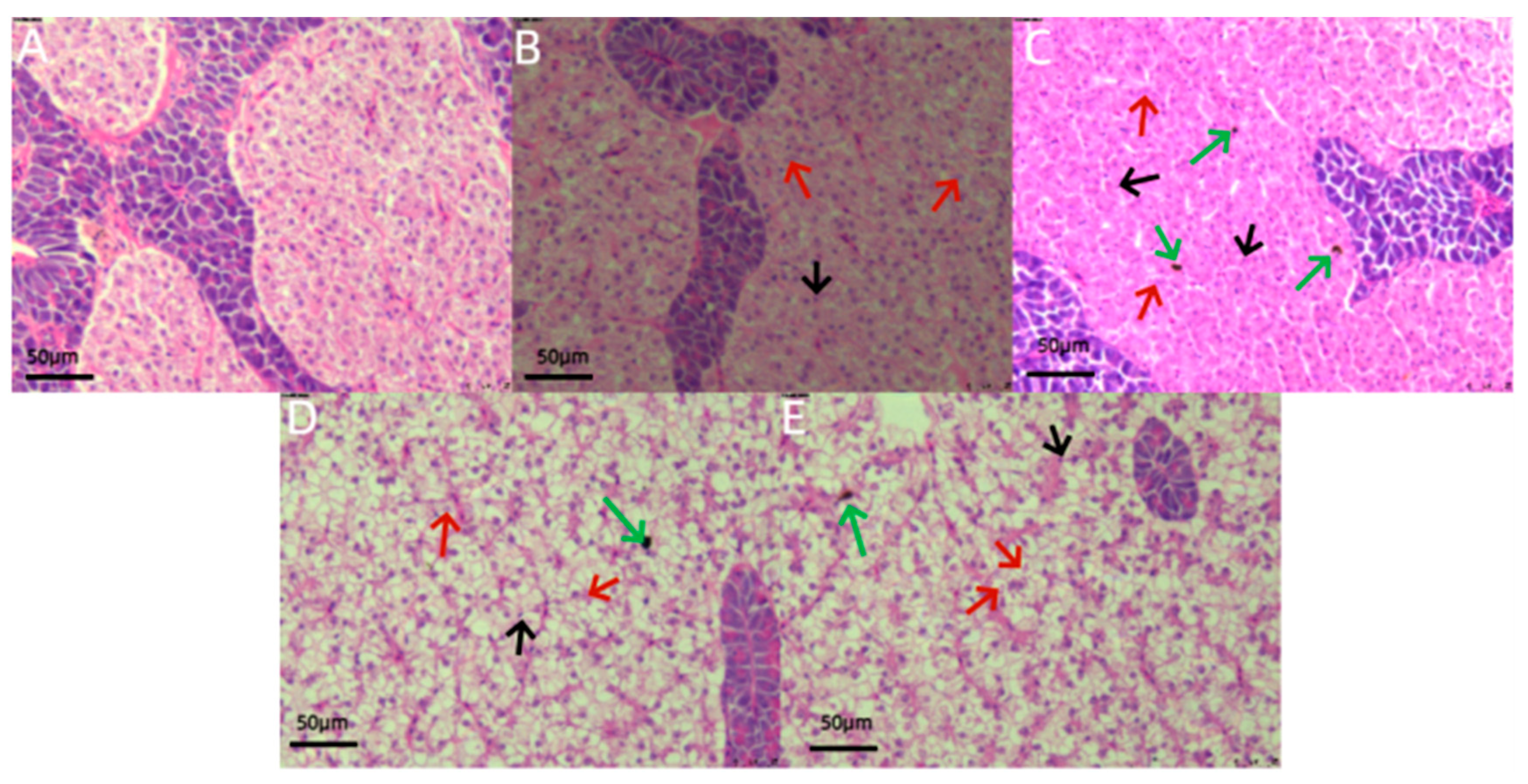
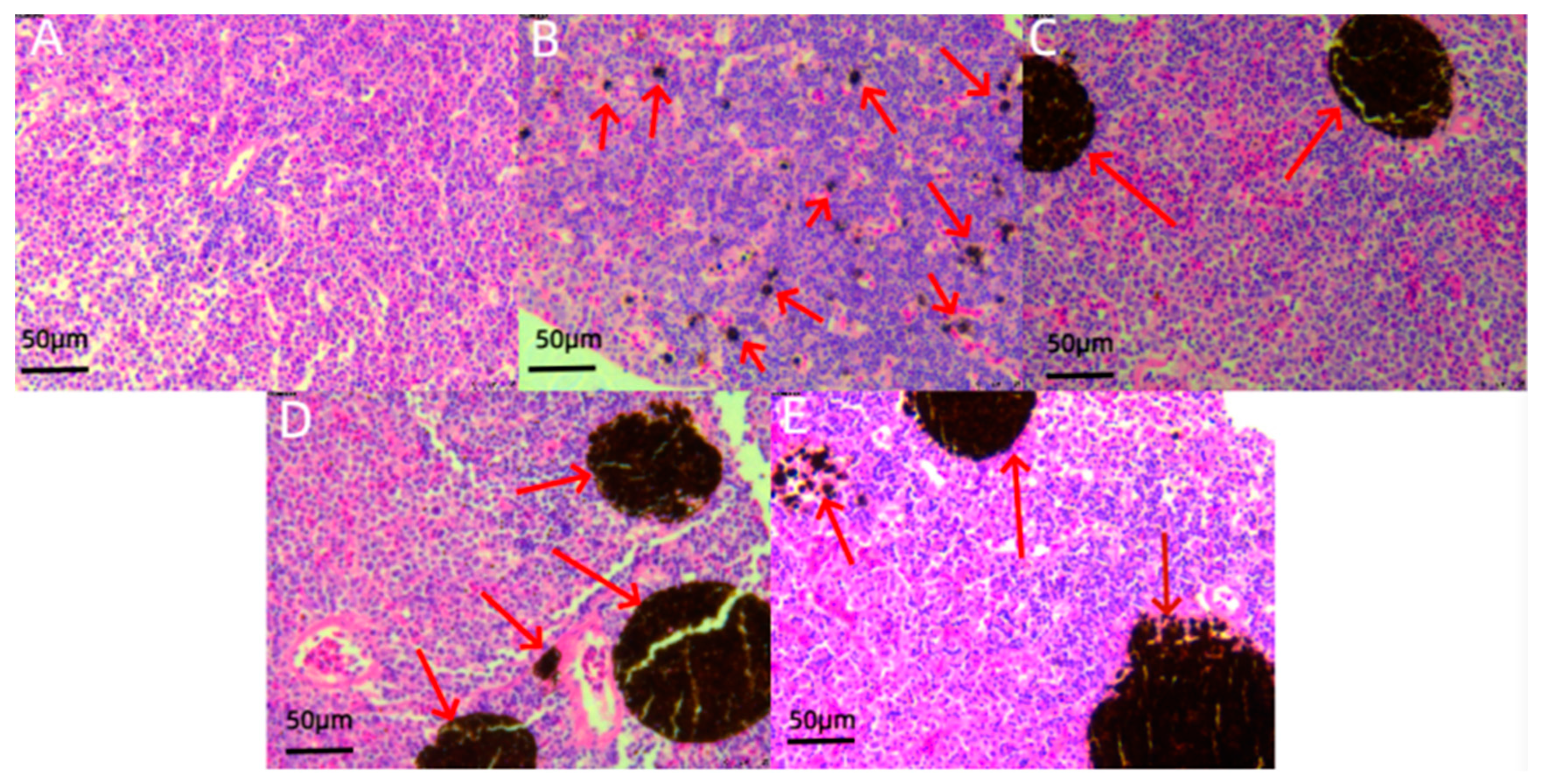
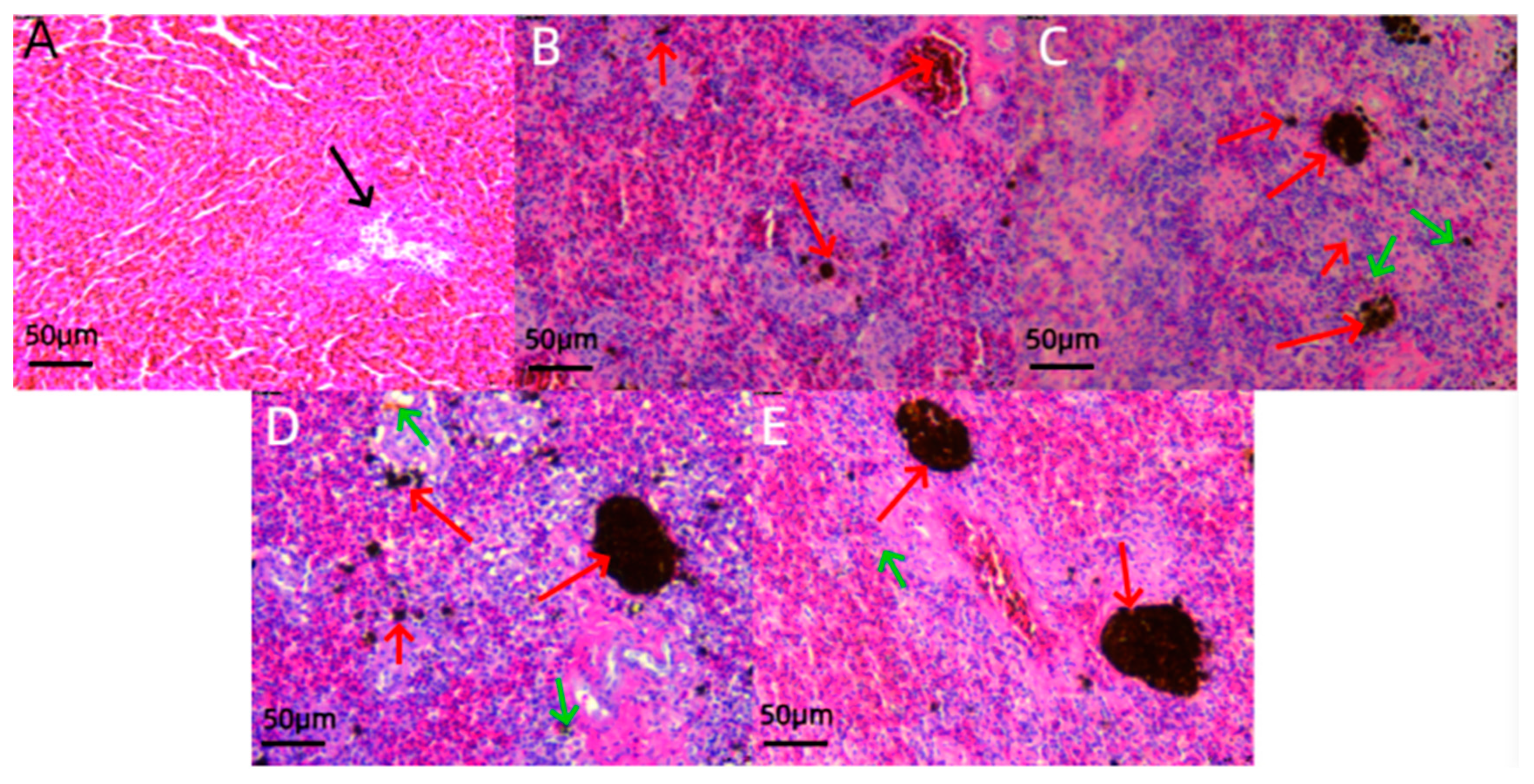
3.3. Histological Analysis
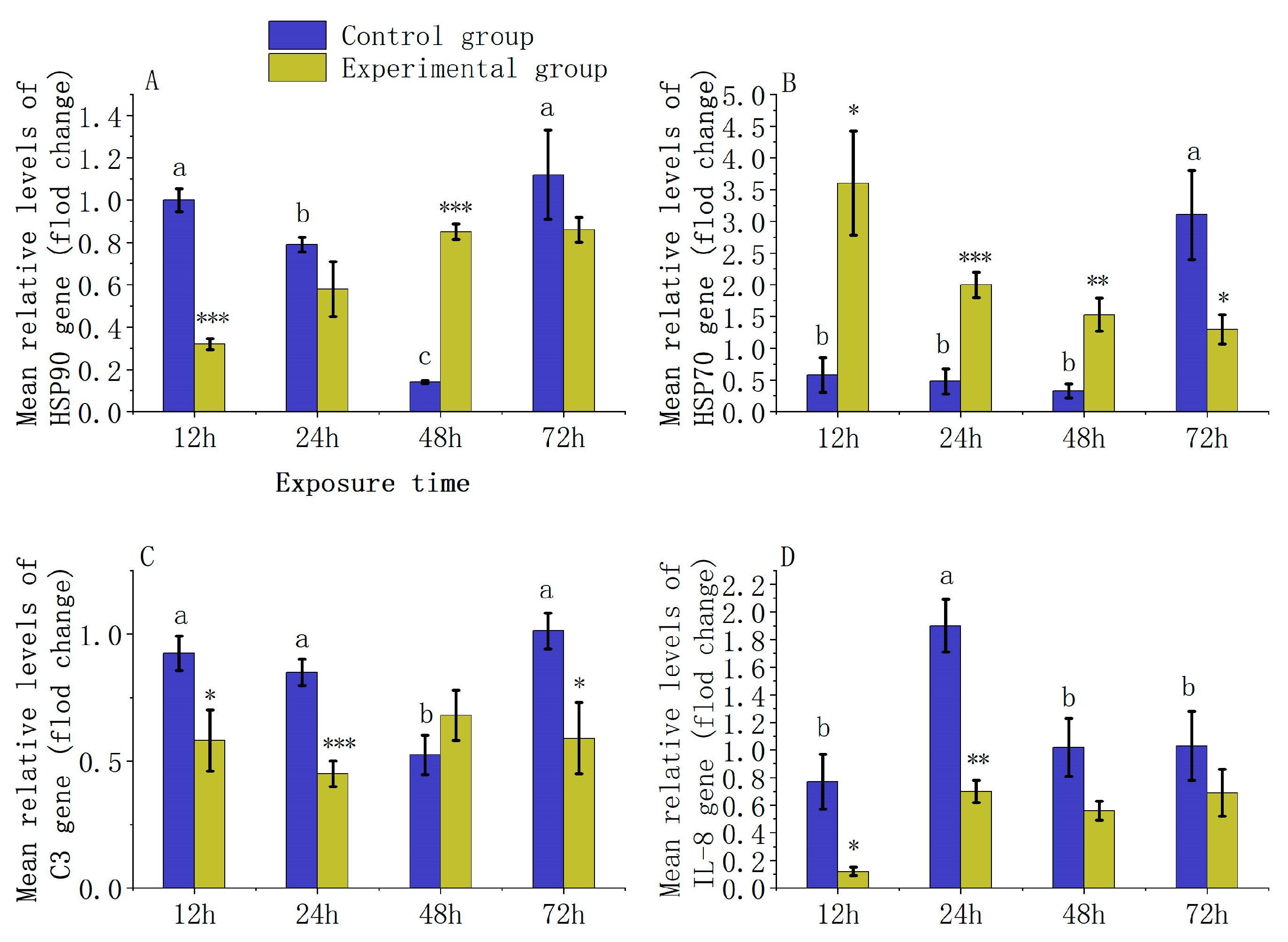
3.4. Analysis of Immune Gene Expression Levels
4. Discussion
4.1. Effect of Chemical Pesticide Exposure on Liver Antioxidant Enzyme Activity in Fish
4.2. Effect of Chemical Pesticide Exposure on Serum Biochemical Indexes in Fish
4.3. Effects of Chemical Pesticide Exposure on Organs and Tissues
4.4. Changes in the Expression of Immune Genes in Fish by Chemical Pesticides
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Ethics Statement
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yue, G.H.; Zhu, Z.Y.; Lo, L.C.; Wang, C.M.; Lin, G.; Feng, F.; Pang, H.Y.; Li, J.; Gong, P.; Liu, H.M. Genetic Variation and Population Structure of Asian Seabass (Lates calcarifer) in the Asia-Pacific Region. Aquaculture 2009, 293, 22–28. [Google Scholar] [CrossRef]
- Ye, B.; Wan, Z.; Wang, L.; Pang, H.; Wen, Y.; Liu, H.; Liang, B.; Lim, H.S.; Jiang, J.; Yue, G. Heritability of Growth Traits in the Asian Seabass (Lates calcarifer). Aquac. Fish. 2017, 2, 112–118. [Google Scholar] [CrossRef]
- Gunasekara, A.S.; Rubin, A.L.; Goh, K.S.; Spurlock, F.C.; Tjeerdema, R.S. Environmental Fate and Toxicology of Carbaryl. Rev. Environ. Contam. Toxicol. 2008, 196, 95–121. [Google Scholar] [PubMed]
- Mount, M.E.; Oehme, F.W. Carbaryl: A Literature Review. Residue Reviews: Residues of Pesticides and Other Contaminants in the Total Environment; Springer: Berlin/Heidelberg, Germany, 1981; pp. 1–64. [Google Scholar]
- Xie, H.; Wang, X.; Chen, J.; Li, X.; Jia, G.; Zou, Y.; Zhang, Y.; Cui, Y. Occurrence, Distribution and Ecological Risks of Antibiotics and Pesticides in Coastal Waters around Liaodong Peninsula, China. Sci. Total Environ. 2019, 656, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Patiri, G. Heavy Metals and Pesticide Residues as Quality Determinants for Sustainable Management of Prawns along the Indian Coastline of Tanzani. Master’s Thesis, NM-AIST, Arusha, Tanzania, 2020. [Google Scholar]
- Bruslé, J.; Gonzàlez i Anadon, G. The Structure and Function of Fish Liver. In Fish Morphology; Routledge: London, UK, 2017; pp. 77–93. [Google Scholar]
- Triebskorn, R.; Köhler, H.-R.; Flemming, J.; Braunbeck, T.; Negele, R.-D.; Rahmann, H. Evaluation of Bis (Tri-N-Butyltin) Oxide (Tbto) Neurotoxicity in Rainbow Trout (Oncorhynchus mykiss). I. Behaviour, Weight Increase, and Tin Content. Aquat. Toxicol. 1994, 30, 189–197. [Google Scholar] [CrossRef]
- Triebskorn, R.; Köhler, H.-R.; Honnen, W.; Schramm, M.; Adams, S.M.; Müller, E.F. Induction of Heat Shock Proteins, Changes in Liver Ultrastructure, and Alterations of Fish Behavior: Are These Biomarkers Related and Are They Useful to Reflect the State of Pollution in the Field? J. Aquat. Ecosyst. Stress Recovery 1997, 6, 57–73. [Google Scholar] [CrossRef]
- Laurén, D.J.; Wails, D. Liver Structural Alterations Accompanying Chronic Toxicity in Fishes: Potential Biomarkers of Exposure. In Biomarkers of Environmental Contamination; CRC Press: Boca Raton, FL, USA, 2018; pp. 17–57. [Google Scholar]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular Biomarkers of Oxidative Stress in Aquatic Organisms in Relation to Toxic Environmental Pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef]
- Matos, P.; Fontaı, A.; Peixoto, F.; Carrola, J.; Rocha, E. Biochemical and Histological Hepatic Changes of Nile Tilapia Oreochromis Niloticus Exposed to Carbaryl. Pestic. Biochem. Physiol. 2007, 89, 73–80. [Google Scholar] [CrossRef]
- Boran, H.; Altinok, I.; Capkin, E. Histopathological Changes Induced by Maneb and Carbaryl on Some Tissues of Rainbow Trout, Oncorhynchus mykiss. Tissue Cell 2010, 42, 158–164. [Google Scholar] [CrossRef]
- Labenia, J.S.; Baldwin, D.H.; French, B.L.; Davis, J.W.; Scholz, N.L. Behavioral Impairment and Increased Predation Mortality in Cutthroat Trout Exposed to Carbaryl. Mar. Ecol. Prog. Ser. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Olisah, C.; Rubidge, G.; Human, L.R.D.; Adams, J.B. Tissue Distribution, Dietary Intake and Human Health Risk Assessment of Organophosphate Pesticides in Common Fish Species from South African Estuaries. Mar. Pollut. Bull. 2023, 186, 114466. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Elskus, A.A.; Shepherd, B.S.; Crowley, P.H.; McCarthy, T.M.; Niedzwiecki, J.H.; Sager, T.; Sih, A.; Palmer, B.D. Lethal and Sublethal Effects of Atrazine, Carbaryl, Endosulfan, and Octylphenol on the Streamside Salamander (Ambystoma barbouri). Environ. Toxicol. Chem. Int. J. 2003, 22, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, S.; Palanichamy, S. Sublethal Effects of Carbaryl on Surfacing Behaviour and Food Utilization in the Air-Breathing Fish, Macropodus cupanus. Physiol. Behav. 1982, 29, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Dumbauld, B.R.; Brooks, K.M.; Posey, M.H. Response of an Estuarine Benthic Community to Application of the Pesticide Carbaryl and Cultivation of Pacific Oysters (Crassostrea gigas) in Willapa Bay, Washington. Mar. Pollut. Bull. 2001, 42, 826–844. [Google Scholar] [CrossRef] [PubMed]
- Ángeles Esteban, M. An Overview of the Immunological Defenses in Fish Skin. Int. Sch. Res. Not. 2012, 2012, 853470. [Google Scholar] [CrossRef]
- Kumar, G.; Denslow, N.D. Gene Expression Profiling in Fish Toxicology: A Review. Rev. Environ. Contam. Toxicol. 2017, 241, 1–38. [Google Scholar]
- Boonphakdee, C.; Cheevaporn, V.; Somnuek, C.; Tanaka, K. Gene Expression of Acetylcholinesterase in Hybrid Catfish (Clarias gariepinus X Clarias macrocephalus) Exposed to Chlorpyrifos and Carbaryl. J. Environ. Biol. 2009, 30, 83–88. [Google Scholar]
- Blum, J.L.; Nyagode, B.A.; James, M.O.; Denslow, N.D. Effects of the Pesticide Methoxychlor on Gene Expression in the Liver and Testes of the Male Largemouth Bass (Micropterus salmoides). Aquat. Toxicol. 2008, 86, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, S.; Pang, Q. Vitellogenin Is a Novel Player in Defense Reactions. Fish Shellfish Immunol. 2006, 20, 769–772. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate Immunity of Fish (Overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef]
- Derbalah, A.; Chidya, R.; Kaonga, C.; Iwamoto, Y.; Takeda, K.; Sakugawa, H. Carbaryl Residue Concentrations, Degradation, and Major Sinks in the Seto Inland Sea, Japan. Environ. Sci. Pollut. Res. 2020, 27, 14668–14678. [Google Scholar] [CrossRef] [PubMed]
- El Ayari, T.; Mhadhbi, L.; El Menif, N.T.; El Cafsi, M. Acute Toxicity and Teratogenicity of Carbaryl (Carbamates), Tebufenpyrad (Pyrazoles), Cypermethrin and Permethrin (Pyrethroids) on the European Sea Bass (Dicentrarchus labrax L., 1758) Early Life Stages. Environ. Sci. Pollut. Res. 2022, 29, 66125–66135. [Google Scholar] [CrossRef] [PubMed]
- Beuter, L.-K.; Dören, L.; Hommen, U.; Kotthoff, M.; Schäfers, C.; Ebke, K.P. Testing Effects of Pesticides on Macroinvertebrate Communities in Outdoor Stream Mesocosms Using Carbaryl as Example Test Item. Environ. Sci. Eur. 2019, 31, 1–17. [Google Scholar] [CrossRef]
- Ramos, A.S.; Correia, A.T.; Antunes, S.C.; Gonçalves, F.; Nunes, B. Effect of Acetaminophen Exposure in Oncorhynchus mykiss Gills and Liver: Detoxification Mechanisms, Oxidative Defence System and Peroxidative Damage. Environ. Toxicol. Pharmacol. 2014, 37, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Chen, X.; Liu, S.; Fu, Z.; Han, M.; Wang, Y.; Gu, Z.; Ma, Z. Impact of Fish Density on Water Quality and Physiological Response of Golden Pompano (Trachinotus ovatus) Flingerlings During Transportation. Aquaculture 2019, 507, 260–265. [Google Scholar] [CrossRef]
- Olsvik, P.A.; Lie, K.K.; Jordal, A.-E.O.; Nilsen, T.O.; Hordvik, I. Evaluation of Potential Reference Genes in Real-Time Rt-Pcr Studies of Atlantic Salmon. BMC Mol. Biol. 2005, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Venturino, A.; de D’Angelo, A.M.P. Effects of Carbaryl and Azinphos Methyl on Juvenile Rainbow Trout (Oncorhynchus mykiss) Detoxifying Enzymes. Pestic. Biochem. Physiol. 2007, 88, 134–142. [Google Scholar] [CrossRef]
- Khare, A.; Chhawani, N.; Kumari, K. Glutathione Reductase and Catalase as Potential Biomarkers for Synergistic Intoxication of Pesticides in Fish. Biomarkers 2019, 24, 666–676. [Google Scholar] [CrossRef]
- Pham, B.; Miranda, A.; Allinson, G.; Nugegoda, D. Evaluating the Non-Lethal Effects of Organophosphorous and Carbamate Insecticides on the Yabby (Cherax destructor) Using Cholinesterase (Ache, Bche), Glutathione S-Transferase and Atpase as Biomarkers. Ecotoxicol. Environ. Saf. 2017, 143, 283–288. [Google Scholar] [CrossRef]
- Wdziȩczak, J.; Zaleśna, G.; Wujec, E.; Peres, G. Comparative Studies on Superoxide Dismutase, Catalase and Peroxidase Levels in Erythrocytes and Livers of Different Freshwater and Marine Fish Species. Comp. Biochem. Physiol. Part B: Comp. Biochem. 1982, 73, 361–365. [Google Scholar] [CrossRef]
- Sardella, B.A.; Kültz, D. The Physiological Responses of Green Sturgeon (Acipenser medirostris) to Potential Global Climate Change Stressors. Physiol. Biochem. Zool. 2014, 87, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bagchi, M.; Hassoun, E.A.; Stohs, S.J. In Vitro and in Vivo Generation of Reactive Oxygen Species, DNA Damage and Lactate Dehydrogenase Leakage by Selected Pesticides. Toxicology 1995, 104, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Peres, H.; Santos, S.; Oliva-Teles, A. Blood Chemistry Profile as Indicator of Nutritional Status in European Seabass (Dicentrarchus labrax). Fish Physiol. Biochem. 2014, 40, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, S.; Jiang, H.; Nie, G.; Li, X. Responses of Acid/Alkaline Phosphatase, Lysozyme, and Catalase Activities and Lipid Peroxidation to Mercury Exposure During the Embryonic Development of Goldfish Carassius Auratus. Aquat. Toxicol. 2012, 120, 119–125. [Google Scholar] [CrossRef]
- Rao, J.V. Biochemical Alterations in Euryhaline Fish, Oreochromis mossambicus Exposed to Sub-Lethal Concentrations of an Organophosphorus Insecticide, Monocrotophos. Chemosphere 2006, 65, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.V. Toxic Effects of Novel Organophosphorus Insecticide (Rpr-V) on Certain Biochemical Parameters of Euryhaline Fish, Oreochromis mossambicus. Pestic. Biochem. Physiol. 2006, 86, 78–84. [Google Scholar] [CrossRef]
- Fırat, Ö.; Cogun, H.Y.; Yüzereroğlu, T.A.; Gök, G.; Fırat, Ö.; Kargin, F.; Kötemen, Y. A Comparative Study on the Effects of a Pesticide (Cypermethrin) and Two Metals (Copper, Lead) to Serum Biochemistry of Nile Tilapia, Oreochromis niloticus. Fish Physiol. Biochem. 2011, 37, 657–666. [Google Scholar] [CrossRef]
- Kharat, T.L.; Rokade, K.B.; Shejule, K.B. Effect of Roundup 41%(Glyphosate) on Blood Serum Biochemical Parameters of Freshwater Fish, Rasbora daniconius. J. Environ. Biol. 2020, 41, 222–227. [Google Scholar] [CrossRef]
- Sema, D.-C.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar]
- El-Bahr, S.M. Biochemistry of Free Radicals and Oxidative Stress. Biochemistry 2013, 1, 11. [Google Scholar] [CrossRef]
- Mahboob, S. Environmental Pollution of Heavy Metals as a Cause of Oxidative Stress in Fish: A Review. Life Sci. J. 2013, 10, 336–347. [Google Scholar]
- Perrone, R.D.; Madias, N.E.; Levey, A.S. Serum Creatinine as an Index of Renal Function: New Insights into Old Concepts. Clin. Chem. 1992, 38, 1933–1953. [Google Scholar] [CrossRef]
- Kong, R.Y.C.; Giesy, J.P.; Wu, R.S.; Chen, E.X.; Chiang, M.W.; Lim, P.L.; Yuen, B.B.; Yip, B.W.; Mok, H.O.; Au, D.W. Development of a Marine Fish Model for Studying in Vivo Molecular Responses in Ecotoxicology. Aquat. Toxicol. 2008, 86, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.F. Pesticides Toxicity in Fish: Histopathological and Hemato-Biochemical Aspects—A Review. Emerg. Contam. 2023, 9, 100234. [Google Scholar] [CrossRef]
- Alesci, A.; Cicero, N.; Fumia, A.; Petrarca, C.; Mangifesta, R.; Nava, V.; Cascio, P.L.; Gangemi, S.; Di Gioacchino, M.; Lauriano, E.R. Histological and Chemical Analysis of Heavy Metals in Kidney and Gills of Boops Boops: Melanomacrophages Centers and Rodlet Cells as Environmental Biomarkers. Toxics 2022, 10, 218. [Google Scholar] [CrossRef]
- Mela, M.; Randi, M.A.; Ventura, D.F.; Carvalho, C.E.; Pelletier, E.; Ribeiro, C.A.O. Effects of Dietary Methylmercury on Liver and Kidney Histology in the Neotropical Fish Hoplias malabaricus. Ecotoxicol. Environ. Saf. 2007, 68, 426–435. [Google Scholar] [CrossRef]
- Lebret, T.; Watson, R.W.G.; Molinié, V.; O’Neill, M.; Gabriel, C.; Fitzpatrick, J.M.; Botto, H. Heat Shock Proteins Hsp27, Hsp60, Hsp70, and Hsp90: Expression in Bladder Carcinoma. Cancer 2003, 98, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Bildik, A.; Ekren, G.A.; Akdeniz, G.; Kıral, F. Effect of Enviromental Temperature on Heat Shock Proteins (Hsp30, Hsp70, Hsp90) and Igf-I Mrna Expression in Sparus Aurata. Iran. J. Fish. Sci. 2019, 18, 1014–1024. [Google Scholar]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.-S. A Review on the Involvement of Heat Shock Proteins (Extrinsic chaperones) in Response to Stress Conditions in Aquatic Organisms. Antioxidants 2023, 12, 1444. [Google Scholar] [CrossRef]
- Agus, H.H.; Erkmen, B.; Sümer, S.; Sepici-Dinçel, A.; Erkoç, F. Impact of Dbp on Histology and Expression of Hsp 70 in Gill and Liver Tissue of Cyprinus carpio. Mol. Biol. Rep. 2015, 42, 1409–1417. [Google Scholar] [CrossRef]
- da Rosa, J.G.S.; Koakoski, G.; Piato, A.L.; Bogo, M.R.; Bonan, C.D.; Barcellos, L.J.G. Impaired Brain Star and Hsp 70 Gene Expression in Zebrafish Exposed to Methyl-Parathion Based Insecticide. J. Toxicol. Environ. Health Part A 2016, 79, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Forn-Cuni, G.; Reis, E.S.; Dios, S.; Posada, D.; Lambris, J.D.; Figueras, A.; Novoa, B. The Evolution and Appearance of C3 Duplications in Fish Originate an Exclusive Teleost C3 Gene Form with Anti-Inflammatory Activity. PLoS ONE 2014, 9, e99673. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Zhang, Y.; Fang, Q.; Li, Y.; Li, Y. Toxic Effects of Chlorpyrifos on Lysozyme Activities, the Contents of Complement C3 and Igm, and Igm and Complement C3 Expressions in Common Carp (Cyprinus carpio L.). Chemosphere 2013, 93, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.; Elvitigala, D.A.S.; Godahewa, G.I.; Umasuthan, N.; Whang, I.; Noh, J.K.; Lee, J. Molecular Characterization and Comparative Expression Analysis of Two Teleostean Pro-Inflammatory Cytokines, Il-1β and Il-8, from Sebastes schlegeli. Gene 2016, 575, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, Z.; Yao, H.; Cao, Y.; Xing, H.; Xu, S. Pro-and Anti-Inflammatory Cytokine Expression in Immune Organs of the Common Carp Exposed to Atrazine and Chlorpyrifos. Pestic. Biochem. Physiol. 2014, 114, 8–15. [Google Scholar] [CrossRef]
- Zahran, E.; Risha, E.; Awadin, W.; Palić, D. Acute Exposure to Chlorpyrifos Induces Reversible Changes in Health Parameters of Nile Tilapia (Oreochromis niloticus). Aquat. Toxicol. 2018, 197, 47–59. [Google Scholar] [CrossRef]
| Gene Abbreviation | Primer Sequence (5′–3′) | Amplicon Size (bp) | Accession No. |
|---|---|---|---|
| β-actin | F: AACCAAACGCCCAACAACT | 112 | XM_018667666 |
| R: ATAACTGAAGCCATGCCAATG | |||
| HSP90 | F: ACGATGATGAGCAGTATGCC R: CAAACAGGGTGATGGGGTA | 201 | XM018661637 |
| HSP70 | F: CTGGAGTCCTACGCTTTCAA R: CTTGCTGATGATGGGGTTAC | 204 | HQ646109 |
| C3 | F: AAATGCTGCCATCGTTCC | 175 | XM_018679796 |
| R: CCAGTGACCTTCAGACCAAA | |||
| IL-8 | F: TCTGACTGTTCCTGAGGCTATC R: GACGTCCAATGGGCTTTCT | 92 | XM_018695863 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Fu, Z.; Yu, W.; Bai, Z.; Ma, Z. Toxic Effects of Carbaryl Exposure on Juvenile Asian Seabass (Lates calcarifer). J. Xenobiot. 2024, 14, 923-938. https://doi.org/10.3390/jox14030051
Huang J, Fu Z, Yu W, Bai Z, Ma Z. Toxic Effects of Carbaryl Exposure on Juvenile Asian Seabass (Lates calcarifer). Journal of Xenobiotics. 2024; 14(3):923-938. https://doi.org/10.3390/jox14030051
Chicago/Turabian StyleHuang, Junhua, Zhengyi Fu, Wei Yu, Zemin Bai, and Zhenhua Ma. 2024. "Toxic Effects of Carbaryl Exposure on Juvenile Asian Seabass (Lates calcarifer)" Journal of Xenobiotics 14, no. 3: 923-938. https://doi.org/10.3390/jox14030051
APA StyleHuang, J., Fu, Z., Yu, W., Bai, Z., & Ma, Z. (2024). Toxic Effects of Carbaryl Exposure on Juvenile Asian Seabass (Lates calcarifer). Journal of Xenobiotics, 14(3), 923-938. https://doi.org/10.3390/jox14030051







