Short-Term Immunotoxic Effects of an Anti-Cancer Drug (Etoposide) on the Freshwater Pondsnail Lymnaea stagnalis †
Introduction
Materials and Methods
Experimental conditions and chemical exposure
Hemolymph collection and flow cytometry analysis
Statistical analysis
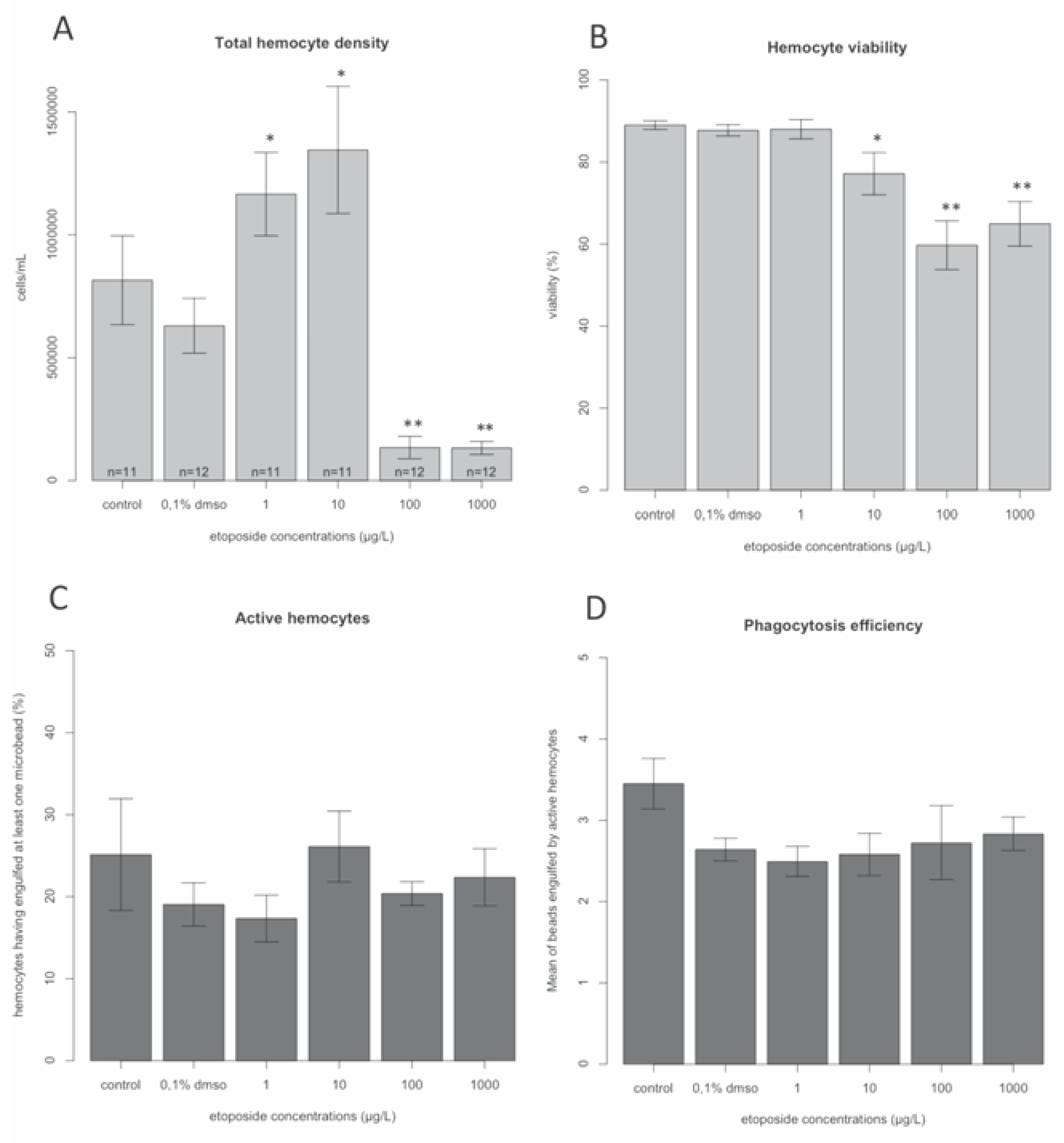
Results and Discussion
Conclusions
Acknowledgments
References
- Kummerer, K. The presence of pharmaceuticals in the environment due to human use-present knowledge and future challenges. J Environ Manage 2009, 90, 2354–66. [Google Scholar] [CrossRef] [PubMed]
- Christensen, FM. Pharmaceuticals in the environment—A human risk? Regul Toxicol Pharmacol 1998, 28, 212–21. [Google Scholar] [CrossRef] [PubMed]
- Matozzo, V. Effects of pharmaceuticals on immune parameters of aquatic invertebrates. ISJ, 2014; [In press]. [Google Scholar]
- Dagher, R; Johnson, J; Williams, G; Keegan, P; Pazdur, R. Accelerated approval of oncology products: A decade of experience. J Natl Cancer Inst 2004, 96, 1500–9. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M; Mayer-Nicolai, C; Pfaff, O. Approval probabilities and regulatory review patterns for anticancer drugs in the European Union. Crit Rev Oncol Hematol 2013, 87, 112–21. [Google Scholar] [CrossRef] [PubMed]
- Apolone, G; Joppi, R; Bertele, V; Garattini, S. Ten years of marketing approvals of anticancer drugs in Europe: Regulatory policy and guidance documents need to find a balance between different pressures. Br J Cancer 2005, 93, 504–9. [Google Scholar] [CrossRef] [PubMed]
- Besse, JP; Latour, JF; Garric, J. Anticancer drugs in surface waters: What can we say about the occurrence and environmental significance of cytotoxic, cytostatic and endocrine therapy drugs? Environ Int 2012, 39, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Catastini, C; Mullot, J-U; Boukari, S; Mazellier, P; Levi, Y; Cervantes, P; et al. Assessment of antineoplastic drugs in effluents of two hospitals (Identification de molécules anticancéreuses dans les effluents hospitaliers). J Eur Hydro 2008, 39, 80–171. [Google Scholar]
- Cancer Care Ontario. Etoposide mono graph. 2013. Available online: https://www. cancercare.on.ca/.
- Hande, KRPJW; Noone, RM; Wilkinson, GR; Greco, FA; Wolff, SN. Pharmacokinetics of high-dose etoposide (VP-16-213) administered to cancer patients. Cancer Res 1984, 44, 379–382. [Google Scholar] [PubMed]
- OECD. DRPDoML-CTT, in OECD Series on Testing and Assessment No. 121, Organisation for Economic Co-operation and Development: Paris, 2010; 182.
- Galloway, T; Depledge, M. Immunotoxicity in invertebrates: Measurement and ecotoxicological relevance. Ecotoxicology 2001, 10, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Team, RC. R: A language and environment for statistical computing; R. Foundation for Statistical Computing: Vienna, 2014; Available online: http://www.R-project.org/.
- Wozniak, AJ; Ross, WE. DNA Damage as a basis for 4’-Demethylepipodophyllotoxin-9-(4,6-O-ethylidene-β-d-glucopyranoside) (etoposide) cytotoxicity. Cancer Res 1983, 43, 120–4. [Google Scholar] [PubMed]
- Russo, J; Madec, L; Brehélin, M. Haemocyte lysosomal fragility facing an environmental reality: A toxicological perspective with atrazine and Lymnaea stagnalis (Gastropoda, Pulmonata) as a test case. Ecotoxicol Environ Saf 2009, 72, 1719–26. [Google Scholar]
- Gust, M; Fortier, M; Garric, J; Fournier, M; Gagne, F. Effects of short-term exposure to environmentally relevant concentrations of different pharmaceutical mixtures on the immune response of the pond snail Lymnaea stagnalis. Sci Total Environ 2013, 445–446, 210–8. [Google Scholar] [CrossRef] [PubMed]
- Gust, M; Fortier, M; Garric, J; Fournier, M; Gagne, F. Immunotoxicity of surface waters contaminated by municipal effluents to the snail Lymnaea stagnalis. Aquat Toxicol 2013, 126, 393–403. [Google Scholar] [CrossRef] [PubMed]
© 2024 by the authors. Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 4.0 License (CC BY-NC 4.0).
Share and Cite
Boisseaux, P.; Gust, M.; Betoulle, S.; Garric, J. Short-Term Immunotoxic Effects of an Anti-Cancer Drug (Etoposide) on the Freshwater Pondsnail Lymnaea stagnalis. J. Xenobiot. 2014, 4, 4894. https://doi.org/10.4081/xeno.2014.4894
Boisseaux P, Gust M, Betoulle S, Garric J. Short-Term Immunotoxic Effects of an Anti-Cancer Drug (Etoposide) on the Freshwater Pondsnail Lymnaea stagnalis. Journal of Xenobiotics. 2014; 4(2):4894. https://doi.org/10.4081/xeno.2014.4894
Chicago/Turabian StyleBoisseaux, P., M. Gust, S. Betoulle, and J. Garric. 2014. "Short-Term Immunotoxic Effects of an Anti-Cancer Drug (Etoposide) on the Freshwater Pondsnail Lymnaea stagnalis" Journal of Xenobiotics 4, no. 2: 4894. https://doi.org/10.4081/xeno.2014.4894
APA StyleBoisseaux, P., Gust, M., Betoulle, S., & Garric, J. (2014). Short-Term Immunotoxic Effects of an Anti-Cancer Drug (Etoposide) on the Freshwater Pondsnail Lymnaea stagnalis. Journal of Xenobiotics, 4(2), 4894. https://doi.org/10.4081/xeno.2014.4894



