4-Nonylphenol Disrupts Osmoregulation in the European Sea-Bass Dicentrarchus labrax †
Introduction
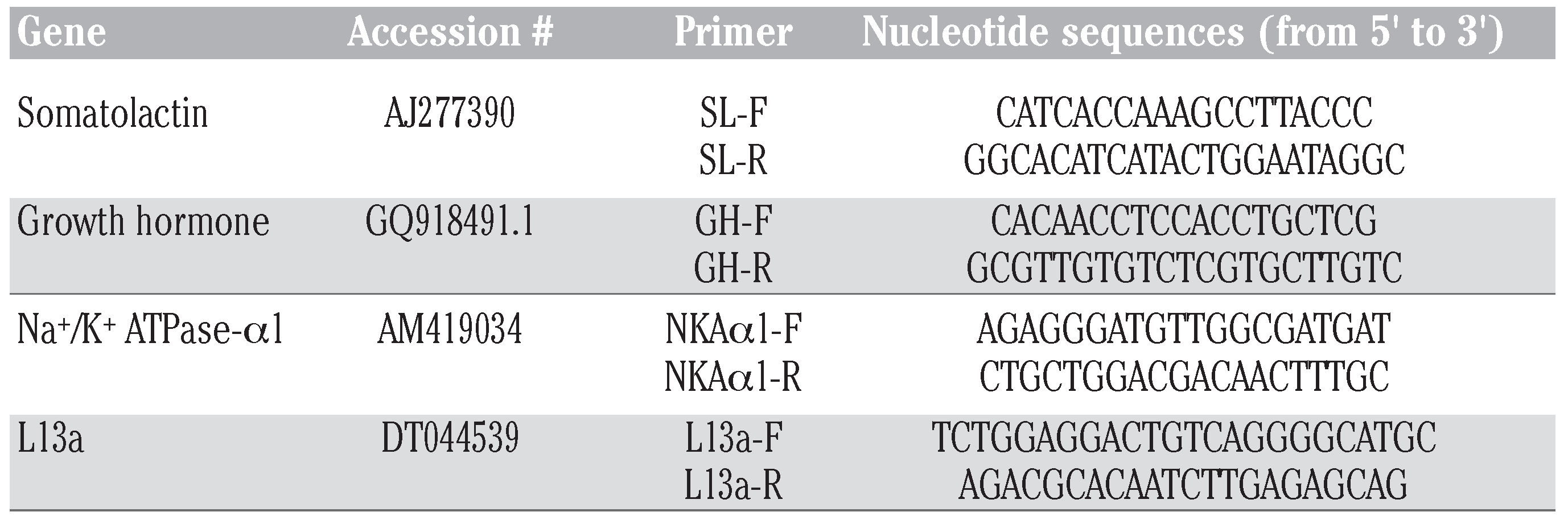
Materials and Methods
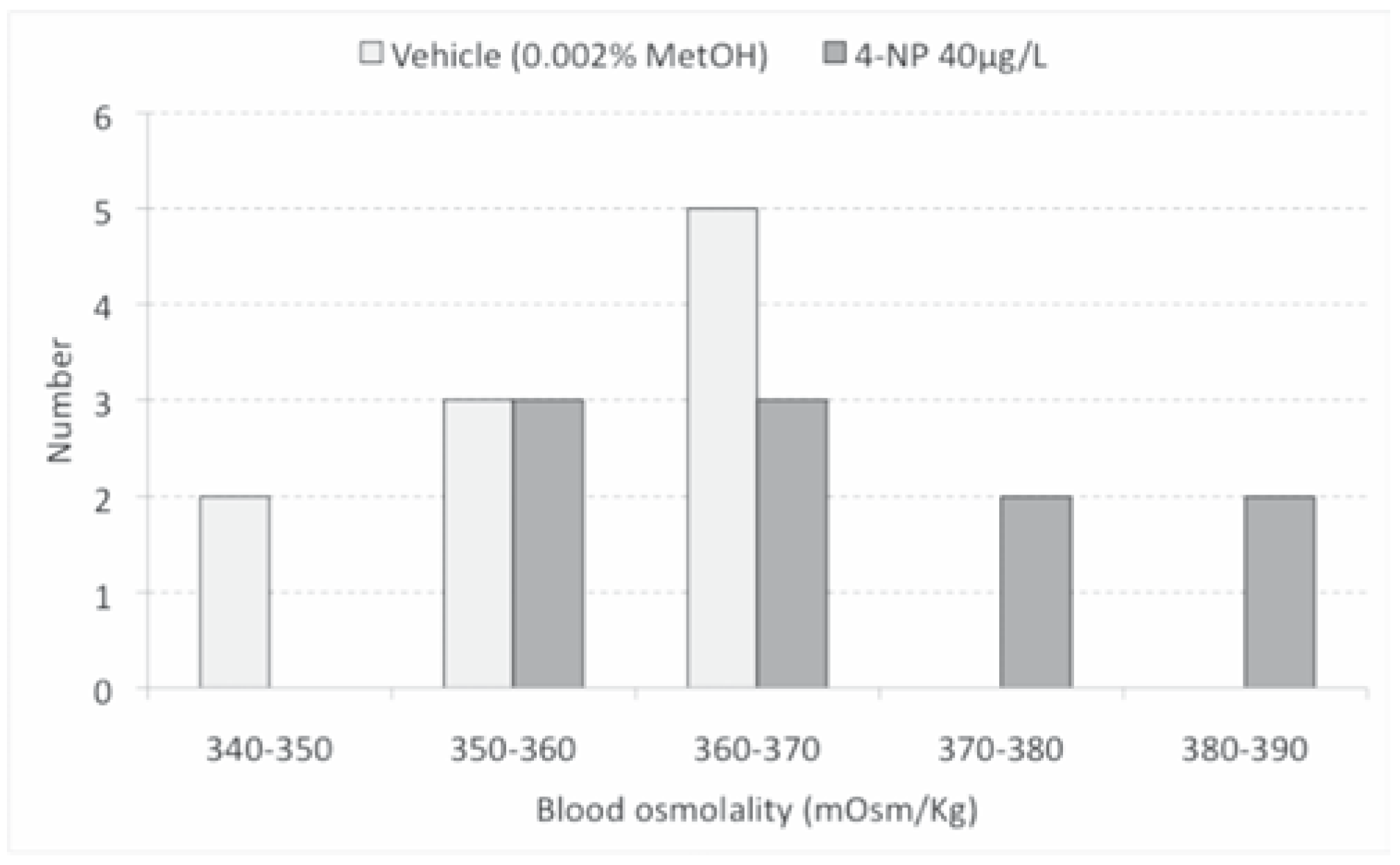
Results and Discussion
References
- Yadetie, F.; Arukwe, A.; Goksoyr, A.; Male, R. Induction of hepatic estrogen receptor in juvenile Atlantic salmon in vivo by the environmental estrogen, 4-nonylphenol. Sci Total Environ 1999, 233, 201–10. [Google Scholar] [CrossRef] [PubMed]
- Liber, K.; Knuth, M.L.; Stay, F.S. An integrated evaluation of the persistence and effects of 4-nonylphenol in an experimental littoral ecosystem. Environ Toxicol Chem 1999, 18, 357–62. [Google Scholar]
- David, A.; Fenet, H.; Gomez, E. Alkylphenols in marine environments: Distribution monitoring strategies and detection considerations. Mar Pollut Bull 2009, 58, 953–60. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, G.; Schwaiger, J.; Negele, R.; Fent, K. Effects of long-term nonylphenol exposure on gonadal development and biomarkers of estrogenicity in juvenile rainbow trout Oncorhynchus mykiss. Aquat Toxicol 2002, 60, 203–21. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S.; Tyler, C. Endocrine disruption in wild freshwater fish. Pure Appl Chem 2003, 75, 2219–34. [Google Scholar] [CrossRef]
- Arsenault, J.T.M.; Fairchild, W.L.; MacLatchy, D.L.; Burridge, L.; Haya, K.; Brown, S.B. Effects of water-borne 4-nonylphenol and 17β-estradiol exposures during parr-smolt transformation on growth and plasma IGFI of Atlantic salmon (Salmo salar L.). Aquat Toxicol 2004, 66, 255–65. [Google Scholar]
- McCormick, S.D.; O’Dea, M.F.; Moeckel, A.M.; Lerner, D.T.; Bjornsson, B.T. Endocrine disruption of parr-smolt transformation and seawater tolerance of Atlantic salmon by 4-nonylphenol and 17beta-estradiol. Gen Comp Endocrinol 2005, 142, 280–8. [Google Scholar]
- Lerner, D.; Sheridan, M.; McCormick, S. Estrogenic compounds decrease growth hormone receptor abundance and alter osmoregulation in Atlantic salmon. Gen Comp Endocrinol 2012, 179, 196–204. [Google Scholar] [CrossRef]
- Hanson, A.M.; Kittilson, J.D.; Martin, L.E.; Sheridan, M.A. Environmental estrogens inhibit growth of rainbow trout (Oncorhynchus mykiss) by modulating the growth hormone-insulin-like growth factor system. Gen Comp Endocrinol 2014, 196, 130–8. [Google Scholar] [CrossRef]
- Varsamos, S.; Xuereb, B.; Commes, T.; Flik, G.; Spanings-Pierrot, C. Pituitary hormone mRNA expression in European sea bass Dicentrarchus labrax in seawater and following acclimation to fresh water. J. Endocrinology 2006, 191, 473–80. [Google Scholar] [CrossRef]
- Bonga, W. The stress response in fish. Physiol Rev 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Mancera, J.M.; McCormick, S.D. Osmoregulatory actions of the GH/IGF axis in non-salmonid teleosts. Comp Biochem Physiol B 1998, 121, 43–8. [Google Scholar] [CrossRef]
- McCormick, S.D. Endocrine control of osmoregulation in Teleost Fish. Am Zool 2001, 41, 781–94. [Google Scholar] [CrossRef]
- Tanghe, T.; Verstraete, W. Adsorption of nonylphenol onto granular activated carbon. Water Air Soil Poll 2001, 131, 61–72. [Google Scholar] [CrossRef]
- Mitter, K.; Kotoulas, G.; Magoulas, A.; Mulero, V.; Sepulcre, P.; Figueras, A.; et al. Evaluation of candidate reference genes for QPCR during ontogenesis and of immune-rele-vant tissues of European seabass (Dicentrarchus labrax). Comp Biochem Physiol B 2009, 153, 340–47. [Google Scholar] [CrossRef]
- Carrera, E.P.; Garcia-Lopez, A.; Martin del Rio, Mdel, P.; Martinez-Rodriguez, G.; Sole, M.; et al. Effects of 17beta-estradiol and 4-nonylphenol on osmoregulation and hepatic enzymes in gilthead sea bream (Sparus auratus). Comp Biochem Physiol C 2007, 145, 210–7. [Google Scholar]
- Madsen, S.S.; Skovbolling, S.; Nielsen, C.; Korsgaard, B. 17-Beta estradiol and 4-nonylphenol delay smolt development and downstream migration in Atlantic salmon, Salmo salar. Aquat Toxicol 2004, 68, 109–20. [Google Scholar] [CrossRef]
- Lerner, D.T.; Bjornsson, B.T.; McCormick, S.D. Aqueous exposure to 4-nonylphenol and 17beta-estradiol increases stress sensitivity and disrupts ion regulatory ability of juvenile Atlantic salmon. Environ Toxicol Chem 2007, 26, 1433–40. [Google Scholar] [CrossRef]
- Nebel, C.; Romestand, B.; Nègre-Sadargues, G.; Grousset, E.; Aujoulat, F.; Bacal, J.; et al. Differential freshwater adaptation in juvenile sea-bass Dicentrarchus labrax: Gill and urinary system involvement. J Exp Biol 2005, 208, 3859–71. [Google Scholar] [CrossRef]
- Bossus, M.; Charmantier, G.; Blondeau-Bidet, E.; Valletta, B.; Boulo, V.; Lorin-Nebel, C. The ClC-3 chloride channel and osmoregulation in the European Sea Bass, Dicentrarchus labrax. J Comp Physiol B 2013, 183, 641–62. [Google Scholar] [CrossRef]
- Agustsson, T.; Sundell, K.; Sakamoto, T.; Ando, M.; Bjornsson, B.T. Pituitary gene expression of somatolactin, prolactin, and growth hormone during Atlantic salmon parrsmolt transformation. Aquaculture 2003, 222, 229–38. [Google Scholar] [CrossRef]
- Wan, G.; Chan, K.M. A study of somatolactin actions by ectopic expression in transgenic zebrafish larvae. J Mol Endocrinol 2010, 45, 301–15. [Google Scholar] [CrossRef] [PubMed]

© Copyright C. Lorin-Nebel et al., 2014. Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BY-NC 3.0).
Share and Cite
Lorin-Nebel, C.; Budzinski, H.; Le Ménach, K.; Devier, M.H.; Charmantier, G.; Gros, R.; Grousset, E.; Blondeau-Bidet, E.; Farcy, E. 4-Nonylphenol Disrupts Osmoregulation in the European Sea-Bass Dicentrarchus labrax. J. Xenobiot. 2014, 4, 4905. https://doi.org/10.4081/xeno.2014.4905
Lorin-Nebel C, Budzinski H, Le Ménach K, Devier MH, Charmantier G, Gros R, Grousset E, Blondeau-Bidet E, Farcy E. 4-Nonylphenol Disrupts Osmoregulation in the European Sea-Bass Dicentrarchus labrax. Journal of Xenobiotics. 2014; 4(2):4905. https://doi.org/10.4081/xeno.2014.4905
Chicago/Turabian StyleLorin-Nebel, C., H. Budzinski, K. Le Ménach, M.H. Devier, G. Charmantier, R. Gros, E. Grousset, E. Blondeau-Bidet, and E. Farcy. 2014. "4-Nonylphenol Disrupts Osmoregulation in the European Sea-Bass Dicentrarchus labrax" Journal of Xenobiotics 4, no. 2: 4905. https://doi.org/10.4081/xeno.2014.4905
APA StyleLorin-Nebel, C., Budzinski, H., Le Ménach, K., Devier, M. H., Charmantier, G., Gros, R., Grousset, E., Blondeau-Bidet, E., & Farcy, E. (2014). 4-Nonylphenol Disrupts Osmoregulation in the European Sea-Bass Dicentrarchus labrax. Journal of Xenobiotics, 4(2), 4905. https://doi.org/10.4081/xeno.2014.4905




