The Soil–Plant Continuity of Rare Earth Elements: Insights into an Enigmatic Class of Xenobiotics and Their Interactions with Plant Structures and Processes
Abstract
1. An Introduction to Rare Earth Elements
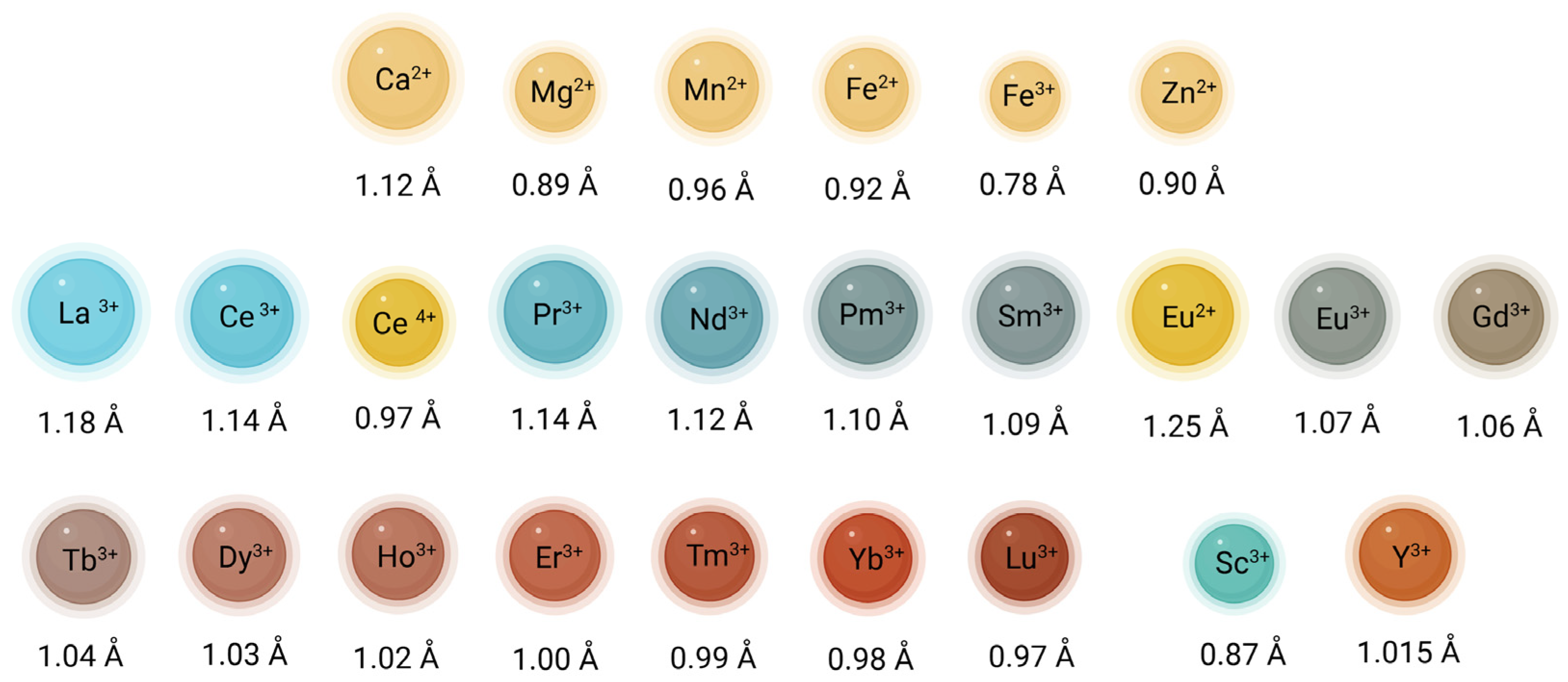
2. Basic Chemical Information About REEs
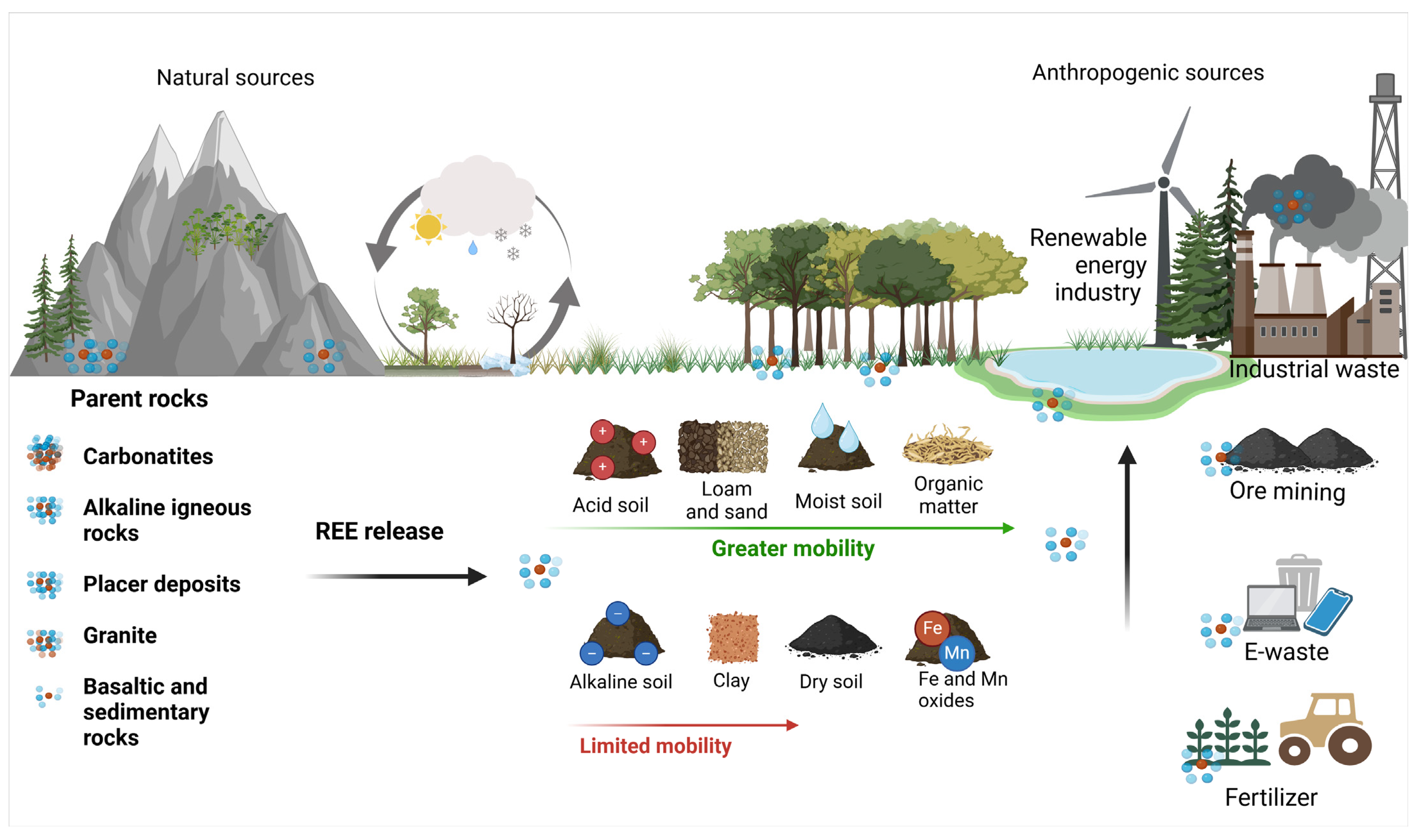
3. Geochemistry of REEs in Soils
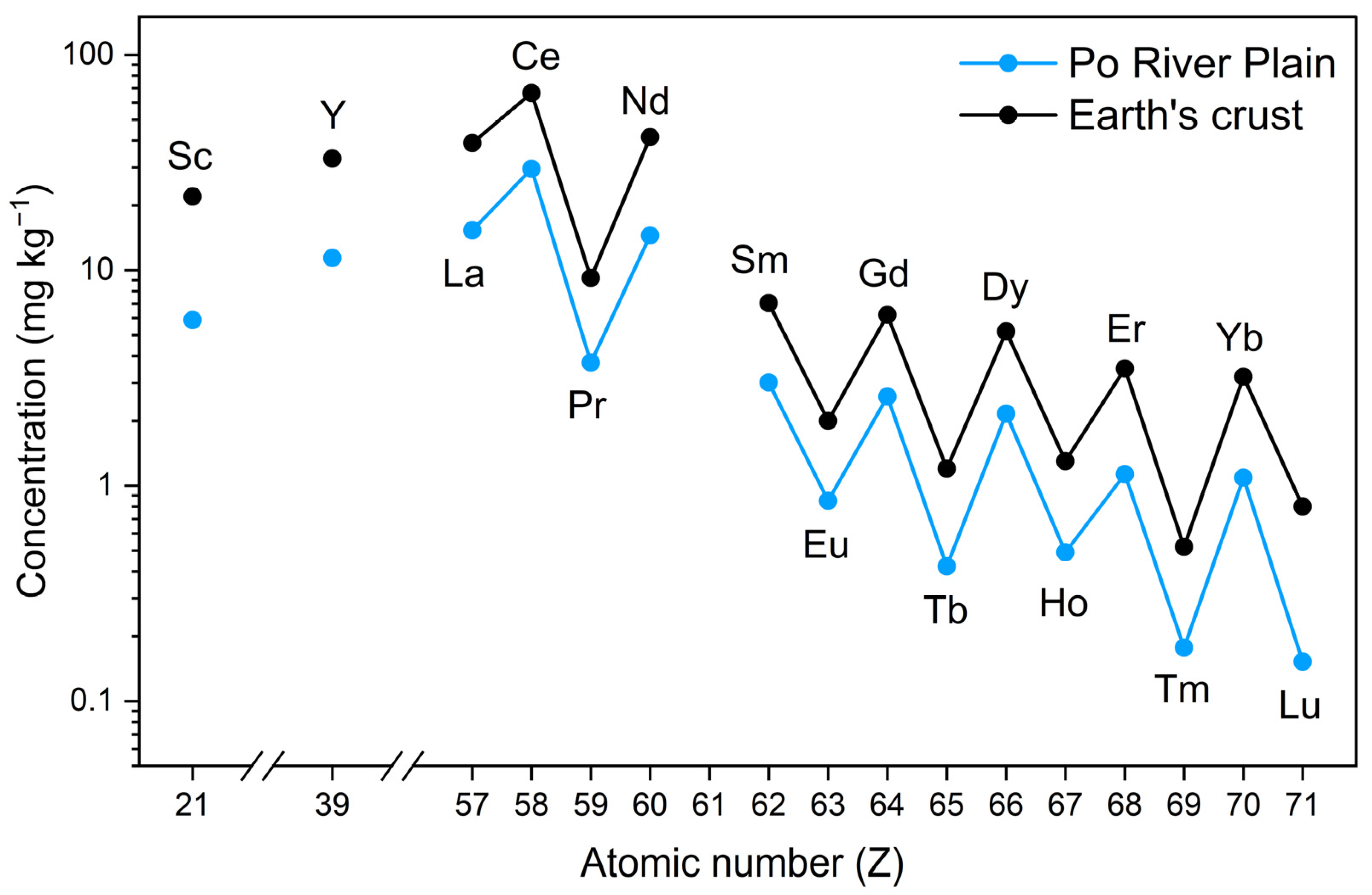
3.1. Influence of Parent Rocks
3.2. Geochemical Processes Affecting REE Mobility and Bioavailability in Soil
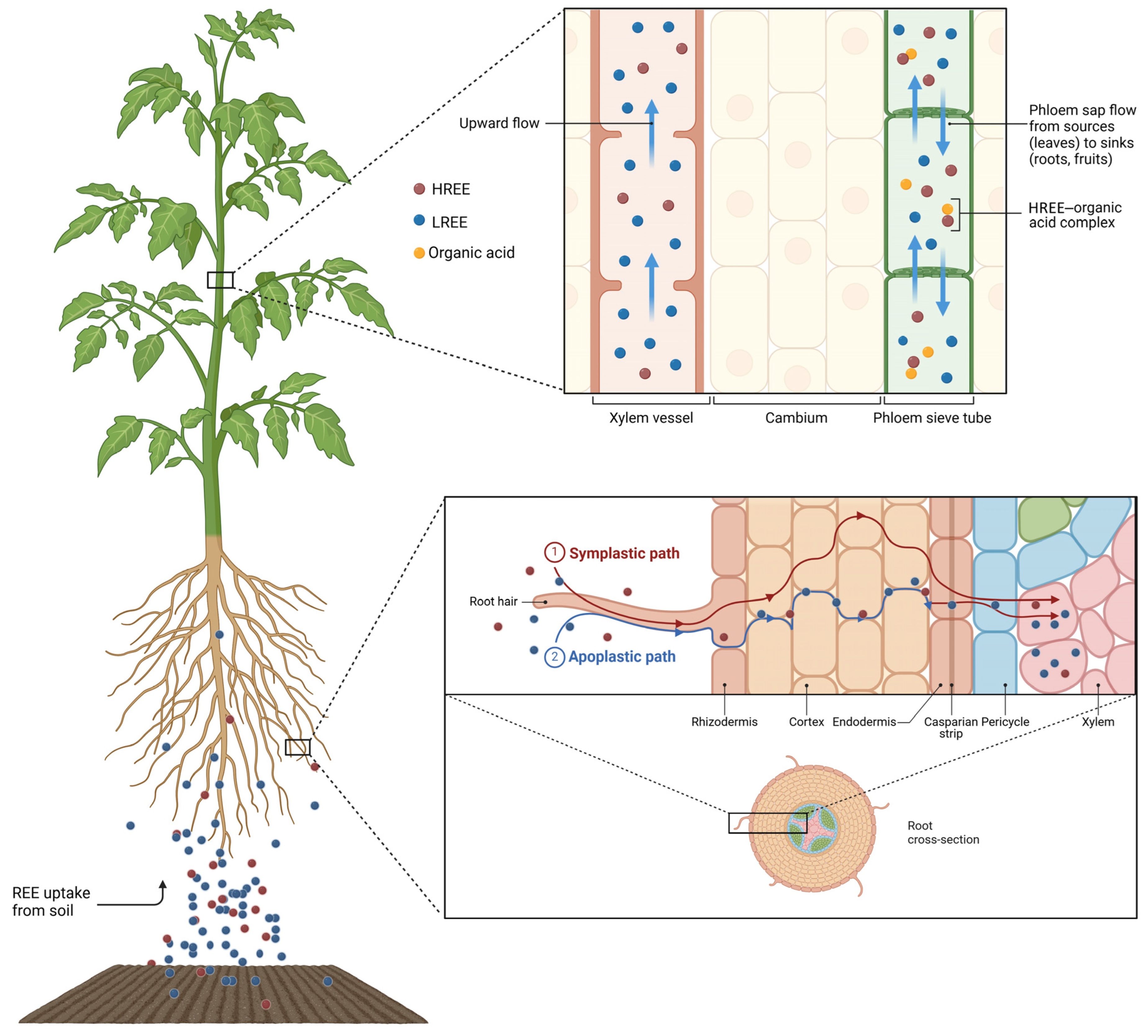
4. Absorption and Fractionation of REEs in Plants
4.1. What Happens Around the Roots: Events in the Rhizosphere
4.2. Root Surface Interactions
4.3. Upon Entering the Root
4.4. The Route for REE Translocation to the Shoot and Their Fractionation
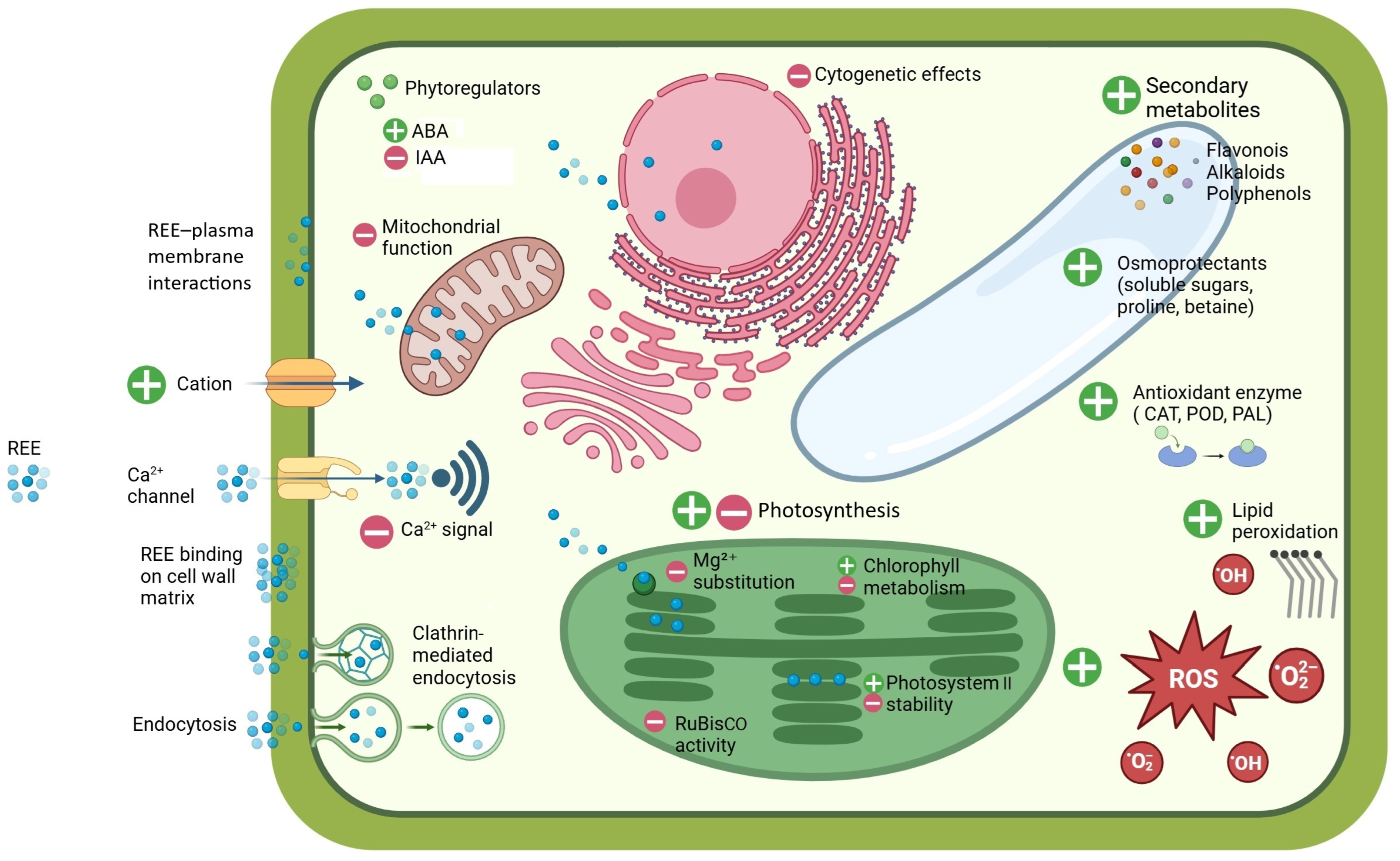
5. Impacts of REEs on Plant Processes
5.1. Hormetic Action of REEs on Plants
5.2. Interference of REEs with Ca2⁺-Mediated Signalling
5.3. Effects of REEs on General Plant Metabolism
5.4. Effects of REEs on Photosynthesis
5.5. Effects of REEs on Cell Structures
5.6. Effects of REEs on Plant Growth, Development, and Cytogenetics
6. Conclusions and Future Directions for Research
- (a)
- The simultaneous chemical interaction of REEs with soil phosphates, sulfates, and carboxyl groups of pectins at the root surface, and whether the plant cell is able to actively modulate it, for example, through pectin (de)methylation;
- (b)
- REEs and mineral nutrition, whether a general framework can be developed to understand how REEs can improve the uptake of specific microelements, including a probable cooperative role with mycorrhizal fungi;
- (c)
- Whether and how REE fractionation inside the plant is dependent on REE redistribution through the phloem and is related to different mechanisms of phloem (un)loading;
- (d)
- The Eu anomalies in plant organs relative to the soil, whether it is related to the redox chemistry of Eu within the plant cell;
- (e)
- The central role of ROS generated by REEs inside the plant cell, from which most of the beneficial changes could ensue;
- (f)
- The REEs as photosynthetic membrane stabilizers, disentangling whether it is an indirect (antioxidant induction) and/or direct (photosystem stability or other) effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tyler, G. Rare Earth Elements in Soil and Plant Systems—A Review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Ribeiro, P.G.; De Oliveira, C.; Guerra, M.B.B.; De Carvalho, T.S.; Martins, G.C.; Pereira, W.V.D.S.; Ramos, S.J.; Guilherme, L.R.G. Rare Earths as Emerging Trace Element Contaminants in the Soil. Curr. Pollut. Rep. 2024, 10, 443–458. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, C.; Chen, M.; Du, W.; Xu, X. Geobiochemistry Characteristics of Rare Earth Elements in Soil and Ground Water: A Case Study in Baotou, China. Sci. Rep. 2020, 10, 11740. [Google Scholar] [CrossRef]
- Aide, M. Lanthanide Soil Chemistry and Its Importance in Understanding Soil Pathways: Mobility, Plant Uptake, and Soil Health. In Lanthanides; IntechOpen: London, UK, 2018; ISBN 978-1-78985-010-9. [Google Scholar]
- Rim, K.-T. Effects of Rare Earth Elements on the Environment and Human Health: A Literature Review. Toxicol. Environ. Health Sci. 2016, 8, 189–200. [Google Scholar] [CrossRef]
- Walters, A.; Lusty, P. Rare Earth Elements. Available online: https://nora.nerc.ac.uk/id/eprint/17448 (accessed on 12 December 2024).
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The Story of Rare Earth Elements (REEs): Occurrences, Global Distribution, Genesis, Geology, Mineralogy and Global Production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Klinger, J.M. A Historical Geography of Rare Earth Elements: From Discovery to the Atomic Age. Extr. Ind. Soc. 2015, 2, 572–580. [Google Scholar] [CrossRef]
- Diao, H.; Yang, H.; Tan, T.; Ren, G.; You, M.; Wu, L.; Yang, M.; Bai, Y.; Xia, S.; Song, S.; et al. Navigating the Rare Earth Elements Landscape: Challenges, Innovations, and Sustainability. Miner. Eng. 2024, 216, 108889. [Google Scholar] [CrossRef]
- Jiménez-Ballesta, R.; Higueras, P.L.; García Navarro, F.J. Rare Earths in Soils. In Frontier Studies in Soil Science; Núñez-Delgado, A., Ed.; Springer International Publishing: Cham, Switzerland, 2024; pp. 43–77. ISBN 978-3-031-50503-4. [Google Scholar]
- Peng, X.-X.; Wang, M.-X.; Zhang, J.-L. Emerging Frontiers in Rare-Earth Element Chemical Biology. Coord. Chem. Rev. 2024, 519, 216096. [Google Scholar] [CrossRef]
- Hoshino, M.; Sanematsu, K.; Watanabe, Y. Chapter 279—REE Mineralogy and Resources. In Handbook on the Physics and Chemistry of Rare Earths; Jean-Claude, B., Vitalij, K.P., Eds.; Including Actinides; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 129–291. [Google Scholar]
- Tao, Y.; Shen, L.; Feng, C.; Yang, R.; Qu, J.; Ju, H.; Zhang, Y. Distribution of Rare Earth Elements (REEs) and Their Roles in Plant Growth: A Review. Environ. Pollut. 2022, 298, 118540. [Google Scholar] [CrossRef]
- Prudêncio, M.I.; Braga, M.A.S.; Gouveia, M.A. REE Mobilization, Fractionation and Precipitation During Weathering of Basalts. Chem. Geol. 1993, 107, 251–254. [Google Scholar] [CrossRef]
- Aide, M.; Aide, C. Rare Earth Elements: Their Importance in Understanding Soil Genesis. ISRN Soil Sci. 2012, 1, 783876. [Google Scholar] [CrossRef]
- Nikanorov, A. Oddo–Harkins Evenness Rule as an Indication of the Abundances of Chemical Elements in the Earth’s Hydrosphere and Estimations of the Nature of Cosmic Bodies. Geochem. Int. 2016, 54, 464–469. [Google Scholar] [CrossRef]
- Lide, D.R.; Baysinger, G.; Chemistry, S.; Berger, L.I.; Goldberg, R.N.; Kehiaian, H.V. CRC Handbook of Chemistry and Physics; CRC press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Cotton, S. Lanthanide and Actinide Chemistry, 2nd ed.; Wiley: Hoboken, NJ, USA, 2024; ISBN 978-1-118-87346-5. [Google Scholar]
- Grosjean, N.; Purwadi, I.; Sirguey, C.; Chalot, M.; Le Jean, M.; Van Der Ent, A.; Blaudez, D. Rare Earth Elements in Plants: Transfer, Transport, Accumulation, Impacts and Perspectives. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2024; Volume 109, pp. 19–61. ISBN 978-0-443-15825-4. [Google Scholar]
- Cotton, S.A.; Raithby, P.R. Systematics and Surprises in Lanthanide Coordination Chemistry. Coord. Chem. Rev. 2017, 340, 220–231. [Google Scholar] [CrossRef]
- Cotton, S. Two Centuries of the Rare Earths. Chim. Nouv. 2020, 133, 1–12. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physic, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Sager, M.; Wiche, O. Rare Earth Elements (REE): Origins, Dispersion, and Environmental Implications—A Comprehensive Review. Environments 2024, 11, 24. [Google Scholar] [CrossRef]
- Dostal, J. Rare Earth Element Deposits of Alkaline Igneous Rocks. Resources 2017, 6, 34. [Google Scholar] [CrossRef]
- Lesnov, F.P. Rare Earth Elements in Ultramafic and Mafic Rocks and Their Minerals: Main Types of Rocks: Rock-Forming Minerals; CRC Press/Balkema: London, UK, 2010; ISBN 978-0-415-57890-5. [Google Scholar]
- Wu, Z.; Chen, Y.; Wang, Y.; Xu, Y.; Lin, Z.; Liang, X.; Cheng, H. Review of Rare Earth Element (REE) Adsorption on and Desorption from Clay Minerals: Application to Formation and Mining of Ion-Adsorption REE Deposits. Ore Geol. Rev. 2023, 157, 105446. [Google Scholar] [CrossRef]
- Coppin, F.; Berger, G.; Bauer, A.; Castet, S.; Loubet, M. Sorption of Lanthanides on Smectite and Kaolinite. Chem. Geol. 2002, 182, 57–68. [Google Scholar] [CrossRef]
- Marques Fernandes, M.; Vér, N.; Baeyens, B. Predicting the Uptake of Cs, Co, Ni, Eu, Th and U on Argillaceous Rocks Using Sorption Models for Illite. Appl. Geochem. 2015, 59, 189–199. [Google Scholar] [CrossRef]
- Yang, J.; Torres, M.; McManus, J.; Algeo, T.J.; Hakala, J.A.; Verba, C. Controls on Rare Earth Element Distributions in Ancient Organic-Rich Sedimentary Sequences: Role of Post-Depositional Diagenesis of Phosphorus Phases. Chem. Geol. 2017, 466, 533–544. [Google Scholar] [CrossRef]
- Caetano, M.; Prego, R.; Vale, C.; de Pablo, H.; Marmolejo-Rodríguez, J. Record of Diagenesis of Rare Earth Elements and Other Metals in a Transitional Sedimentary Environment. Mar. Chem. 2009, 116, 36–46. [Google Scholar] [CrossRef]
- Bayon, G.; Toucanne, S.; Skonieczny, C.; André, L.; Bermell, S.; Cheron, S.; Dennielou, B.; Etoubleau, J.; Freslon, N.; Gauchery, T.; et al. Rare Earth Elements and Neodymium Isotopes in World River Sediments Revisited. Geochim. Cosmochim. Acta 2015, 170, 17–38. [Google Scholar] [CrossRef]
- Miruo, L.; Yanzhong, W.; Yingchang, C.; Shuping, W.; Qiangwang, X.; Xiuyu, D. Sources of Ca2+ in the Major Carbonate Cements in Eocene Sandstones and Conglomerates: Evidence from Sr Isotopes, Sr/Ca Ratios, and Rare-Earth Elements. Mar. Pet. Geol. 2020, 120, 104568. [Google Scholar] [CrossRef]
- Mihajlovic, J.; Rinklebe, J. Rare Earth Elements in German Soils—A Review. Chemosphere 2018, 205, 514–523. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R.G. Rare Earth Elements in the Soil Environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Maulana, A.; Yonezu, K.; Watanabe, K. Geochemistry of Rare Earth Elements (REE) in the Weathered Crusts from the Granitic Rocks in Sulawesi Island, Indonesia. J. Earth Sci. 2014, 25, 460–472. [Google Scholar] [CrossRef]
- Liang, X.; Wu, P.; Wei, G.; Yang, Y.; Ji, S.; Ma, L.; Zhou, J.; Tan, W.; Zhu, J.; Takahashi, Y. Enrichment and Fractionation of Rare Earth Elements (REEs) in Ion-Adsorption-Type REE Deposits: Constraints of an Iron (Hydr)Oxide–Clay Mineral Composite. Am. Mineral. J. Earth Planet. Mater. 2025, 110, 114–135. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, H.; Lin, X.; Liang, X.; Yamaguchi, A.; Zhu, J.; Takahashi, Y.; Zhu, R. Distribution of Rare Earth Elements (REEs) in Supergene Environment around a Typical Ion Adsorption–Type REE Deposit. Ore Geol. Rev. 2023, 162, 105721. [Google Scholar] [CrossRef]
- Catrouillet, C.; Guenet, H.; Pierson-Wickmann, A.-C.; Dia, A.; Bouhnik-Le Coz, M.; Deville, S.; Lenne, Q.; Suko, Y.; Davranche, M. Rare Earth Elements as Tracers of Active Colloidal Organic Matter Composition. Environ. Chem. 2020, 17, 133–139. [Google Scholar] [CrossRef]
- Tadayon, Y.; Vantelon, D.; Gigault, J.; Dia, A.; Pattier, M.; Dutruch, L.; Davranche, M. Rare Earth Elements Interaction with Iron-Organic Matter Colloids as a Control of the REE Environmental Dissemination. J. Colloid Interface Sci. 2024, 655, 70–79. [Google Scholar] [CrossRef]
- Li, W.; Nakada, R.; Takahashi, Y.; Gaschnig, R.M.; Hu, Y.; Shakouri, M.; Rudnick, R.L.; Liu, X.-M. Cerium Geochemical Composition of the Upper Continental Crust through Time: Implications for Tracing Past Surface Redox Conditions. Geochim. Cosmochim. Acta 2023, 359, 20–29. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P. Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar] [CrossRef]
- Laveuf, C.; Cornu, S. A Review on the Potentiality of Rare Earth Elements to Trace Pedogenetic Processes. Geoderma 2009, 154, 1–12. [Google Scholar] [CrossRef]
- Yongliang, X.; Yusheng, Z. The Mobility of Rare-Earth Elements during Hydrothermal Activity: A Review. Chin. J. Geochem. 1991, 10, 295–306. [Google Scholar] [CrossRef]
- Åström, M.; Corin, N. Distribution of Rare Earth Elements in Anionic, Cationic and Particulate Fractions in Boreal Humus-Rich Streams Affected by Acid Sulphate Soils. Water Res. 2003, 37, 273–280. [Google Scholar] [CrossRef]
- Åström, M. Abundance and Fractionation Patterns of Rare Earth Elements in Streams Affected by Acid Sulphate Soils. Chem. Geol. 2001, 175, 249–258. [Google Scholar] [CrossRef]
- Nyman, A.; Johnson, A.; Yu, C.; Dopson, M.; Åström, M. Multi-Element Features of Active Acid Sulfate Soils across the Swedish Coastal Plains. Appl. Geochem. 2023, 152, 105653. [Google Scholar] [CrossRef]
- Wiche, O.; Zertani, V.; Hentschel, W.; Achtziger, R.; Midula, P. Germanium and Rare Earth Elements in Topsoil and Soil-Grown Plants on Different Land Use Types in the Mining Area of Freiberg (Germany). J. Geochem. Explor. 2017, 175, 120–129. [Google Scholar] [CrossRef]
- D’Antone, C.; Punturo, R.; Vaccaro, C. Rare Earth Elements Distribution in Grapevine Varieties Grown on Volcanic Soils: An Example from Mount Etna (Sicily, Italy). Environ. Monit. Assess. 2017, 189, 160. [Google Scholar] [CrossRef]
- Pepi, S.; Sansone, L.; Chicca, M.; Marrocchino, E.; Vaccaro, C. Distribution of Rare Earth Elements in Soil and Grape Berries of Vitis vinifera Cv. “Glera”. Environ. Monit. Assess. 2016, 188, 477. [Google Scholar] [CrossRef]
- Balaram, V. Rare Earth Elements: A Review of Applications, Occurrence, Exploration, Analysis, Recycling, and Environmental Impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Jiménez-Ballesta, R.; Bravo, S.; Amoros, J.A.; Pérez-de-los-Reyes, C.; García-Pradas, J.; Sanchez, M.; García-Navarro, F.J. Occurrence of Some Rare Earth Elements in Vineyard Soils Under Semiarid Mediterranean Environment. Environ. Monit. Assess. 2021, 194, 341. [Google Scholar] [CrossRef]
- Punturo, R.; D’Antone, C.; Pepi, S.; Vaccaro, C. Rare Earth Elements Absorption Patterns in Grapevine “Vitis vinifera L.” Cultivated in Carbonate Terrains (South-Eastern Sicily, Italy). Environ. Earth Sci. 2018, 77, 801. [Google Scholar] [CrossRef]
- Charlesworth, S.; De Miguel, E.; Ordóñez, A. A Review of the Distribution of Particulate Trace Elements in Urban Terrestrial Environments and Its Application to Considerations of Risk. Environ. Geochem. Health 2011, 33, 103–123. [Google Scholar] [CrossRef]
- Galhardi, J.A.; Leles, B.P.; de Mello, J.W.V.; Wilkinson, K.J. Bioavailability of Trace Metals and Rare Earth Elements (REE) from the Tropical Soils of a Coal Mining Area. Sci. Total Environ. 2020, 717, 134484. [Google Scholar] [CrossRef]
- Huang, Z.-Y.; Wu, C.-Y.; Hseu, Z.-Y. Rare earth elements in tea garden soils and their bioavailability to tea buds in Taiwan. Sci. Total Environ. 2023, 893, 164895. [Google Scholar] [CrossRef]
- Hovey, J.L.; Dittrich, T.M.; Allen, M.J. Coordination Chemistry of Surface-Associated Ligands for Solid–Liquid Adsorption of Rare-Earth Elements. J. Rare Earths 2023, 41, 1–18. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, G.; Zhou, C.; Luo, J.; Lin, J.; Zhao, X. Rehabilitation Effect of the Combined Application of Bamboo Biochar and Coal Ash on Ion-Adsorption-Type Rare Earth Tailings. J. Soils Sediments 2020, 20, 3351–3357. [Google Scholar] [CrossRef]
- Liu, W.-S.; van der Ent, A.; Erskine, P.D.; Morel, J.L.; Echevarria, G.; Spiers, K.M.; Montargès-Pelletier, E.; Qiu, R.-L.; Tang, Y.-T. Spatially Resolved Localization of Lanthanum and Cerium in the Rare Earth Element Hyperaccumulator Fern Dicranopteris linearis from China. Environ. Sci. Technol. 2020, 54, 2287–2294. [Google Scholar] [CrossRef]
- Hayat, S.; Faraz, A.; Faizan, M. Root Exudates: Composition and Impact on Plant–Microbe Interaction; Ahmad, I., Husain, F.M., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 179–193. [Google Scholar]
- He, C.; Feng, Y.; Deng, Y.; Lin, L.; Cheng, S. A Systematic Review and Meta-Analysis on the Root Effects and Toxic Mechanisms of Rare Earth Elements. Chemosphere 2024, 363, 142951. [Google Scholar] [CrossRef]
- Hu, R.; Beguiristain, T.; De Junet, A.; Leyval, C. No Significant Transfer of the Rare Earth Element Samarium from Spiked Soil to Alfalfa by Funneliformis mosseae. Mycorrhiza 2020, 30, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Watanabe, S.; Gao, F.; Dubos, C. Iron Nutrition in Plants: Towards a New Paradigm? Plants 2023, 12, 384. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.K.; Singh, B. Uninhibited Biosynthesis and Release of Phytosiderophores in the Presence of Heavy Metal (HM) Favors HM Remediation. Environ. Sci. Pollut. Res. 2017, 24, 9407–9416. [Google Scholar] [CrossRef]
- Kaliakin, D.S.; Sobrinho, J.A.; Monteiro, J.H.S.K.; de Bettencourt-Dias, A.; Cantu, D.C. Solution Structure of a Europium-Nicotianamine Complex Supports That Phytosiderophores Bind Lanthanides. Phys. Chem. Chem. Phys. 2021, 23, 4287–4299. [Google Scholar] [CrossRef]
- Schwabe, R.; Dittrich, C.; Kadner, J.; Rudi Senges, C.H.; Bandow, J.E.; Tischler, D.; Schlömann, M.; Levicán, G.; Wiche, O. Secondary Metabolites Released by the Rhizosphere Bacteria Arthrobacter oxydans and Kocuria rosea Enhance Plant Availability and Soil–Plant Transfer of Germanium (Ge) and Rare Earth Elements (REEs). Chemosphere 2021, 285, 131466. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Z.; Pan, H.; Bai, Y.; Hu, Y.; Jin, S. Effects of Rare Earth Elements on Bacteria in Rhizosphere, Root, Phyllosphere and Leaf of Soil–Rice Ecosystem. Sci. Rep. 2022, 12, 2089. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Z.; Hao, B.; Diao, F.; Zhang, J.; Bao, Z.; Guo, W. Arbuscular Mycorrhizal Fungi Alter Microbiome Structure of Rhizosphere Soil to Enhance Maize Tolerance to La. Ecotoxicol. Environ. Saf. 2021, 212, 111996. [Google Scholar] [CrossRef]
- Chang, Q.; Diao, F.; Wang, Q.; Pan, L.; Dang, Z.; Guo, W. Effects of Arbuscular Mycorrhizal Symbiosis on Growth, Nutrient and Metal Uptake by Maize Seedlings (Zea mays L.) Grown in Soils Spiked with Lanthanum and Cadmium. Environ. Pollut. 2018, 241, 607–615. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy Metal and Metalloid Toxicity in Horticultural Plants: Tolerance Mechanism and Remediation Strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular Mycorrhizal Fungi-Induced Mitigation of Heavy Metal Phytotoxicity in Metal Contaminated Soils: A Critical Review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Hu, Z.-H.; Yan, T.-X.; Lu, R.-R.; Peng, C.-L.; Li, S.-S.; Jing, Y.-X. Arbuscular Mycorrhizal Fungi Alleviate Cd Phytotoxicity by Altering Cd Subcellular Distribution and Chemical Forms in Zea mays. Ecotoxicol. Environ. Saf. 2019, 171, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Diao, F.; Hao, B.; Xu, L.; Jia, B.; Hou, Y.; Ding, S.; Guo, W. Multiomics Reveals Claroideoglomus etunicatum Regulates Plant Hormone Signal Transduction, Photosynthesis and La Compartmentalization in Maize to Promote Growth under La Stress. Ecotoxicol. Environ. Saf. 2023, 262, 115128. [Google Scholar] [CrossRef]
- Ding, S.; Liang, T.; Zhang, C.; Yan, J.; Zhang, Z. Accumulation and Fractionation of Rare Earth Elements (REEs) in Wheat: Controlled by Phosphate Precipitation, Cell Wall Absorption and Solution Complexation. J. Exp. Bot. 2005, 56, 2765–2775. [Google Scholar] [CrossRef] [PubMed]
- Jalali, J.; Lebeau, T. The Role of Microorganisms in Mobilization and Phytoextraction of Rare Earth Elements: A Review. Front. Environ. Sci. 2021, 9, 688430. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, Q.; Yang, L.; Huang, B. Subcellular Distribution of Rare Earth Elements and Characterization of Their Binding Species in a Newly Discovered Hyperaccumulator Pronephrium simplex. Talanta 2006, 70, 26–31. [Google Scholar] [CrossRef]
- Liang, T.; Ding, S.; Song, W.; Chong, Z.; Zhang, C.; Li, H. A Review of Fractionations of Rare Earth Elements in Plants. J. Rare Earths 2008, 26, 7–15. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier/Academic Press: Cambridge, MA, USA, 2012; ISBN 978-0-12-384905-2. [Google Scholar]
- Zhu, X.F.; Wang, Z.W.; Wan, J.X.; Sun, Y.; Wu, Y.R.; Li, G.X.; Shen, R.F.; Zheng, S.J. Pectin Enhances Rice (Oryza sativa) Root Phosphorus Remobilization. J. Exp. Bot. 2015, 66, 1017–1024. [Google Scholar] [CrossRef]
- Qi, W.; Ye, T.; Xiaolong, Z.; Xiaoying, D.; Jixing, X.; Renfang, S.; Xiaofang, Z. Pectin Methylesterases Enhance Root Cell Wall Phosphorus Remobilization in Rice. Rice Sci. 2022, 29, 179–188. [Google Scholar] [CrossRef]
- Shan, X.; Wang, H.; Zhang, S.; Zhou, H.; Zheng, Y.; Yu, H.; Wen, B. Accumulation and Uptake of Light Rare Earth Elements in a Hyperaccumulator Dicropteris dichotoma. Plant Sci. 2003, 165, 1343–1353. [Google Scholar] [CrossRef]
- Brioschi, L.; Steinmann, M.; Lucot, E.; Pierret, M.C.; Stille, P.; Prunier, J.; Badot, P.M. Transfer of Rare Earth Elements (REE) from Natural Soil to Plant Systems: Implications for the Environmental Availability of Anthropogenic REE. Plant Soil 2013, 366, 143–163. [Google Scholar] [CrossRef]
- Naseer, S.; Lee, Y.; Lapierre, C.; Franke, R.; Nawrath, C.; Geldner, N. Casparian Strip Diffusion Barrier in Arabidopsis Is Made of a Lignin Polymer without Suberin. Proc. Natl. Acad. Sci. USA 2012, 109, 10101–10106. [Google Scholar] [CrossRef] [PubMed]
- Barberon, M. The Endodermis as a Checkpoint for Nutrients. New Phytol. 2017, 213, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, G.; Thomson, W.W.; Leonard, R.T. The Casparian Strip as a Barrier to the Movement of Lanthanum in Corn Roots. Science 1974, 183, 670–671. [Google Scholar] [CrossRef] [PubMed]
- DuPont, F.M.; Leonard, R.T. The Use of Lanthanum to Study the Functional Development of the Casparian Strip in Corn Roots. Protoplasma 1977, 91, 315–323. [Google Scholar] [CrossRef]
- Lawton, J.R.; Todd, A.; Naidoo, D.K. Preliminary Investigations into the Structure of the Roots of the Mangroves, Avicennia marina and Bruguiera gymnorrhiza, in Relation to Ion Uptake. New Phytol. 1981, 88, 713–722. [Google Scholar] [CrossRef]
- Zadokar, A.; Negi, S.; Kumar, P.; Bhargava, B.; Sharma, R.; Irfan, M. Molecular Insights into Rare Earth Element (REE)-Mediated Phytotoxicity and Its Impact on Human Health. Environ. Sci. Pollut. Res. 2023, 30, 84829–84849. [Google Scholar] [CrossRef]
- Dhara, A.; Raichaudhuri, A. ABCG Transporter Proteins with Beneficial Activity on Plants. Phytochemistry 2021, 184, 112663. [Google Scholar] [CrossRef]
- de Oliveira, C.; Ramos, S.J.; Siqueira, J.O.; Faquin, V.; de Castro, E.M.; Amaral, D.C.; Techio, V.H.; Coelho, L.C.; e Silva, P.H.P.; Schnug, E.; et al. Bioaccumulation and Effects of Lanthanum on Growth and Mitotic Index in Soybean Plants. Ecotoxicol. Environ. Saf. 2015, 122, 136–144. [Google Scholar] [CrossRef]
- Diatloff, E.; Smith, F.W.; Asher, C.J. Effects of Lanthanum and Cerium on the Growth and Mineral Nutrition of Corn and Mungbean. Ann. Bot. 2008, 101, 971–982. [Google Scholar] [CrossRef]
- Huang, G.; Wang, L.; Sun, Z.; Li, X.; Zhou, Q.; Huang, X. Combined Effects of Lanthanum(III) and Elevated Ultraviolet-B Radiation on Root Nitrogen Nutrient in Soybean Seedlings. Biol. Trace Elem. Res. 2015, 163, 224–234. [Google Scholar] [CrossRef]
- Yuan, M.; Guo, M.-N.; Liu, W.-S.; Liu, C.; Van Der Ent, A.; Morel, J.L.; Huot, H.; Zhao, W.-Y.; Wei, X.-G.; Qiu, R.-L.; et al. The Accumulation and Fractionation of Rare Earth Elements in Hydroponically Grown Phytolacca americana L. Plant Soil 2017, 421, 67–82. [Google Scholar] [CrossRef]
- Gong, B.; He, E.; Qiu, H.; Li, J.; Ji, J.; Zhao, L.; Cao, X. Phytotoxicity of Individual and Binary Mixtures of Rare Earth Elements (Y, La, and Ce) in Relation to Bioavailability. Environ. Pollut. 2019, 246, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Fehlauer, T.; Collin, B.; Angeletti, B.; Negahi, M.M.; Dentant, C.; Chaurand, P.; Lallemand, C.; Levard, C.; Rose, J. Multiscale Imaging on Saxifraga paniculata Provides New Insights into Yttrium Uptake by Plants. Sci. Rep. 2022, 12, 18268. [Google Scholar] [CrossRef]
- Zheng, H.-X.; Liu, W.-S.; Sun, D.; Zhu, S.-C.; Li, Y.; Yang, Y.-L.; Liu, R.-R.; Feng, H.-Y.; Cai, X.; Cao, Y.; et al. Plasma-Membrane-Localized Transporter NREET1 Is Responsible for Rare Earth Element Uptake in Hyperaccumulator Dicranopteris linearis. Environ. Sci. Technol. 2023, 57, 6922–6933. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhou, Q.; Yang, G.; Ding, X.L.; Li, X.; Cai, C.X.; Zhang, Z.; Wei, H.Y.; Lu, T.H.; et al. Rare Earth Elements Activate Endocytosis in Plant Cells. Proc. Natl. Acad. Sci. USA 2014, 111, 12936–12941. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, X.; Ben, Y.; Zhang, S.; Wang, L.; Zhou, Q.; Huang, X. Enrichment Process of Lanthanum as a Nonessential Trace Element in Leaf Cells of Lettuce (Lactuca sativa L.). J. Rare Earths 2022, 40, 1969–1976. [Google Scholar] [CrossRef]
- Ben, Y.; Cheng, M.; Liu, Y.; Wang, L.; Yang, Q.; Huang, X.; Zhou, Q. The Stimulatory Effect and Mechanism of Low-Dose Lanthanum on Soybean Leaf Cells. J. Hazard. Mater. 2023, 441, 129924. [Google Scholar] [CrossRef]
- Brown, P.H.; Rathjen, A.H.; Graham, R.D.; Tribe, D.E. Chapter 92—Rare Earth Elements in Biological Systems. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 1990; Volume 13, pp. 423–452. [Google Scholar]
- Kaur, P.; Mahajan, M.; Gambhir, H.; Khan, A.; Khan, M.I.R. Rare Earth Metallic Elements in Plants: Assessing Benefits, Risks and Mitigating Strategies. Plant Cell Rep. 2024, 43, 216. [Google Scholar] [CrossRef]
- He, D.; Guo, T.; Peng, C.; Li, J.; Wang, F. Foliar Application of Lanthanum Promotes Growth and Phytoremediation Potential Solanum nigrum L. J. Environ. Manag. 2023, 334, 117259. [Google Scholar] [CrossRef]
- He, D.; Xia, B.; Zhou, Q.; Wang, L.; Huang, X. Rare Earth Elements Regulate the Endocytosis and DNA Methylation in Root Cells of Arabidopsis thaliana. Chemosphere 2019, 227, 522–532. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, M.; Chu, Y.; Li, X.; Chen, D.D.Y.; Huang, X.; Zhou, Q. Responses of Plant Calmodulin to Endocytosis Induced by Rare Earth Elements. Chemosphere 2016, 154, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Kötschau, A.; Büchel, G.; Einax, J.W.; von Tümpling, W.; Merten, D. Sunflower (Helianthus annuus): Phytoextraction Capacity for Heavy Metals on a Mining-Influenced Area in Thuringia, Germany. Environ. Earth Sci. 2014, 72, 2023–2031. [Google Scholar] [CrossRef]
- Ramírez-Olvera, S.M.; Trejo-Téllez, L.I.; García-Morales, S.; Pérez-Sato, J.A.; Gómez-Merino, F.C. Cerium Enhances Germination and Shoot Growth, and Alters Mineral Nutrient Concentration in Rice. PLoS ONE 2018, 13, e0194691. [Google Scholar] [CrossRef]
- Fu, Y.; Li, F.; Xu, T.; Cai, S.; Chu, W.; Qiu, H.; Sha, S.; Cheng, G.; Xu, Q. Bioaccumulation, Subcellular, and Molecular Localization and Damage to Physiology and Ultrastructure in Nymphoides peltata (Gmel.) O. Kuntze Exposed to Yttrium. Environ. Sci. Pollut. Res. 2014, 21, 2935–2942. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Zhou, Q. Effects of Rare Earth Elements on the Distribution of Mineral Elements and Heavy Metals in Horseradish. Chemosphere 2008, 73, 314–319. [Google Scholar] [CrossRef]
- Wiche, O.; Dittrich, C.; Pourret, O.; Monei, N.; Heim, J.; Lambers, H. Relationships between Carboxylate-Based Nutrient-Acquisition Strategies, Phosphorus-Nutritional Status and Rare Earth Element Accumulation in Plants. Plant Soil 2023, 489, 645–666. [Google Scholar] [CrossRef]
- Gao, Y.; Zeng, F.; Yi, A.; Ping, S.; Jing, L. Research of the Entry of Rare Earth Elements Eu3+ and La3+ into Plant Cell. Biol. Trace Elem. Res. 2003, 91, 253–265. [Google Scholar] [CrossRef]
- Han, F.; Shan, X.-Q.; Zhang, J.; Xie, Y.-N.; Pei, Z.-G.; Zhang, S.-Z.; Zhu, Y.-G.; Wen, B. Organic Acids Promote the Uptake of Lanthanum by Barley Roots. New Phytol. 2005, 165, 481–492. [Google Scholar] [CrossRef]
- Ding, S.; Liang, T.; Wang, L.J.; Sun, Q. Characteristics of Translocation and Fractionation of Rare Earth Elements in Soil-Wheat System. J. Agro-Environ. Sci. Chin. 2003, 22, 519–523. [Google Scholar]
- Fu, F.; Akagi, T.; Yabuki, S.; Iwaki, M. The Variation of REE (Rare Earth Elements) Patterns in Soil-Grown Plants: A New Proxy for the Source of Rare Earth Elements and Silicon in Plants. Plant Soil 2001, 235, 53–64. [Google Scholar] [CrossRef]
- Luo, Z.-B.; He, J.; Polle, A.; Rennenberg, H. Heavy Metal Accumulation and Signal Transduction in Herbaceous and Woody Plants: Paving the Way for Enhancing Phytoremediation Efficiency. Biotechnol. Adv. 2016, 34, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Erenoglu, B.; Nikolic, M.; Römheld, V.; Cakmak, I. Uptake and Transport of Foliar Applied Zinc (65Zn) in Bread and Durum Wheat Cultivars Differing in Zinc Efficiency. Plant Soil 2002, 241, 251–257. [Google Scholar] [CrossRef]
- Ando, Y.; Nagata, S.; Yanagisawa, S.; Yoneyama, T. Copper in Xylem and Phloem Saps from Rice (Oryza sativa): The Effect of Moderate Copper Concentrations in the Growth Medium on the Accumulation of Five Essential Metals and a Speciation Analysis of Copper-Containing Compounds. Funct. Plant Biol. 2012, 40, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.R.; Sammons, R.D.; Grabiak, R.C. A Speciation Model of Essential Trace Metal Ions in Phloem. J. Inorg. Biochem. 2012, 116, 140–150. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, S.; Yan, S.; Lei, S.; Gao, Y.; Chen, K.; Shi, X.; Yuan, M.; Yao, H. The Translocation and Fractionation of Rare Earth Elements (REEs) via the Phloem in Phytolacca americana L. Environ. Sci. Pollut. Res. 2023, 30, 114044–114055. [Google Scholar] [CrossRef]
- Ernst, W.H.O. Instrumental Element and Multi–Element Analysis of Plant Samples. Methods and Applications. B. Markert. Plant Ecol. 1998, 136, 250–251. [Google Scholar] [CrossRef]
- Grosjean, N.; Le Jean, M.; Berthelot, C.; Chalot, M.; Gross, E.M.; Blaudez, D. Accumulation and Fractionation of Rare Earth Elements Are Conserved Traits in the Phytolacca Genus. Sci. Rep. 2019, 9, 18458. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, K.; Lei, S.; Gao, Y.; Yan, S.; Yuan, M. Rare Earth Elements (REEs) Adsorption and Detoxification Mechanisms in Cell Wall Polysaccharides of Phytolacca americana L. Plants 2023, 12, 1981. [Google Scholar] [CrossRef]
- Hu, Z.; Haneklaus, S.; Sparovek, G.; Schnug, E. Rare Earth Elements in Soils. Commun. Soil Sci. Plant Anal. 2006, 37, 1381–1420. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Feng, L.; Chen, Z.; Owens, G.; Chen, Z. Uptake and Transport Mechanisms of Rare Earth Hyperaccumulators: A Review. J. Environ. Manag. 2024, 351, 119998. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, C.; Liu, W.-S.; Guo, M.-N.; Morel, J.L.; Huot, H.; Yu, H.-J.; Tang, Y.-T.; Qiu, R.-L. Accumulation and Fractionation of Rare Earth Elements (REEs) in the Naturally Grown Phytolacca americana L. in Southern China. Int. J. Phytorem. 2018, 20, 415–423. [Google Scholar] [CrossRef]
- Ding, S.; Liang, T.; Yan, J.; Zhang, Z.; Huang, Z.; Xie, Y. Fractionations of Rare Earth Elements in Plants and Their Conceptive Model. Sci. China C Life Sci. 2007, 50, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Liang, T. Fractionation Mechanisms of Rare Earth Elements (REEs) in Hydroponic Wheat: An Application for Metal Accumulation by Plants. Environ. Sci. Technol. 2006, 40, 2686–2691. [Google Scholar] [CrossRef]
- Wang, H.; Shan, X.; Zhang, S.; Wen, B. Preliminary Characterization of a Light-Rare-Earth-Element-Binding Peptide of a Natural Perennial Fern Dicranopteris dichotoma. Anal. Bioanal. Chem. 2003, 376, 49–52. [Google Scholar] [CrossRef]
- Pourret, O.; van der Ent, A.; Hursthouse, A.; Irawan, D.E.; Liu, H.; Wiche, O. The ‘Europium Anomaly’ in Plants: Facts and Fiction. Plant Soil 2022, 476, 721–728. [Google Scholar] [CrossRef]
- Barbera, M.; Zuddas, P.; Piazzese, D.; Oddo, E.; Lopes, F.; Censi, P.; Saiano, F. Accumulation of Rare Earth Elements in Common Vine Leaves Is Achieved through Extraction from Soil and Transport in the Xylem Sap. Commun. Earth Environ. 2023, 4, 291. [Google Scholar] [CrossRef]
- Ding, S.; Liang, T.; Zhang, C.; Yan, J.; Zhang, Z.; Sun, Q. Role of Ligands in Accumulation and Fractionation of Rare Earth Elements in Plants. Biol. Trace Elem. Res. 2005, 107, 73–86. [Google Scholar] [CrossRef]
- Krzciuk, K.; Gałuszka, A. Presence and Possible Origin of Positive Eu Anomaly in Shoot Samples of Juncus effusus L. J. Trace Elem. Med. Biol. 2020, 58, 126432. [Google Scholar] [CrossRef]
- Censi, P.; Saiano, F.; Pisciotta, A.; Tuzzolino, N. Geochemical Behaviour of Rare Earths in Vitis vinifera Grafted onto Different Rootstocks and Growing on Several Soils. Sci. Total Environ. 2014, 473–474, 597–608. [Google Scholar] [CrossRef]
- Song, K.; Gao, J.; Li, S.; Sun, Y.; Sun, H.; An, B.; Hu, T.; He, X. Experimental and Theoretical Study of the Effects of Rare Earth Elements on Growth and Chlorophyll of Alfalfa (Medicago sativa L.) Seedling. Front. Plant Sci. 2021, 12, 731838. [Google Scholar] [CrossRef]
- Egler, S.G.; Niemeyer, J.C.; Correia, F.V.; Saggioro, E.M. Effects of Rare Earth Elements (REE) on Terrestrial Organisms: Current Status and Future Directions. Ecotoxicology 2022, 31, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, F.; Thomas, P.J.; Pagano, G.; Perono, G.A.; Oral, R.; Lyons, D.M.; Toscanesi, M.; Trifuoggi, M. Review of Rare Earth Elements as Fertilizers and Feed Additives: A Knowledge Gap Analysis. Arch. Environ. Contam. Toxicol. 2021, 81, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Kastori, R.; Putnik-Delić, M.; Maksimović, I. Rare Earth Elements Application in Agriculture. Acta Agric. Serbica 2023, 28, 87–95. [Google Scholar] [CrossRef]
- Guo, B.S.; Zhu, W.M.; Xiong, P.K.; Ji, Y.J.; Liu, Z.; Wu, Z.M. Rare Earth Elements in Agriculture; Agricultural Scientific Technological Press: Beijing, China, 1988. [Google Scholar]
- Andrew, J.R. Plant Growth Stimulators Comprising Metal Ions and Long-Chain Alkyl Carboxylic Acids and Alts and Derivatives Thereof. UK Patent GB 2118158, 2 March 1983. [Google Scholar]
- Zhu, Z.; Liu, C.-Q.; Wang, Z.-L.; Liu, X.; Li, J. Rare Earth Elements Concentrations and Speciation in Rainwater from Guiyang, an Acid Rain Impacted Zone of Southwest China. Chem. Geol. 2016, 442, 23–34. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Mu, Y. Effects of Rare-Earth Fertilizers on the Emission of Nitrous Oxide from Agricultural Soils in China. Atmos. Environ. 2008, 42, 3882–3887. [Google Scholar] [CrossRef]
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.A.; Shafiq, M.; Yi, H.; Jilani, G.; Javed, R.; et al. Cryptic Footprints of Rare Earth Elements on Natural Resources and Living Organisms. Environ. Int. 2019, 127, 785–800. [Google Scholar] [CrossRef]
- He, E.; Qiu, H. Lanthanum and Cerium Disrupt Similar Biological Pathways and Interact Synergistically in Triticum aestivum as Revealed by Metabolomic Profiling and Quantitative Modeling. J. Hazard. Mater. 2022, 426, 127831. [Google Scholar] [CrossRef]
- de Oliveira, C.; Ramos, S.J.; Dinali, G.S.; de Carvalho, T.S.; Martins, F.A.D.; Faquin, V.; Castro, E.M.D.; Sarkis, J.E.S.; Siqueira, J.O.; Guilherme, L.R.G. Biostimulant Response of Foliar Application of Rare Earth Elements on Physiology, Growth, and Yield of Rice. Plants 2024, 13, 1435. [Google Scholar] [CrossRef]
- Samal, R.R.; Subudhi, U. Biochemical and Biophysical Interaction of Rare Earth Elements with Biomacromolecules: A Comprehensive Review. Chemosphere 2024, 357, 142090. [Google Scholar] [CrossRef]
- Ozturk, M.; Metin, M.; Altay, V.; Prasad, M.N.V.; Gul, A.; Bhat, R.A.; Darvash, M.A.; Hasanuzzaman, M.; Nahar, K.; Unal, D.; et al. Role of Rare Earth Elements in Plants. Plant Mol. Biol. Report. 2023, 41, 345–368. [Google Scholar] [CrossRef]
- Squier, T.C.; Bigelow, D.J.; Fernandez-Belda, F.J.; Demeis, L.; Inesi, G. Calcium and lanthanide binding in the sarcoplasmic reticulum ATPase. J. Biol. Chem. 1990, 265, 13713–13720. [Google Scholar] [CrossRef] [PubMed]
- Kotelnikova, A.; Fastovets, I.; Rogova, O.; Volkov, D.S.; Stolbova, V. Toxicity Assay of Lanthanum and Cerium in Solutions and Soil. Ecotoxicol. Environ. Saf. 2019, 167, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.; Kircheva, N.; Dobrev, S.; Angelova, S.; Dudev, T. Lanthanides as Calcium Mimetic Species in Calcium-Signaling/Buffering Proteins: The Effect of Lanthanide Type on the Ca2+/Ln3+ Competition. Int. J. Mol. Sci. 2023, 24, 6297. [Google Scholar] [CrossRef] [PubMed]
- Gutenthaler, S.M.; Tsushima, S.; Steudtner, R.; Gailer, M.; Hoffmann-Röder, A.; Drobot, B.; Daumann, L.J. Lanmodulin Peptides—Unravelling the Binding of the EF-Hand Loop Sequences Stripped from the Structural Corset. Inorg. Chem. Front. 2022, 9, 4009–4021. [Google Scholar] [CrossRef]
- Dridi, N.; Brito, P.; Bouslimi, H.; Ferreira, R.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Physiological and Biochemical Behaviours and Antioxidant Response of Helianthus annuus under Lanthanum and Cerium Stress. Sustainability 2022, 14, 4153. [Google Scholar] [CrossRef]
- Ozaki, T.; Ambe, S.; Abe, T.; Francis, A.J. Competitive Inhibition and Selectivity Enhancement by Ca in the Uptake of Inorganic Elements (Be, Na, Mg, K, Ca, Sc, Mn, Co, Zn, Se, Rb, Sr, Y, Zr, Ce, Pm, Gd, Hf) by Carrot (Daucus carota Cv. U.S. Harumakigosun). Biol. Trace Elem. Res. 2005, 103, 69–82. [Google Scholar] [CrossRef]
- Tähtiharju, S.; Sangwan, V.; Monroy, A.F.; Dhindsa, R.S.; Borg, M. The Induction of Kin Genes in Cold-Acclimating Arabidopsis thaliana. Evidence of a Role for Calcium. Planta 1997, 203, 442–447. [Google Scholar] [CrossRef]
- Yang, X.-C.; Sachs, F. Block of Stretch-Activated Ion Channels in Xenopus Oocytes by Gadolinium and Calcium Ions. Science 1989, 243, 1068–1071. [Google Scholar] [CrossRef]
- Schwenke, H.; Wagner, E. A New Concept of Root Exudation. Plant Cell Environ. 1992, 15, 289–299. [Google Scholar] [CrossRef]
- Dreßler, L.; Michel, F.; Thondorf, I.; Mansfeld, J.; Golbik, R.; Ulbrich-Hofmann, R. Metal Ions and Phosphatidylinositol 4,5-Bisphosphate as Interacting Effectors of α-Type Plant Phospholipase D. Phytochemistry 2017, 138, 57–64. [Google Scholar] [CrossRef]
- Zeng, F.; Tian, H.E.; Wang, Z.; An, Y.; Gao, F.; Zhang, L.; Li, F.; Shan, L. Effect of Rare Earth Element Europium on Amaranthin Synthesis in Amarathus caudatus Seedlings. Biol. Trace Elem. Res. 2003, 93, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, S.; Tokunaga, T.; Kamon, E.; Takenaka, Y.; Koshimizu, S.; Watanabe, M.; Ishimizu, T. Lanthanum Supplementation Alleviates Tomato Root Growth Suppression under Low Light Stress. Plants 2023, 12, 2663. [Google Scholar] [CrossRef] [PubMed]
- Alp, F.N.; Arikan, B.; Ozfidan-Konakci, C.; Gulenturk, C.; Yildiztugay, E.; Turan, M.; Cavusoglu, H. Hormetic Activation of Nano-Sized Rare Earth Element Terbium on Growth, PSII Photochemistry, Antioxidant Status and Phytohormone Regulation in Lemna minor. Plant Physiol. Biochem. 2023, 194, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Shan, C.; Zhao, H.; Jia, G.; Chen, D. Lanthanum Improves the Cadmium Tolerance of Zea mays Seedlings by the Regulation of Ascorbate and Glutathione Metabolism. Biol. Plant. 2017, 61, 551–556. [Google Scholar] [CrossRef]
- Zicari, M.A.; d’Aquino, L.; Paradiso, A.; Mastrolitti, S.; Tommasi, F. Effect of Cerium on Growth and Antioxidant Metabolism of Lemna minor L. Ecotoxicol. Environ. Saf. 2018, 163, 536–543. [Google Scholar] [CrossRef]
- Bouslimi, H.; Ferreira, R.; Dridi, N.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Effects of Barium Stress in Brassica juncea and Cakile Maritima: The Indicator Role of Some Antioxidant Enzymes and Secondary Metabolites. Phyton—Int. J. Exp. Bot. 2020, 90, 145–158. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, Y.; Qiu, N.; Hu, F.; Sheng, L.; Wang, R.; Cao, F. Ce3+ Induces Flavonoids Accumulation by Regulation of Pigments, Ions, Chlorophyll Fluorescence and Antioxidant Enzymes in Suspension Cells of Ginkgo biloba L. Plant Cell Tissue Organ Cult. 2015, 123, 283–296. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, K.; Wang, F.; Zhao, X.; Bai, R.; Liu, B. Effects of Rare Earth Elements on Growth and Determination of Secondary Metabolites under In Vitro Conditions in Salvia miltiorrhiza. HortScience 2020, 55, 310–316. [Google Scholar] [CrossRef]
- Idrees, M.; Hassan, I.U.; Naeem, M.; Ali, A.; Aftab, T.; Khan, M.M.A. The Accumulation and Degradation of Alkaloids in Catharanthus roseus Supported by Various External Agents Under Different Environmental Conditions. In Catharanthus Roseus: Current Research and Future Prospects; Naeem, M., Aftab, T., Khan, M.M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 321–329. ISBN 978-3-319-51620-2. [Google Scholar]
- Kovaříková, M.; Tomášková, I.; Soudek, P. Rare Earth Elements in Plants. Biol. Plant. 2019, 63, 20–32. [Google Scholar] [CrossRef]
- Peng, X.; He, J.-Y. The Inhibitory Effect of Ca2+ on the Flavonoid Production of Tetrastigma hemsleyanum Suspension Cells Induced by Metal Elicitors. In Vitro Cell. Dev. Biol. Plant 2013, 49, 550–559. [Google Scholar] [CrossRef]
- Abuelsoud, W.; Madany, M.M.Y.; Sheteiwy, M.S.; Korany, S.M.; Alsharef, E.; AbdElgawad, H. Alleviation of Gadolinium Stress on Medicago by Elevated Atmospheric CO2 Is Mediated by Changes in Carbohydrates, Anthocyanin, and Proline Metabolism. Plant Physiol. Biochem. 2023, 202, 107925. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Fallah Imani, A.; Gomarian, M.; Ghorbanpour, M.; Ramak, P.; Chavoshi, S. Foliar-Applied Nano-Cerium Dioxide Differentially Affect Morpho-Physiological Traits and Essential Oil Profile of Salvia mirzayanii Rech. f. & Esfand under Drought Stress and Post-Stress Recovery Conditions. Plant Physiol. Biochem. 2023, 203, 108046. [Google Scholar] [CrossRef]
- Elbasan, F.; Ozfidan-Konakci, C.; Yildiztugay, E.; Kucukoduk, M. Rare-Earth Element Scandium Improves Stomatal Regulation and Enhances Salt and Drought Stress Tolerance by up-Regulating Antioxidant Responses of Oryza sativa. Plant Physiol. Biochem. 2020, 152, 157–169. [Google Scholar] [CrossRef]
- Dridi, N.; Ferreira, R.; Bouslimi, H.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Assessment of Tolerance to Lanthanum and Cerium in Helianthus annuus Plant: Effect on Growth, Mineral Nutrition, and Secondary Metabolism. Plants 2022, 11, 988. [Google Scholar] [CrossRef]
- Babula, P.; Klejdus, B.; Kovacik, J.; Hedbavny, J.; Hlavna, M. Lanthanum Rather than Cadmium Induces Oxidative Stress and Metabolite Changes in Hypericum perforatum. J. Hazard. Mater. 2015, 286, 334–342. [Google Scholar] [CrossRef]
- Zhu, Y.; Che, R.; Dong, Z.; Guo, T.; He, X.; Li, J.; Wang, F. Metabolomics Reveals the Potential Mechanism of La(III) Promoting Enrichment of Sodium Hydrogen Arsenate and Roxarsone in Solanum nigrum L. Sci. Total Environ. 2024, 953, 175990. [Google Scholar] [CrossRef]
- Zhu, L.; Song, L.; Gao, Y.; Qian, J.; Zhang, X.; Li, S. Effects of Lanthanum on the Growth and Essential Oil Components of Lavender under Osmotic Stress. J. Rare Earths 2018, 36, 891–897. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, W.-H.; Hu, Q.; Guo, Y.-Q. Improvement of Indole Alkaloid Production in Catharanthus roseus Cell Cultures by Osmotic Shock. Biotechnol. Lett. 2000, 22, 1227–1231. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Mei, X. Stimulation of Taxol Production and Excretion in Taxus Spp Cell Cultures by Rare Earth Chemical Lanthanum. J. Biotechnol. 2001, 85, 67–73. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, B.; Wang, X.; Yuan, X.; Wang, Y. Promotion of the Growth of Crocus Sativus Cells and the Production of Crocin by Rare Earth Elements. Biotechnol. Lett. 2004, 26, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kunert, K.J.; Foyer, C.H. Chapter Three—The Ascorbate/Glutathione Cycle. In Advances in Botanical Research; Mittler, R., Breusegem, F.V., Eds.; Oxidative Stress Response in Plants; Academic Press: Cambridge, MA, USA, 2023; Volume 105, pp. 77–112. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Gjata, I.; Tommasi, F.; De Leonardis, S.; Dipierro, N.; Paciolla, C. Cytological Alterations and Oxidative Stress Induced by Cerium and Neodymium in Lentil Seedlings and Onion Bulbs. Front. Environ. Sci. 2022, 10, 969162. [Google Scholar] [CrossRef]
- Xin, P.; Shuang-Lin, Z.; Jun-Yao, H.; Li, D. Influence of Rare Earth Elements on Metabolism and Related Enzyme Activity and Isozyme Expression in Tetrastigma hemsleyanum Cell Suspension Cultures. Biol. Trace Elem. Res. 2013, 152, 82–90. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, Q. The Endogenous Hormones in Soybean Seedlings under the Joint Actions of Rare Earth Element La(III) and Ultraviolet-B Stress. Biol. Trace Elem. Res. 2009, 132, 270–277. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Zhou, Q.; Huang, X. Effects of Terbium (III) on Signaling Molecules in Horseradish. Biol. Trace Elem. Res. 2015, 164, 122–129. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, J.; Wang, Y. Changes in Endogenous Hormone Levels and Redox Status during Enhanced Adventitious Rooting by Rare Earth Element Neodymium of Dendrobium densiflorum Shoot Cuttings. J. Rare Earths 2008, 26, 869–874. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Zhang, P.; Wan, J.; Wang, R.; Xu, J. Lanthanum Inhibits Primary Root Growth by Repressing Auxin Carrier Abundances in Arabidopsis. Front. Plant Sci. 2017, 8, 1661. [Google Scholar] [CrossRef]
- Hong, F.; Wei, Z.; Zhao, G. Mechanism of Lanthanum Effect on Chlorophyll of Spinach. Sci. China C Life Sci. 2002, 45, 166–176. [Google Scholar] [CrossRef]
- Wei, Z.; Hong, F.; Yin, M.; Li, H.; Hu, F.; Zhao, G.; Wong, J.W. Structural Differences between Light and Heavy Rare Earth Elment Binding Chlorophylls in Naturally Grown Fern Dicranopteris linearis. Biol. Trace Elem. Res. 2005, 106, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Gao, W.; Chen, G. Toxic Effects of Lanthanum(III) on Photosynthetic Performance of Rice Seedlings: Combined Chlorophyll Fluorescence, Chloroplast Structure and Thylakoid Membrane Protein Assessment. Ecotoxicol. Environ. Saf. 2023, 267, 115627. [Google Scholar] [CrossRef]
- Tao, Y.; Zhao, G.; Yang, J.; Ikeda, S.; Jiang, J.; Hu, T.; Chen, W.; Wei, Z.; Hong, F. Determination of Double Decker Sandwich Structured La-Substituted Chlorophyll a by EXAFS. J. Synchrotron Radiat. 2001, 8, 996–997. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Mukherjee, S.; Al-Munqedhi, B.M.A.; Kumar, R.; Kalaji, H.M. Salicylic Acid and Silicon Impart Resilience to Lanthanum Toxicity in Brassica juncea L. Seedlings. Plant Growth Regul. 2023, 100, 453–466. [Google Scholar] [CrossRef]
- Jimbo, H.; Izuhara, T.; Hihara, Y.; Hisabori, T.; Nishiyama, Y. Light-Inducible Expression of Translation Factor EF-Tu during Acclimation to Strong Light Enhances the Repair of Photosystem II. Proc. Natl. Acad. Sci. USA 2019, 116, 21268–21273. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. Protein Synthesis Is the Primary Target of Reactive Oxygen Species in the Photoinhibition of Photosystem II. Physiol. Plant. 2011, 142, 35–46. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Li, Y.; Sun, J.; Zhou, Q.; Huang, X. Insight into Mechanism of Lanthanum (III) Induced Damage to Plant Photosynthesis. Ecotoxicol. Environ. Saf. 2016, 127, 43–50. [Google Scholar] [CrossRef]
- Yin, H.; Wang, J.; Zeng, Y.; Shen, X.; He, Y.; Ling, L.; Cao, L.; Fu, X.; Peng, L.; Chun, C. Effect of the Rare Earth Element Lanthanum (La) on the Growth and Development of Citrus Rootstock Seedlings. Plants 2021, 10, 1388. [Google Scholar] [CrossRef]
- Shevela, D.; Kern, J.F.; Govindjee, G.; Messinger, J. Solar Energy Conversion by Photosystem II: Principles and Structures. Photosynth. Res. 2023, 156, 279–307. [Google Scholar] [CrossRef]
- Joshi, M.K.; Mohanty, P. Chlorophyll a Fluorescence as a Probe of Heavy Metal Ion Toxicity in Plants. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 637–661. ISBN 978-1-4020-3218-9. [Google Scholar]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazar, D.; Kromdijk, J.; Govindjee, G. Chlorophyll a Fluorescence Induction: Can Just a One-Second Measurement Be Used to Quantify Abiotic Stress Responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does It Make Any Difference the Fact to Be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently Asked Questions about in Vivo Chlorophyll Fluorescence: Practical Issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently Asked Questions about Chlorophyll Fluorescence, the Sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Mathur, S.; Jajoo, A.; Mehta, P.; Bharti, S. Analysis of Elevated Temperature-Induced Inhibition of Photosystem II Using Chlorophyll a Fluorescence Induction Kinetics in Wheat Leaves (Triticum aestivum). Plant Biol. 2011, 13, 1–6. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. On the Relation between the Kautsky Effect (Chlorophyll a Fluorescence Induction) and Photosystem II: Basics and Applications of the OJIP Fluorescence Transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherland, 2004; pp. 321–362. ISBN 978-1-4020-3218-9. [Google Scholar]
- Tsimilli-Michael, M. Revisiting JIP-Test: An Educative Review on Concepts, Assumptions, Approximations, Definitions and Terminology. Photosynthetica 2019, 57, 90–107. [Google Scholar] [CrossRef]
- Lazár, D. Parameters of Photosynthetic Energy Partitioning. J. Plant Physiol. 2015, 175, 131–147. [Google Scholar] [CrossRef]
- Ferroni, L.; Živčak, M.; Kovar, M.; Colpo, A.; Pancaldi, S.; Allakhverdiev, S.I.; Brestič, M. Fast Chlorophyll a Fluorescence Induction (OJIP) Phenotyping of Chlorophyll-Deficient Wheat Suggests That an Enlarged Acceptor Pool Size of Photosystem I Helps Compensate for a Deregulated Photosynthetic Electron Flow. J. Photochem. Photobiol. B Biol. 2022, 234, 112549. [Google Scholar] [CrossRef]
- Garab, G. Revisiting the Nonregulatory, Constitutive Nonphotochemical Quenching of the Absorbed Light Energy in Oxygenic Photosynthetic Organisms. Photosynthetica 2024, 62, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Garab, G.; Magyar, M.; Sipka, G.; Lambrev, P.H. New Foundations for the Physical Mechanism of Variable Chlorophyll a Fluorescence. Quantum Efficiency versus the Light-Adapted State of Photosystem II. J. Exp. Bot. 2023, 74, 5458–5471. [Google Scholar] [CrossRef] [PubMed]
- Schansker, G.; Ohnishi, M.; Furutani, R.; Miyake, C. Identification of Twelve Different Mineral Deficiencies in Hydroponically Grown Sunflower Plants on the Basis of Short Measurements of the Fluorescence and P700 Oxidation/Reduction Kinetics. Front. Plant Sci. 2022, 13, 894607. [Google Scholar] [CrossRef]
- Schreiber, U. Letter to the Editor. Photosynthetica 2024, 62, 314–317. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kalaji, H.M.; Govindjee. Photosynthetic Responses of Sun- and Shade-Grown Barley Leaves to High Light: Is the Lower PSII Connectivity in Shade Leaves Associated with Protection against Excess of Light? Photosynth. Res. 2014, 119, 339–354. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Olsovska, K.; Allakhverdiev, S.I. Effect of Photosystem I Inactivation on Chlorophyll a Fluorescence Induction in Wheat Leaves: Does Activity of Photosystem I Play Any Role in OJIP Rise? J. Photochem. Photobiol. B Biol. 2015, 152, 318–324. [Google Scholar] [CrossRef]
- Cui, W.; Kamran, M.; Song, Q.; Zuo, B.; Jia, Z.; Han, Q. Lanthanum Chloride Improves Maize Grain Yield by Promoting Photosynthetic Characteristics, Antioxidants Enzymes and Endogenous Hormone at Reproductive Stages. J. Rare Earths 2019, 37, 781–790. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, T.; Xie, Y.; Lv, Q.; Qiu, L. Alleviatory Effect of Rare Earth Micro-Fertilizer on Photosystem II (PSII) Photoinhibition in Pseudostellaria heterophylla Leaves at Photosynthetic Midday Depression. J. Rare Earths 2022, 40, 1156–1164. [Google Scholar] [CrossRef]
- Longoni, F.P.; Goldschmidt-Clermont, M. Thylakoid Protein Phosphorylation in Chloroplasts. Plant Cell Physiol. 2021, 62, 1094–1107. [Google Scholar] [CrossRef]
- Rantala, M.; Rantala, S.; Aro, E.-M. Composition, Phosphorylation and Dynamic Organization of Photosynthetic Protein Complexes in Plant Thylakoid Membrane. Photochem. Photobiol. Sci. 2020, 19, 604–619. [Google Scholar] [CrossRef]
- Barber, J. An Explanation for the Relationship between Salt-Induced Thylakoid Stacking and the Chlorophyll Fluorescence Changes Associated with Changes in Spillover of Energy from Photosystem II to Photosystem I. FEBS Lett. 1980, 118, 1–10. [Google Scholar] [CrossRef]
- Chow, W.S.; Kim, E.-H.; Horton, P.; Anderson, J.M. Granal Stacking of Thylakoid Membranes in Higher Plant Chloroplasts: The Physicochemical Forces at Work and the Functional Consequences That Ensue. Photochem. Photobiol. Sci. 2005, 4, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Murata, N. Control of Excitation Transfer in Photosynthesis. II. Magnesium Ion-Dependent Distribution of Excitation Energy between Two Pigment Systems in Spinach Chloroplasts. Biochim. Biophys. Acta 1969, 189, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kai, S.; Ohnishi, A.; Tsumura, N.; Ishikawa, T.; Hori, H.; Morita, N.; Ishikawa, Y. Quality Control of PSII: Behavior of PSII in the Highly Crowded Grana Thylakoids Under Excessive Light. Plant Cell Physiol. 2014, 55, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Horton, P.; Kim, E.-H.; Chow, W.S. Towards Elucidation of Dynamic Structural Changes of Plant Thylakoid Architecture. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3515–3524. [Google Scholar] [CrossRef]
- Grieco, M.; Suorsa, M.; Jajoo, A.; Tikkanen, M.; Aro, E.-M. Light-Harvesting II Antenna Trimers Connect Energetically the Entire Photosynthetic Machinery—Including Both Photosystems II and I. Biochim. Biophys. Acta BBA—Bioenerg. 2015, 1847, 607–619. [Google Scholar] [CrossRef]
- Kim, E.; Yokono, M.; Tsugane, K.; Ishii, A.; Noda, C.; Minagawa, J. Formation of a Stable PSI–PSII Megacomplex in Rice That Conducts Energy Spillover. Plant Cell Physiol. 2023, 64, 858–865. [Google Scholar] [CrossRef]
- Stirbet, A. Excitonic Connectivity between Photosystem II Units: What Is It, and How to Measure It? Photosynth. Res. 2013, 116, 189–214. [Google Scholar] [CrossRef]
- Albanese, P.; Melero, R.; Engel, B.D.; Grinzato, A.; Berto, P.; Manfredi, M.; Chiodoni, A.; Vargas, J.; Sorzano, C.Ó.S.; Marengo, E.; et al. Pea PSII-LHCII Supercomplexes Form Pairs by Making Connections across the Stromal Gap. Sci. Rep. 2017, 7, 10067. [Google Scholar] [CrossRef]
- Aliprandi, E.; Demaria, S.; Colpo, A.; Brestič, M.; Živčak, M.; Martina, A.; Pancaldi, S.; Baldisserotto, C.; Ferroni, L. Thylakoid Ultrastructural Variations in Chlorophyll-Deficient Wheat: Aberrations or Structural Acclimation? Planta 2024, 259, 90. [Google Scholar] [CrossRef]
- Gu, L.; Grodzinski, B.; Han, J.; Marie, T.; Zhang, Y.-J.; Song, Y.C.; Sun, Y. Granal Thylakoid Structure and Function: Explaining an Enduring Mystery of Higher Plants. New Phytol. 2022, 236, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Höhner, R.; Pribil, M.; Herbstová, M.; Lopez, L.S.; Kunz, H.-H.; Li, M.; Wood, M.; Svoboda, V.; Puthiyaveetil, S.; Leister, D.; et al. Plastocyanin Is the Long-Range Electron Carrier between Photosystem II and Photosystem I in Plants. Proc. Natl. Acad. Sci. USA 2020, 117, 15354–15362. [Google Scholar] [CrossRef] [PubMed]
- Caspy, I.; Nelson, N. Structure of the Plant Photosystem I. Biochem. Soc. Trans. 2018, 46, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.; Jain, A.; Ebersberger, I.; Teige, M. An Evolutionary View on Thylakoid Protein Phosphorylation Uncovers Novel Phosphorylation Hotspots with Potential Functional Implications. J. Exp. Bot. 2016, 67, 3883–3896. [Google Scholar] [CrossRef]
- Chen, W.-J.; Gu, Y.-H.; Zhao, G.-W.; Tao, Y.; Luo, J.-P.; Hu, T.-D. Effects of Rare Earth Ions on Activity of RuBPcase in Tobacco. Plant Sci. 2000, 152, 145–151. [Google Scholar] [CrossRef]
- Hong, F.; Liu, C.; Zheng, L.; Wang, X.; Wu, K.; Song, W.; Lü, S.; Tao, Y.; Zhao, G. Formation of Complexes of Rubisco-Rubisco Activase from La3+, Ce3+ Treatment Spinach. Sci. China Ser. B Chem. 2005, 48, 67–74. [Google Scholar] [CrossRef]
- Liu, C.; Hong, F.; Tao, Y.; Liu, T.; Xie, Y.; Xu, J.; Li, Z. The Mechanism of the Molecular Interaction between Cerium (III) and Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase (Rubisco). Biol. Trace Elem. Res. 2011, 143, 1110–1120. [Google Scholar] [CrossRef]
- Heiner, Z.; Zeise, I.; Elbaum, R.; Kneipp, J. Insight into Plant Cell Wall Chemistry and Structure by Combination of Multiphoton Microscopy with Raman Imaging. J. Biophotonics 2018, 11, e201700164. [Google Scholar] [CrossRef]
- Backer, R.; Solomon, D. REAP Supplemental Fertilizer Improves Greenhouse Crop Yield. bioRxiv 2020. [Google Scholar] [CrossRef]
- Salgado, O.; Teodoro, J.; Alvarenga, J.; Oliveira, C.; de Carvalho, T.; Domiciano, D.; Marchiori, P.; Guilherme, L. Cerium Alleviates Drought-Induced Stress in Phaseolus vulgaris. J. Rare Earths 2019, 38, 324–331. [Google Scholar] [CrossRef]
- Jiao, Y.; He, D.; Zhang, S.; Cheng, M.; Chen, S.; Dong, T.; Wang, L.; Huang, X. Lanthanum Interferes with the Fundamental Rhythms of Stomatal Opening, Expression of Related Genes, and Evapotranspiration in Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2024, 281, 116576. [Google Scholar] [CrossRef]
- Wang, F.-F.; Lian, H.-L.; Kang, C.-Y.; Yang, H.-Q. Phytochrome B Is Involved in Mediating Red Light-Induced Stomatal Opening in Arabidopsis thaliana. Mol. Plant 2010, 3, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, S.; Takemiya, A.; Sakamoto, T.; Kurata, T.; Tsutsumi, T.; Kinoshita, T.; Shimazaki, K. The Plasma Membrane H+-ATPase AHA1 Plays a Major Role in Stomatal Opening in Response to Blue Light. Plant Physiol. 2016, 171, 2731–2743. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S. Addressing Lanthanum Toxicity in Plants: Sources, Uptake, Accumulation, and Mitigation Strategies. Sci. Total Environ. 2024, 929, 172560. [Google Scholar] [CrossRef]
- Cheng, M.; Zhou, Q.; Wang, L.; Jiao, Y.; Liu, Y.; Tan, L.; Zhu, H.; Nagawa, S.; Wei, H.; Yang, Z.; et al. A New Mechanism by Which Environmental Hazardous Substances Enhance Their Toxicities to Plants. J. Hazard. Mater. 2022, 421, 126802. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A Review on Drought Stress in Plants: Implications, Mitigation and the Role of Plant Growth Promoting Rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Wason, M.S.; Zhao, J. Cerium Oxide Nanoparticles: Potential Applications for Cancer and Other Diseases. Am. J. Transl. Res. 2013, 5, 126–131. [Google Scholar]
- Laur, J.; Hacke, U.G. The Role of Water Channel Proteins in Facilitating Recovery of Leaf Hydraulic Conductance from Water Stress in Populus trichocarpa. PLoS ONE 2014, 9, e111751. [Google Scholar] [CrossRef]
- Yue, L.; Ma, C.; Zhan, X.; White, J.C.; Xing, B. Molecular Mechanisms of Maize Seedling Response to La2O3 NP Exposure: Water Uptake, Aquaporin Gene Expression and Signal Transduction. Environ. Sci. Nano 2017, 4, 843–855. [Google Scholar] [CrossRef]
- Yue, L.; Chen, F.; Yu, K.; Xiao, Z.; Yu, X.; Wang, Z.; Xing, B. Early Development of Apoplastic Barriers and Molecular Mechanisms in Juvenile Maize Roots in Response to La2O3 Nanoparticles. Sci. Total Environ. 2019, 653, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wu, M.; Wang, X.; Zhang, Y.; Jiang, F.; Liu, Y.; Dai, J. Membrane Permeability Transition and Dysfunction of Rice Mitochondria Effected by Er(III). J. Membr. Biol. 2015, 248, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fu, Y.; Min, H.; Cai, S.; Sha, S.; Cheng, G. Laboratory Assessment of Uptake and Toxicity of Lanthanum (La) in the Leaves of Hydrocharis dubia (Bl.) Backer. Environ. Sci. Pollut. Res. 2012, 19, 3950–3958. [Google Scholar] [CrossRef]
- Solymosi, K.; Bertrand, M. Soil Metals, Chloroplasts, and Secure Crop Production: A Review. Agron. Sustain. Dev. 2012, 32, 245–272. [Google Scholar] [CrossRef]
- Weiner, E.; Pinskey, J.M.; Nicastro, D.; Otegui, M.S. Electron Microscopy for Imaging Organelles in Plants and Algae. Plant Physiol. 2022, 188, 713–725. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Z.; Fang, H.; Jiang, H.; Yang, G.; Chen, L.; Zhou, X.; Hu, B.; Qin, C.; Hu, G.; et al. Plantorganelle Hunter Is an Effective Deep-Learning-Based Method for Plant Organelle Phenotyping in Electron Microscopy. Nat. Plants 2023, 9, 1760–1775. [Google Scholar] [CrossRef]
- Colpo, A.; Molinari, A.; Boldrini, P.; Živčak, M.; Brestič, M.; Demaria, S.; Baldisserotto, C.; Pancaldi, S.; Ferroni, L. Thylakoid Membrane Appression in the Giant Chloroplast of Selaginella Martensii Spring: A Lycophyte Challenges Grana Paradigms in Shade-Adapted Species. Plant Sci. 2023, 336, 111833. [Google Scholar] [CrossRef]
- Mazur, R.; Mostowska, A.; Kowalewska, Ł. How to Measure Grana—Ultrastructural Features of Thylakoid Membranes of Plant Chloroplasts. Front. Plant Sci. 2021, 12, 756009. [Google Scholar] [CrossRef]
- Sharma, S.; Shree, B.; Aditika; Sharma, A.; Irfan, M.; Kumar, P. Nanoparticle-Based Toxicity in Perishable Vegetable Crops: Molecular Insights, Impact on Human Health and Mitigation Strategies for Sustainable Cultivation. Environ. Res. 2022, 212, 113168. [Google Scholar] [CrossRef]
- Espindola, M.C.G.; Menezes, N.L.D.; Barbieri, A.P.P. Efeito do cério na qualidade fisiológica de sementes de milho e no desempenho agronômico das plantas. Biosci. J Online 2013, 29, 1501–1507. [Google Scholar]
- Sun, Q.; Sun, L.; Feng, S.; Guo, S. Effect of La3+ on Seed Germination and Seedling Growth of Salvia miltiorrhiza. J. Rare Earths 2018, 36, 898–902. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Hu, T.; Li, W.; Xue, S. Effects of Lanthanum on Abscisic Acid Regulation of Root Growth in Arabidopsis. J. Rare Earths 2014, 32, 78–82. [Google Scholar] [CrossRef]
- d’Aquino, L.; Morgana, M.; Carboni, M.A.; Staiano, M.; Antisari, M.V.; Re, M.; Lorito, M.; Vinale, F.; Abadi, K.M.; Woo, S.L. Effect of Some Rare Earth Elements on the Growth and Lanthanide Accumulation in Different Trichoderma Strains. Soil Biol. Biochem. 2009, 41, 2406–2413. [Google Scholar] [CrossRef]
- Fashui, H.; Ling, W.; Chao, L. Study of Lanthanum on Seed Germination and Growth of Rice. Biol. Trace Elem. Res. 2003, 94, 273–286. [Google Scholar] [CrossRef]
- Xu, Q.-M.; Chen, H. Antioxidant Responses of Rice Seedling to Ce4+ under Hydroponic Cultures. Ecotoxicol. Environ. Saf. 2011, 74, 1693–1699. [Google Scholar] [CrossRef]
- Qiu, L. Review on the Effects of Rare Earth Elements on Seed Germination: Review on the Effects of Rare Earth Elements on Seed Germination. Chin. J. Eco-Agric. 2008, 16, 529–533. [Google Scholar] [CrossRef]
- Tung, M.H.T.; Hien, T.T.T.; Sharmaa, A.; Cam, N.T.D.; Manh, N.V.; Khan, D.T.; Son, N.L.T.; Chi, N.T.P.L.; Ha, D.T.N. Investigating the Synergistic Effects of Nano CeO2 and Pr2O3 Rare Earth Element Oxides as Fertilizers on the Growth of Salvia miltiorrhiza Bunge. ChemistrySelect 2024, 9, e202402662. [Google Scholar] [CrossRef]
- Drobkov, A.A.; Drobkov, A.A. Influence of Cerium, Lanthanum and Samarium on Development of Peas. Dokl. Akad. Nauk SSSR 1941, 32, 669–670. [Google Scholar]
- Pang, X.; Li, D.; Peng, A. Application of Rare-Earth Elements in the Agriculture of China and Its Environmental Behavior in Soil. Environ. Sci. Pollut. Res. 2002, 9, 143. [Google Scholar] [CrossRef]
- Ozaki, T.; Enomoto, S.; Minai, Y.; Ambe, S.; Ambe, F.; Makide, Y. Beneficial Effect of Rare Earth Elements on the Growth of Dryopteris erythrosora. J. Plant Physiol. 2000, 156, 330–334. [Google Scholar] [CrossRef]
- He, Y.; Loh, C. Cerium and Lanthanum Promote Floral Initiation and Reproductive Growth of Arabidopsis thaliana. Plant Sci. Int. J. Exp. Plant Biol. 2000, 159, 117–124. [Google Scholar] [CrossRef]
- Emmanuel, E.S.C.; Anandkumar, B.; Natesan, M.; Maruthamuthu, S. Efficacy of Rare Earth Elements on the Physiological and Biochemical Characteristics of Zea mays L. Aust. J. Crop Sci. 2010, 4, 289–294. [Google Scholar]
- Stadler, J.; Vogel, M.; Steudtner, R.; Drobot, B.; Kogiomtzidis, A.L.; Weiss, M.; Walther, C. The Chemical Journey of Europium(III) through Winter Rye (Secale cereale L.): Understanding through Mass Spectrometry and Chemical Microscopy. Chemosphere 2023, 313, 137252. [Google Scholar] [CrossRef]
- Rueda-López, I.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Peralta-Sánchez, M.G.; Ramírez-Olvera, S.M. Neodymium and Zinc Stimulate Growth, Biomass Accumulation and Nutrient Uptake of Lettuce Plants in Hydroponics. Folia Hortic. 2024, 36, 283–297. [Google Scholar] [CrossRef]
- Xie, Z.B.; Zhu, J.G.; Chu, H.Y.; Zhang, Y.L.; Zeng, Q.; Ma, H.L.; Cao, Z.H. Effect of Lanthanum on Rice Production, Nutrient Uptake, and Distribution. J. Plant Nutr. 2002, 25, 2315–2331. [Google Scholar] [CrossRef]
- Bergson, P.; Lipkind, G.; Lee, S.P.; Duban, M.-E.; Hanck, D. Verapamil Block of T-Type Calcium Channels. Mol. Pharmacol. 2010, 79, 411–419. [Google Scholar] [CrossRef]
- Ruíz-Herrera, L.F.; Sánchez-Calderón, L.; Herrera-Estrella, L.; López-Bucio, J. Rare Earth Elements Lanthanum and Gadolinium Induce Phosphate-Deficiency Responses in Arabidopsis thaliana Seedlings. Plant Soil 2012, 353, 231–247. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ikka, T.; Kimura, K.; Yasuda, O.; Koyama, H. Characterisation of Lanthanum Toxicity for Root Growth of Arabidopsis thaliana from the Aspect of Natural Genetic Variation. Funct. Plant Biol. 2007, 34, 984–994. [Google Scholar] [CrossRef]
- Gjata, I.; van Drimmelen, C.K.E.; Tommasi, F.; Paciolla, C.; Heise, S. Impact of Rare Earth Elements in Sediments on the Growth and Photosynthetic Efficiency of the Benthic Plant Myriophyllum aquaticum. J. Soils Sediments 2024, 24, 3814–3823. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Q.; Lu, T.; Ding, X.; Huang, X. Molecular and Cellular Mechanism of the Effect of La(III) on Horseradish Peroxidase. J. Biol. Inorg. Chem. 2010, 15, 1063–1069. [Google Scholar] [CrossRef]
- Guo, T.; He, D.; Liu, Y.; Li, J.; Wang, F. Lanthanum Promotes Solanum nigrum L. Growth and Phytoremediation of Cadmium and Lead through Endocytosis: Physiological and Biochemical Response, Heavy Metal Uptake and Visualization. Sci. Total Environ. 2024, 912, 168915. [Google Scholar] [CrossRef]
- Wu, M.; Wang, P.-Y.; Sun, L.-G.; Zhang, J.-J.; Yu, J.; Wang, Y.-W.; Chen, G.-X. Alleviation of Cadmium Toxicity by Cerium in Rice Seedlings Is Related to Improved Photosynthesis, Elevated Antioxidant Enzymes and Decreased Oxidative Stress. Plant Growth Regul. 2014, 74, 251–260. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, W.; Zhu, L.; Chen, J. Effects of LaCl3 on the Growth and Photosynthetic Characteristics of Fny-Infected Tobacco Seedlings. J. Rare Earths 2012, 30, 725–730. [Google Scholar] [CrossRef]
- Thiruvengadam, R.; Easwaran, M.; Rethinam, S.; Madasamy, S.; Siddiqui, S.A.; Kandhaswamy, A.; Venkidasamy, B. Boosting Plant Resilience: The Promise of Rare Earth Nanomaterials in Growth, Physiology, and Stress Mitigation. Plant Physiol. Biochem. 2024, 208, 108519. [Google Scholar] [CrossRef]
- Hu, X.; Ding, Z.; Wang, X.; Chen, Y.; Dai, L. Effects of Lanthanum and Cerium on the Vegetable Growth of Wheat (Triticum aestivum L.) Seedlings. Bull. Environ. Contam. Toxicol. 2002, 69, 0727–0733. [Google Scholar] [CrossRef]
- Shi, K.; Liu, C.; Liu, D.; Lyu, K.; Chen, J.; Wang, X. The Accumulation and Effect of Rare Earth Element Neodymium on the Root of Rice Seedlings. Environ. Sci. Pollut. Res. 2021, 28, 48656–48665. [Google Scholar] [CrossRef]
- Xue, Z.; Chen, Z.; Wang, Y.; Sun, W. Proteomic Analysis Reveals the Association between the Pathways of Glutathione and α-Linolenic Acid Metabolism and Lanthanum Accumulation in Tea Plants. Molecules 2023, 28, 1124. [Google Scholar] [CrossRef]
- Szabó, S.; Zavanyi, G.; Koleszár, G.; del Castillo, D.; Oláh, V.; Braun, M. Phytoremediation, Recovery and Toxic Effects of Ionic Gadolinium Using the Free-Floating Plant Lemna gibba. J. Hazard. Mater. 2023, 458, 131930. [Google Scholar] [CrossRef]
- Carpenter, D.; Boutin, C.; Allison, J.E.; Parsons, J.L.; Ellis, D.M. Uptake and Effects of Six Rare Earth Elements (REEs) on Selected Native and Crop Species Growing in Contaminated Soils. PLoS ONE 2015, 10, e0129936. [Google Scholar] [CrossRef]
- Rodrigues, E.S.; Montanha, G.S.; Marques, J.P.R.; de Almeida, E.; Yabuki, L.N.M.; Menegário, A.A.; Pereira de Carvalho, H.W. Foliar Application of Rare Earth Elements on Soybean (Glycine max (L)): Effects on Biometrics and Characterization of Phytotoxicity. J. Rare Earths 2020, 38, 1131–1139. [Google Scholar] [CrossRef]
- Wang, L.; He, J.; Yang, Q.; Li, X.; Wei, H.; Chen, D.D.Y.; Huang, X. A Preliminary Study on the Effects of Lanthanum (III) on Plant Vitronectin-like Protein and Its Toxicological Basis. Ecotoxicol. Environ. Saf. 2017, 145, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Davranche, M.; Vantelon, D.; Bau, M.; Johannesson, K.H. Editorial: Further Rare Earth Elements Environmental Dissemination: Observation, Analysis, and Impacts. Front. Earth Sci. 2023, 11, 1182827. [Google Scholar] [CrossRef]
- Jiang, W.; Zou, J.; Yue, J.; Liu, D. Effects of Cadmium Stress on Root Tip Cells and Some Physiological Indexes in Allium cepa Var. Agrogarum L. Acta Biol. Cracoviensia. Ser. Bot. 2012, 54, 129–141. [Google Scholar]
- Jiang, D.G.; Yang, J.; Zhang, S.; Yang, D.J. A Survey of 16 Rare Earth Elements in the Major Foods in China. Biomed. Environ. Sci. 2012, 25, 267–271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martina, A.; Ferroni, L.; Marrocchino, E. The Soil–Plant Continuity of Rare Earth Elements: Insights into an Enigmatic Class of Xenobiotics and Their Interactions with Plant Structures and Processes. J. Xenobiot. 2025, 15, 46. https://doi.org/10.3390/jox15020046
Martina A, Ferroni L, Marrocchino E. The Soil–Plant Continuity of Rare Earth Elements: Insights into an Enigmatic Class of Xenobiotics and Their Interactions with Plant Structures and Processes. Journal of Xenobiotics. 2025; 15(2):46. https://doi.org/10.3390/jox15020046
Chicago/Turabian StyleMartina, Angela, Lorenzo Ferroni, and Elena Marrocchino. 2025. "The Soil–Plant Continuity of Rare Earth Elements: Insights into an Enigmatic Class of Xenobiotics and Their Interactions with Plant Structures and Processes" Journal of Xenobiotics 15, no. 2: 46. https://doi.org/10.3390/jox15020046
APA StyleMartina, A., Ferroni, L., & Marrocchino, E. (2025). The Soil–Plant Continuity of Rare Earth Elements: Insights into an Enigmatic Class of Xenobiotics and Their Interactions with Plant Structures and Processes. Journal of Xenobiotics, 15(2), 46. https://doi.org/10.3390/jox15020046






