Evaluation of Clastogenic and Aneugenic Action of Two Bio-Insecticides Using Allium Bioassay
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Insecticide Solutions
2.3. Phytotoxicity Assay
2.4. Cytogenotoxicity Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Root Germination and Growth Inhibition
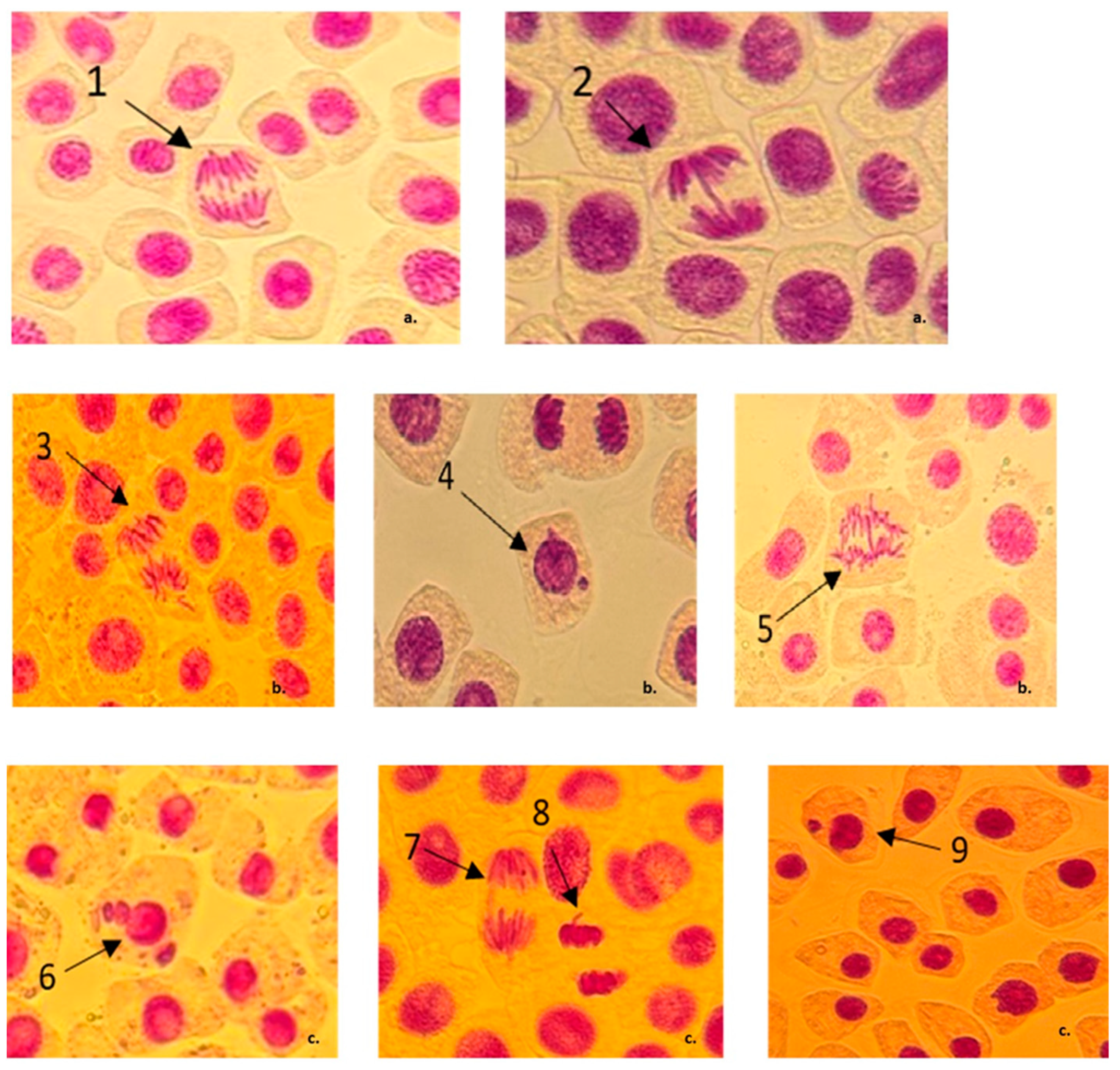
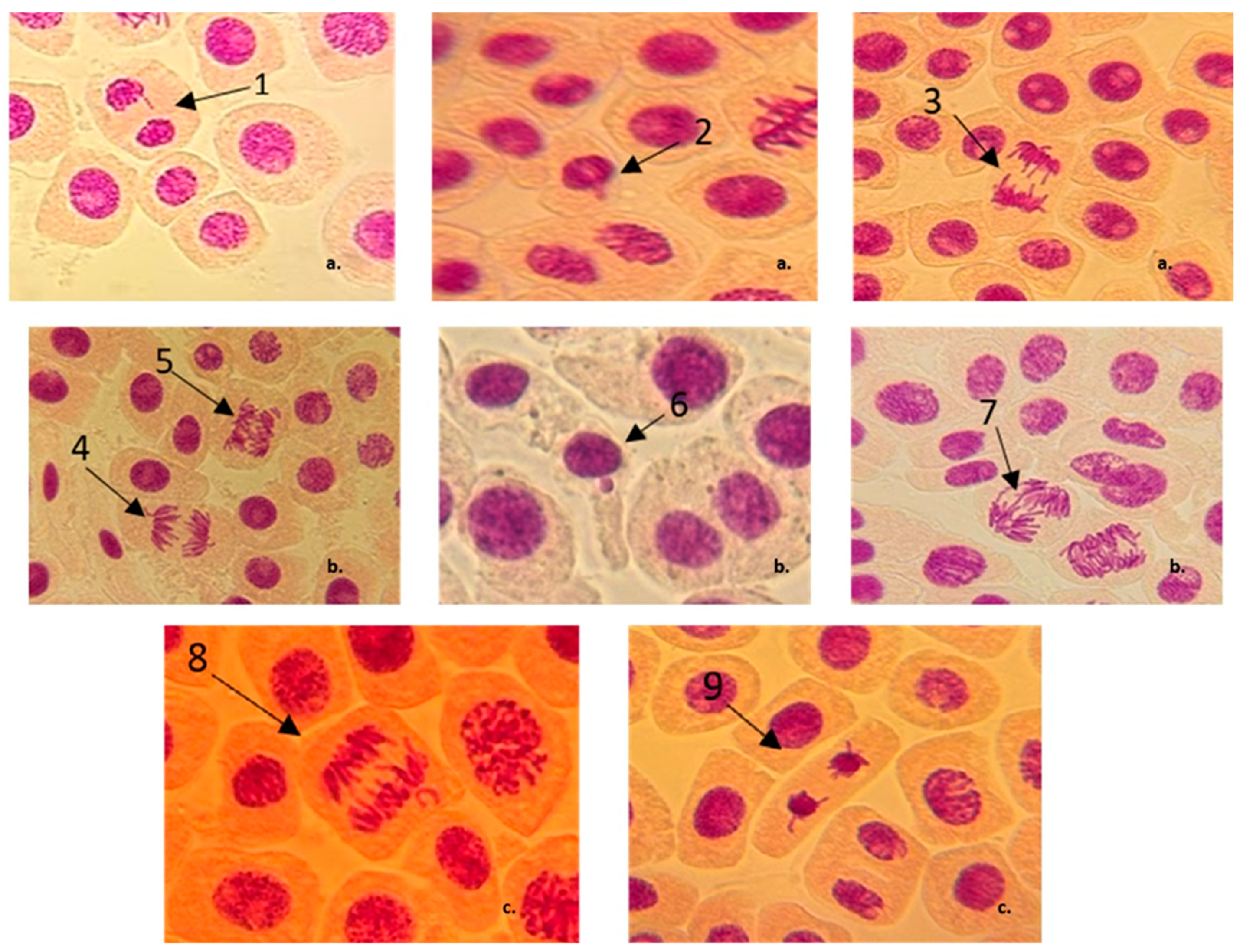
3.2. Chromosomal Aberrations and Micronuclei Induction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pesticides: The Basics. Available online: https://www.hse.gov.uk/pesticides/about-pesticides.htm (accessed on 23 November 2024).
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Hayes, T.B.; Hansen, M.; Kapuscinski, A.R.; Locke, K.A.; Barnosky, A. From silent spring to silent night: Agrochemicals and the anthropocene. Elem. Sci. Anth. 2017, 5, 57. [Google Scholar] [CrossRef]
- Bernardes, M.F.F.; Pazin, M.; Pereira, L.C.; Dorta, D.J. Impact of Pesticides on Environmental and Human Health. In Toxicology Studies—Cells, Drugs and Environment; Andreazza, A.C., Scola, G., Eds.; IntechOpen: London, UK, 2015; pp. 195–233. [Google Scholar]
- Hernández, A.F.; Gil, F.; Lacasaña, M.; Rodríguez-Barranco, M.; Tsatsakis, A.M.; Requena, M.; Alarcón, R. Pesticide exposure and genetic variation in xenobiotic-metabolizing enzymes interact to induce biochemical liver damage. Food Chem. Toxicol. 2013, 61, 144–151. [Google Scholar] [CrossRef]
- Antle, J.M.; Pingali, P.L. Pesticides, productivity, and farmer health: A Philippine case study. Am. J. Agric. Econ. 1994, 76, 418–430. [Google Scholar] [CrossRef]
- Dasgupta, S.; Meisner, C.; Wheeler, D.; Xuyen, K.; Thi Lam, N. Pesticide poisoning of farm workers-implications of blood test results from Vietnam. Int. J. Hyg. Environ. Health 2007, 210, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.; Schreinemachers, P.; Ingwersen, J.; Sangchan, W.; Grovermann, C.; Berger, T. Agricultural pesticide use in mountainous areas of Thailand and Vietnam: Towards reducing exposure and rationalizing use. In Sustainable Land Use and Rural Development in Southeast Asia: Innovations and Policies for Mountainous Areas; Springer Environmental Science and Engineering; Fröhlich, H.L., Schreinemachers, P., Stahr, K., Clemens, C., Eds.; Springer: Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2013; pp. 156–180. [Google Scholar] [CrossRef]
- Sarkar, S.; Gil, J.D.B.; Keeley, J.; Jansen, K. The use of pesticides in developing countries and their impact on health and the right to food. In Study Retrieved by the DEVE Committee; European Union: Brussels, Belgium, 2021; pp. 10–47. Available online: https://library.wur.nl/WebQuery/wurpubs/fulltext/548765 (accessed on 27 November 2024).
- Teklu, B.M.; Haileslassie, A.; Mekuria, W. Pesticides as Water Pollutants and Level of Risks to Environment and People: An Example from Central Rift Valley of Ethiopia, Environment, Development and Sustainability: A Multidisciplinary Spproach to the Theory and Practice of Sustainable Development; Springer: Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2022; Volume 24, pp. 5275–5294. [Google Scholar] [CrossRef]
- Thakur, N.; Kaur, S.; Tomar, P.; Thakur, S.; Yadav, A.N. Microbial biopesticides: Current status and advancement for sustainable agriculture and environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Rastegari, A.A., Yadav, A.N., Yadav, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–282. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Biopesticides. Available online: https://www.epa.gov/pesticides/biopesticides (accessed on 23 November 2024).
- United States Environmental Protection Agency. What are Biopesticides? Available online: https://www.epa.gov/ingredients-used-pesticide-products/what-are-biopesticides (accessed on 23 November 2024).
- Wend, K.; Zorrilla, L.; Freimoser, F.M.; Gallet, A. Microbial pesticides—Challenges and future perspectives for testing and safety assessment with respect to human health. Environ. Health 2024, 23, 49. [Google Scholar] [CrossRef]
- Yadav, H.; Kumar, R.; Sankhla, M.S. Residues of pesticides and heavy metals in crops resulting in toxic effects on living organism. J. Seybold. Rep. 2020, 1533, 9211. [Google Scholar] [CrossRef]
- Kumar, S.R.; Sankhla, M.S.; Rajeev Kumar, R.; Sonone, S.S. Impact of pesticide toxicity in aquatic environment. Biointerface Res. Appl. Chem. 2021, 11, 10131–10140. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3755402 (accessed on 21 November 2024).
- Ayoub, N.; Nawal, L.; Soumaya, R.; Nadia, I.; Amrani Souad, E. Evaluation of the phytotoxicity of a pesticide (TRACTOR 10E) based on Alpha-cypermethrin in two plant species: Lentils (Lens culinaris) and watercress (Lepidium sativum). Pollution 2023, 9, 1386–1395. [Google Scholar] [CrossRef]
- Alves, T.A.; Roberto, C.E.O.; Pinheiro, P.F.; Alves, T.A.; Henrique, M.K.C.; Ferreira, A.; Clarindo, W.R.; Praça-Fontes, M.M. Searching an auxinic herbicide to use as positive control in toxicity assays. An. Acad. Bras. Cienc. 2021, 93, e20181262. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.F.; Costa, A.V.; Alves, T.A.; Galter, I.N.; Pinheiro, C.A.; Pereira, A.F.; Oliveira, C.M.; Fontes, M.M.P. Phytotoxicity and cytotoxicity of essential oil from leaves of Plectranthus amboinicus, carvacrol, and thymol in plant bioassays. J. Agric. Food Chem. 2015, 63, 8981–8990. [Google Scholar] [CrossRef] [PubMed]
- Leme, D.M.; Marin-Morales, M.A. Allium cepa test in environmental monitoring: A review on its application. Mutat. Res. 2009, 682, 71–81. [Google Scholar] [CrossRef]
- Albertini, R.J.; Anderson, D.; Douglas, G.R.; Hagmar, L.; Hemmink, K.; Merlo, F.; Natarajan, A.T.; Norppa, H.; Shuker, D.E.; Tice, R.; et al. IPCS guideline for the monitoring of genotoxic effects of carcinogens in humans. Int. Programme Chem. Saf. Mutat. Res. 2000, 463, 111–172. [Google Scholar] [CrossRef] [PubMed]
- Leme, D.M.; Angelis, D.F.; Marin-Morales, M.A. Action mechanisms of petroleum hydrocarbons present in waters impacted by an oil spill on the genetic material of Allium cepa root cells. Aquat. Toxicol. 2008, 88, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.C.C.; Mazzeo, D.E.C.; Marin-Morales, M.A. Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pest. Biochem. Physiol. 2007, 88, 252–259. [Google Scholar] [CrossRef]
- Ateeq, B.; Adul Farrah, M.; Ali, M.N.; Ahmad, W. Clastogenicity of pentachlorophenol, 2-4-D and butachlor evaluated by Allium rot tip test. Mutat. Res. 2002, 514, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Bolle, P.; Mastrangelo, S.; Tucci, P.; Evandri, M.G. Clastogenicity of atrazine assessed with the Allium cepa test. Environ. Mol. Mutagen. 2004, 43, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.; Raj, A.; Markandeya, A.S. Biodegradation of Azure-B dye by Serratia liquefaciens and its validation by phytotoxicity, genotoxicity and cytotoxicity studies. Chemosphere 2018, 196, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.; Kalamdhad, A.S. Phytotoxicity and cyto-genotoxicity evaluation of organic and inorganic pollutants containing petroleum refinery wastewater using plant bioassay. Environ. Technol. Innov. 2021, 23, 101651. [Google Scholar] [CrossRef]
- Camilo-Cotrim, C.F.; Bailão, E.F.L.C.; Ondei, L.S.; Carneiro, F.M.; Almeida, L.M. What can the Allium cepa test say about pesticide safety? A review. Environ. Sci. Pollut. Res. 2022, 29, 48088–48104. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, L.K.S.; Saxena, P.N.; Gupta, S.K. Cytogenetic effects of cypermethrin and fenvalerate on the root meristem cells of Allium cepa. Environ. Exp. Bot. 1999, 42, 181–189. [Google Scholar] [CrossRef]
- Firbas, P.; Amon, T. Chromosome damage studies in the onion plant Allium cepa L. Caryologia 2014, 67, 25–35. [Google Scholar] [CrossRef]
- Fiskesjö, G. The Allium test as a standard in environmental monitoring. Hereditas 1985, 102, 99–112. [Google Scholar] [CrossRef]
- Rank, J.; Nielsen, M.H. Evaluation of the Allium anaphase–telophase test in relation to genotoxicity screening of industrial wastewater. Mutat. Res. 1994, 312, 17–24. [Google Scholar] [CrossRef]
- Grant, W.F. Chromosome aberration assays in Allium. Mutat. Res. 1982, 99, 273–291. [Google Scholar] [CrossRef] [PubMed]
- European and Mediterranean Plant Protection Organization Organisation. Annual Report 2014 and Council Recommendations. Bull. OEPP/EPPO Bull. 2015, 44, 265–273. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1111/epp.12256 (accessed on 11 November 2024).
- Fernandes, T.C.C.; Mazzeo, D.E.C.; Marin-Morales, M.A. Origin of nuclear and chromosomal alterations derived from the action of an aneugenic agent—Trifluralin herbicide. Ecotoxicol. Environ. Saf. 2009, 72, 1680–1686. [Google Scholar] [CrossRef]
- Appendix A. Multi-Active Ingredients Product Analysis Deltamethrin. Available online: https://www3.epa.gov/pesticides/endanger/litstatus/effects/redleg-frog/2013/deltamethrin/appendix-a.pdf (accessed on 27 November 2024).
- Almasi, A.; Sabahi, Q.; Talebi, K.; Mardani, A. Laboratory evaluation of the tox-icity of proteus, pymetrozine, deltamethrin, and pirimicarb on lady beetle Hippodamia Variegata (Goeze) (Col.: Coccinellidae). J. Plant Prot. Res. 2013, 53, 142–147. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Cage, S.A.; Proudfoot, A.T.; Vale, J.A. Poisoning due to pyrethroids. Toxicol. Rev. 2005, 24, 93–106. [Google Scholar] [CrossRef]
- Das, R.; Steege, A.; Baron, S.; Beckman, J.; Harrison, R. Pesticide-related illness among migrant farm workers in the United States. Int. J. Occup. Environ. Health 2001, 7, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Tuzmen, N.; Candan, N.; Kaya, E.; Demiryas, P. Biochemical effects of chlorpyrifos and deltamethrin on altered antioxidative defense mechanisms and lipid peroxidation in rat liver. Cell Biochem. Funct. 2008, 26, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.H.; Abbas, Z.K.; Ansari, A.A.; Khan, M.N.; Ansari, W.A. Pesticides and their effects on plants: A case study of deltamethrin. In Agrochemicals in Soil and Environment: Impacts and Remediation; Springer Nature Singapore: Singapore, 2022; pp. 183–193. [Google Scholar]
- Bava, R.; Lupia, C.; Castagna, F.; Ruga, S.; Nucera, S.; Carresi, C.; Caminiti, R.; Bulotta, R.M.; Naccari, C.; Britti, D.; et al. Interaction of Flupyradifurone and Deltamethrin, two pesticides commonly used for plant pest control, in honeybees. Animals 2024, 14, 851. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yang, Y.; Lao, Z.; Zhong, Y.; Zhang, K.; Zhao, S. Acute and chronic toxicity of deltamethrin, permethrin, and dihaloacetylated heterocyclic pyrethroids in mice. Pest Manag. Sci. 2020, 76, 4210–4221. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.A.; Al-Baggou, B.K. Toxicological and neurobehavioral effects of chlorpyrifos and deltamethrin insecticides in mice. Iraqi J. Vet. Sci. 2020, 34, 189–196. [Google Scholar] [CrossRef]
- Fisa cu Date de Securitate. Available online: https://www.glissando.ro/wp-content/uploads/2021/04/LASER-240-SC-24.08.2020.pdf (accessed on 20 November 2024).
- Vander Weele, T.J.; Mathur, M.B. Some desirable properties of the bonferroni correction: Is the bonferroni correction really so bad? Am. J. Epidemiol. 2019, 188, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Gupta, Y. Evaluation of genotoxic potential of commonly used organophosphate pesticides in peripheral blood lymphocytes of rats. Hum. Exp. Toxicol. 2015, 34, 390–400. [Google Scholar] [CrossRef]
- Christudoss, A.C.; Kundu, R.; Dimkpa, C.O.; Mukherjee, A. Time dependent release of microplastics from disposable face masks poses cyto-genotoxic risks in Allium cepa. Ecotoxicol. Environ. Saf. 2024, 280, 116542. [Google Scholar] [CrossRef]
- Ramborger, B.P.; Gomes Paz, M.E.; Cavalheiro Kieling, K.M.; Sigal Carriço, M.R.; Gollino, G.P.; Tavares Costa, M.; Bley Ribeiro, V.; Folmer, V.; Gasparotto Denardin, E.L.; Soares, J.J.; et al. Toxicological parameters of aqueous residue after using Plectranthus neochilus for 2,4-D phytoremediation. Chemosphere 2021, 270, 128638. [Google Scholar] [CrossRef] [PubMed]
- Helps, J.C.; Paveley, N.D.; White, S.; van den Bosch, F. Determinants of optimal insecticide resistance management strategies. J Theor Biol. 2020, 503, 110383. [Google Scholar] [CrossRef]
- Tekalign, K. Phytohormone—Producing Rhizobacteria and Their Role in Plant Growth, New In-Sights into Phytohormones; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Ansari, M.; Devi, B.M.; Sarkar, A.; Chattopadhyay, A.; Satnami, L.; Balu, P.; Choudhary, M.; Shahid, M.A.; Jailani, A.A.K. Microbial exudates as biostimulants: Role in plant growth promotion and stress mitigation. J. Xenobiot. 2023, 13, 572–603. [Google Scholar] [CrossRef]
- Gomis-Cebolla, J.; Berry, C. Bacillus thuringiensis as a biofertilizer in crops and their implications in the control of phytopathogens and insect pests. Pest Manag. Sci. 2023, 79, 2992–3001. [Google Scholar] [CrossRef] [PubMed]
- Hajji-Hedfi, L.; Ibrahim, D.S.; Othmen, S.B.; El-Abeid, S.E.; Hlaoua, W.; Mosa, M.A.; Rhouma, A.; Riad, S.N.; Ghareeb, S.; El-Deriny, M.M.; et al. Production of microbial biostimulants as a response to the modern agricultural need for productivity and pant health. In Microbial Biostimulants, 1st ed.; Taylor & Francis: Oxford, NY, USA; Apple Academic Press: Palm Bay, FL, USA, 2025; pp. 31–78. [Google Scholar] [CrossRef]
- Jensen, C.N.G.; Pang, J.K.Y.; Hahn, C.M.; Gottardi, M.; Husted, S.; Moelbak, L.; Kovács, A.T.; Fimognari, L.; Schulz, A. Differential influence of Bacillus subtilis strains on Arabidopsis root architecture through common and distinct plant hormonal pathways. Plant Sci. 2024, 339, 111936. [Google Scholar] [CrossRef]
- Martínez, S.A.; Dussan-G, J. Lysinibacillus sphaericus plant growth promoter bacteria and lead phytoremediation enhancer with Canavalia ensiformis. Environ. Prog. Sustain. Energy 2017, 37, 276–282. [Google Scholar] [CrossRef]
- Demirtaş, G.; Çavuşoğlu, K.; Aneugenic, Y.E. Clastogenic, and multi-toxic effects of diethyl phthalate exposure. Environ. Sci. Pollut. Res. 2020, 27, 5503–5510. [Google Scholar] [CrossRef] [PubMed]
- Arni, P.; Hertner, T. Chromosomal aberrations in vitro induced by aneugens. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1997, 379, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.Y.; Torres, J.Z. Dissecting the mechanisms of cell division. J. Biol. Chem. 2019, 294, 11382–11390. [Google Scholar] [CrossRef]
- de Souza, R.B.; de Souza, C.P.; Guimarães, J.R. Environmentally realistic concentrations of eprinomectin induce phytotoxic and genotoxic effects in Allium cepa. Environ. Sci. Pollut. Res. 2022, 29, 80983–80993. [Google Scholar] [CrossRef]
- Pampalona, J.; Roscioli, E.; Silkworth, W.T.; Bowden, B.; Genescà, A.; Tusell, L.; Cimini, D. Chromosome bridges maintain kinetochore-microtubule attachment throughout mitosis and rarely break during anaphase. PLoS ONE 2016, 11, e0147420. [Google Scholar] [CrossRef] [PubMed]
- Sabeen, M.; Mahmood, Q.; Ahmad Bhatti, Z.; Faridullah Irshad, M.; Bilal, M.; Hayat, M.T.; Irshad, U.; Ali Akbar, T.; Arslan, M.; Shahid, N. Allium cepa assay based comparative study of selected vegetables and the chromosomal aberrations due to heavy metal accumulation. Saudi. J. Biol. Sci. 2020, 27, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Poot, M. Neocentromeres to the rescue of acentric chromosome fragments. Mol. Syndromol. 2017, 8, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Magdaleno, A.; Paz, M.; Fabrizio de Iorio, A.; Weigandt, C.; Moretton, J. Micronucleus and chromosome aberration frequencies in Allium cepa cells exposed to coastal sediments from a polluted estuarine system. Braz. J. Aquat. Sci. Technol. 2021, 25, 8. [Google Scholar] [CrossRef]
- Panda Brahma, B.; Mohan, M.A.V. Mitogen-activated protein kinase signal transduction and DNA repair network are involved in aluminum-induced DNA damage and adaptive response in root cells of Allium cepa L. Front. Plant Sci. 2014, 5, 256. [Google Scholar] [CrossRef][Green Version]
- Szurman-Zubrzycka, M.; Jędrzejek, P.; Szarejko, I. How Do Plants Cope with DNA Damage? A Concise Review on the DDR Pathway in Plants. Int. J. Mol. Sci. 2023, 24, 2404. [Google Scholar] [CrossRef]
- Akmoutsou, P.; Mademtzoglou, D.; Nakou, I.; Onoufriadis, A.; Papadopoulou, X.; Kounatidis, I.; Frantzios, G.; Papadakis, G.; Vasiliadis, K.; Papadopoulos, N.T.; et al. Evaluation of toxicity and genotoxic effects of spinosad and deltamethrin in Drosophila melanogaster and Bactrocera oleae. Pest Manag. Sci. 2011, 66, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Aciole, E.H.; Guimarães, N.N.; Silva, A.S.; Amorim, E.M.; Nunomura, S.M.; Garcia, A.C.; Cunha, K.S.; Rohde, C. Genetic toxicity of dillapiol and spinosad larvicides in somatic cells of Drosophila melanogaster. Pest Manag. Sci. 2014, 70, 559–565. [Google Scholar] [CrossRef]
- Pereira, B.B.; Caixeta, E.S.; Freitas, P.C.; Santos, V.S.; Limongi, J.E.; de Campos Júnior, E.O.; Campos, C.F.; Souto, H.N.; Rodrigues, T.S.; Morelli, S. Toxicological assessment of spinosad: Implications for integrated control of Aedes aegypti using larvicides and larvivorous fish. J. Toxicol. Environ. Health A 2016, 79, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Besard, L.; Mommaerts, V.; Abdu-Alla, G.; Smagghe, G. Lethal and sublethal side-effect assessment supports a more benign profile of spinetoram compared with spinosad in the bumblebee Bombus terrestris. Pest Manag. Sci. 2011, 67, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.M.; Ottea, J. The effects of spinosad on Culex quinquefasciatus and three nontarget insect species. J. Am. Mosq. Control Assoc. 2013, 29, 346–351. [Google Scholar] [CrossRef]
- Toledo-Ibarra, G.A.; Rodríguez-Sánchez, E.J.; Ventura-Ramón, H.G.; Díaz-Resendiz, K.J.G.; Girón-Pérez, M.I. Cholinergic alterations by exposure to pesticides used in control vector: Guppies fish (Poecilia reticulta) as biological model. Int. J. Environ. Health Res. 2018, 28, 79–89. [Google Scholar] [CrossRef]
- Mendonça, T.P.; Davi de Aquino, J.; Junio da Silva, W.; Mendes, D.R.; Campos, C.F.; Vieira, J.S.; Barbosa, N.P.; Carvalho Naves, M.P.; Olegário de Campos Júnior, E.; Alves de Rezende, A.A.; et al. Genotoxic and mutagenic assessment of spinosad using bioassays with Tradescantia pallida and Drosophila melanogaster. Chemosphere 2019, 222, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Hernandes, N.H.; Zuo, Z.; Wang, J.; Wong, C.O.; Karagas, N.E.; Roessner, U.; Rupasinghe, T.; Robin, C.; Venkatachalam, K.; et al. Low doses of the organic insecticide spinosad trigger lysosomal defects, elevated ROS, lipid dysregulation, and neurodegeneration in flies. eLife 2022, 11, e73812. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). Spinosad Pesticide Fact Sheet No. HJ 501C; Office of Pesticides and Toxic Substances: Washington, DC, USA, 1997. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-110003_19-Jul-99.pdf (accessed on 14 November 2024).
- Yano, B.L.; Bond, D.M.; Novilla, M.N.; McFadden, L.G.; Reasor, M.J. Spinosad insecticide: Subchronic and chronic toxicity and lack of carcinogenicity in Fischer 344 rats. Toxicol. Sci. 2002, 65, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, K.E. Spinosad insecticide: Subchronic and chronic toxicity and lack of carcinogenicity in CD-1 mice. Toxicol. Sci. 2002, 65, 276–287. [Google Scholar] [CrossRef]
- Mansour, S.A.; Mossa, A.H.; Heikal, T.M. Cytogenetic and hormonal alteration in rats exposed to recommended “safe doses” of spinosad and malathion insecticides. IJAB 2008, 10, 9–14. [Google Scholar]
- Asita, A.O.; Mohale, R.R.; Magama, S. Cytotoxicity and genotoxicity of imidacloprid, spinosad and bifenthrin—Myclobutanil combination to Allium cepa root tip meristematic cells. Environ. Nat. Resour. Res. 2022, 12, 1. [Google Scholar] [CrossRef]
- Benaissa, A. Rhizosphere: Role of bacteria to manage plant diseases and sustainable agriculture—A review. J. Basic Microbiol. 2024, 64, 2300361. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Piater, L.A.; Dubery, I.A.; Tugizimana, F.; Mhlongo, M.I. Rhizosphere Tripartite Interactions and PGPR-Mediated Metabolic Reprogramming towards ISR and Plant Priming: A Metabolomics Review. Biology 2022, 11, 346. [Google Scholar] [CrossRef]
- Oliveira-Filho, E.C.; Grisolia, C.K. The Ecotoxicology of microbial insecticides and their toxins in genetically modified crops: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 16495. [Google Scholar] [CrossRef] [PubMed]
- Grisolia, C.K.; Oliveira, R.; Domingues, I.; Oliveira-Filho, E.C.; Monnerat, R.G.; Soares, A.M. Genotoxic evaluation of different delta-endotoxins from Bacillus thuringiensis on zebrafish adults and development in early life stages. Mutat. Res. 2009, 672, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Ghanya, A.N.; Aliki, K.; Francesca, G.; Rachele, D.G.; Hellas, C. Genotoxic and antigenotoxic medicinal plant extracts and their main phytochemicals: “A review”. Front. Pharmacol. 2024, 15, 1448731. [Google Scholar] [CrossRef]

| Experimental Variant | Insecticide Concentration (%) | ||
|---|---|---|---|
| PC | BCP | MP | |
| MRFC (1X) | 0.031 | 0.04 | 2 |
| 1.5 MRFC (1.5X) | 0.046 | 0.06 | 3 |
| 2 MRFC (2X) | 0.062 | 0.08 | 4 |
| Treatment | Root Length (cm) (Mean ± SD) | No. of Roots (Mean ± SD) | Root Growth Inhibition (%) | ||
|---|---|---|---|---|---|
| NC | 24 h | 2.2 ± 1.8 | 30.5 ± 2.04 | - | |
| 48 h | 2.75 ± 1.5 | 37 ± 1 | - | ||
| PC | 1X | 24 h | 1.025 ± 0.6 | 46 ± 1.6 | 53.41 |
| 48 h | 1.075 ± 0.9 | 30.5 ± 1.5 | 60.91 | ||
| 1.5X | 24 h | 0.675 ± 0.4 | 42 ± 0.8 | 69.32 | |
| 48 h | 0.8 ± 0.6 | 35 ± 7.7 | 70.91 | ||
| 2X | 24 h | 0.625 ± 0.3 | 45.5 ± 2.85 | 71.59 | |
| 48 h | 0.55 ± 0.54 | 31 ± 4 | 80.00 | ||
| BCP insecticide | 1X | 24 h | 1.52 ± 0.1 | 41 ± 1.4 | 30.91 |
| 48 h | 0.75 ± 0.1 | 28.5 ± 4.9 | 72.73 | ||
| 1.5X | 24 h | 0.93 ± 0.5 | 20 ± 0.1 | 57.73 | |
| 48 h | 0.82 ± 0.2 | 19.5 ± 9.1 | 70.18 | ||
| 2X | 24 h | 0.68 ± 0.2 | 27.5 ± 3.5 | 69.09 | |
| 48 h | 0.57 ± 0.2 | 13.5 ± 0.7 | 79.27 | ||
| MP insecticide | 1X | 24 h | 1.85 ± 0.7 | 30.5 ± 9.1 | 15.91 |
| 48 h | 2.45 ± 0.7 | 34 ± 7.07 | 10.91 | ||
| 1.5X | 24 h | 1.7 ± 0.6 | 29 ± 8.4 | 22.73 | |
| 48 h | 2.42 ± 0.5 | 33 ± 1.6 | 12.00 | ||
| 2X | 24 h | 2.37 ± 0.4 | 33.5 ± 0.7 | −4.45 | |
| 48 h | 3.3 ± 0.2 | 37.5 ± 0.1 | −20 | ||
| Treatment | TNDC ± SD | MI | Pro. (%) | Meta. (%) | Ana. (%) | Telo. (%) | ||
|---|---|---|---|---|---|---|---|---|
| NC | 24 h | 270 ± 8.9 | 33.75 | 39.62 | 29.97 | 11.4 | 21.85 | |
| 48 h | 362 ± 1.5 | 45.25 | 34.25 | 32.9 | 13.5 | 26.6 | ||
| PC | 1X | 24 h | 241 ± 6.2 | 30.13 | 36.51 | 19.08 | 18.6 | 25.7 |
| 48 h | 168 ± 4.2 a | 21.13 a | 26.62 a | 30.76 a | 21.3 a | 21.3 a | ||
| 1.5X | 24 h | 184 ± 8.4 b | 23 b | 26.1 b | 36.4 b | 17.4 b | 20.1 b | |
| 48 h | 227± 4.4 b | 28.34 b | 24.66 b | 40.08 b | 16.4 b | 18.5 b | ||
| 2X | 24 h | 20 ± 4.3 a | 2.5 a | 15 a | 55 a | 15 a | 15 a | |
| 48 h | 214 ± 9.2 | 26.75 | 28.5 | 36.4 | 14.5 | 20.6 | ||
| BCP | 1X | 24 h | 200 ± 7.5 | 25 | 33 | 21 | 17 | 29 |
| 48 h | 130 ± 6.25 b | 16.25 b | 37.7 b | 25.4 b | 12.3 b | 24.6 b | ||
| 1.5X | 24 h | 166 ± 3.6 b | 20.75 b | 36.1 b | 16.9 b | 17.5 b | 29.5 b | |
| 48 h | 165 ± 3.6 b | 20.62 b | 34.5 b | 22.4 b | 17.6 b | 25.5 b | ||
| 2X | 24 h | 151 ± 1.9 b | 18.8 b | 29.1 b | 25.8 b | 19.9 b | 25.2 b | |
| 48 h | 171 ± 7.8 b | 21.38 b | 36.8 b | 13.7 b | 16.9 b | 27.5 b | ||
| MP | 1X | 24 h | 253 ± 6.2 | 31.62 | 41.89 | 18.97 | 14.62 | 24.5 |
| 48 h | 287 ± 6.9 | 35.87 | 31.35 | 29.96 | 20.55 | 18.11 | ||
| 1.5X | 24 h | 198 ± 6.2 b | 24.75 b | 35.87 b | 22.72 b | 16.66 b | 22.72 b | |
| 48 h | 220 ± 4.4 a | 27.5 a | 37.72 a | 22.72 a | 15.45 a | 24.09 a | ||
| 2X | 24 h | 219 ± 2.1 | 27.37 | 34.7 | 22.83 | 17.8 | 24.65 | |
| 48 h | 221 ± 7.3 | 27.62 | 35.75 | 26.69 | 18.55 | 19.1 | ||
| PC | BCP | MP | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1X | 1.5X | 2X | 1X | 1.5X | 2X | 1X | 1.5X | 2X | ||||||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| Chromosomal bridges | 2 | 0 | 0 | 4 | 0 | 3 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| Vagrant/laggard | 8 | 6 | 4 | 11 | 0 | 5 | 5 | 0 | 8 | 5 | 4 | 11 | 12 | 0 | 7 | 13 | 14 | 2 |
| Other modifications * | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| TCA | 10 | 6 | 5 | 17 | 0 | 8 | 6 | 0 | 8 | 6 | 5 | 11 | 13 | 1 | 9 | 14 | 14 | 2 |
| Cells with 1 MN | 0 | 0 | 0 | 1 | 23 | 1 | 5 | 8 | 6 | 10 | 4 | 29 | 3 | 0 | 2 | 1 | 0 | 0 |
| Cells with 2-more MNs ** | 0 | 0 | 0 | 1 | 6 | 0 | 3 | 0 | 0 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| TCNM | 0 | 0 | 0 | 2 | 29 | 1 | 8 | 8 | 6 | 11 | 4 | 33 | 3 | 0 | 2 | 1 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duta-Cornescu, G.; Dugala, M.L.; Constantin, N.; Pojoga, M.-D.; Simon-Gruita, A. Evaluation of Clastogenic and Aneugenic Action of Two Bio-Insecticides Using Allium Bioassay. J. Xenobiot. 2025, 15, 35. https://doi.org/10.3390/jox15020035
Duta-Cornescu G, Dugala ML, Constantin N, Pojoga M-D, Simon-Gruita A. Evaluation of Clastogenic and Aneugenic Action of Two Bio-Insecticides Using Allium Bioassay. Journal of Xenobiotics. 2025; 15(2):35. https://doi.org/10.3390/jox15020035
Chicago/Turabian StyleDuta-Cornescu, Georgiana, Maria Liliana Dugala, Nicoleta Constantin, Maria-Daniela Pojoga, and Alexandra Simon-Gruita. 2025. "Evaluation of Clastogenic and Aneugenic Action of Two Bio-Insecticides Using Allium Bioassay" Journal of Xenobiotics 15, no. 2: 35. https://doi.org/10.3390/jox15020035
APA StyleDuta-Cornescu, G., Dugala, M. L., Constantin, N., Pojoga, M.-D., & Simon-Gruita, A. (2025). Evaluation of Clastogenic and Aneugenic Action of Two Bio-Insecticides Using Allium Bioassay. Journal of Xenobiotics, 15(2), 35. https://doi.org/10.3390/jox15020035







