Effect of 3β, Hydroxy-lup-20(29)-en-28-oic Acid on 7,12-Dimethylbenz(a) Anthracene Impaired Cellular Homeostasis in Extrahepatic Organs of Sprague Dawley Rats
Abstract
:Introduction
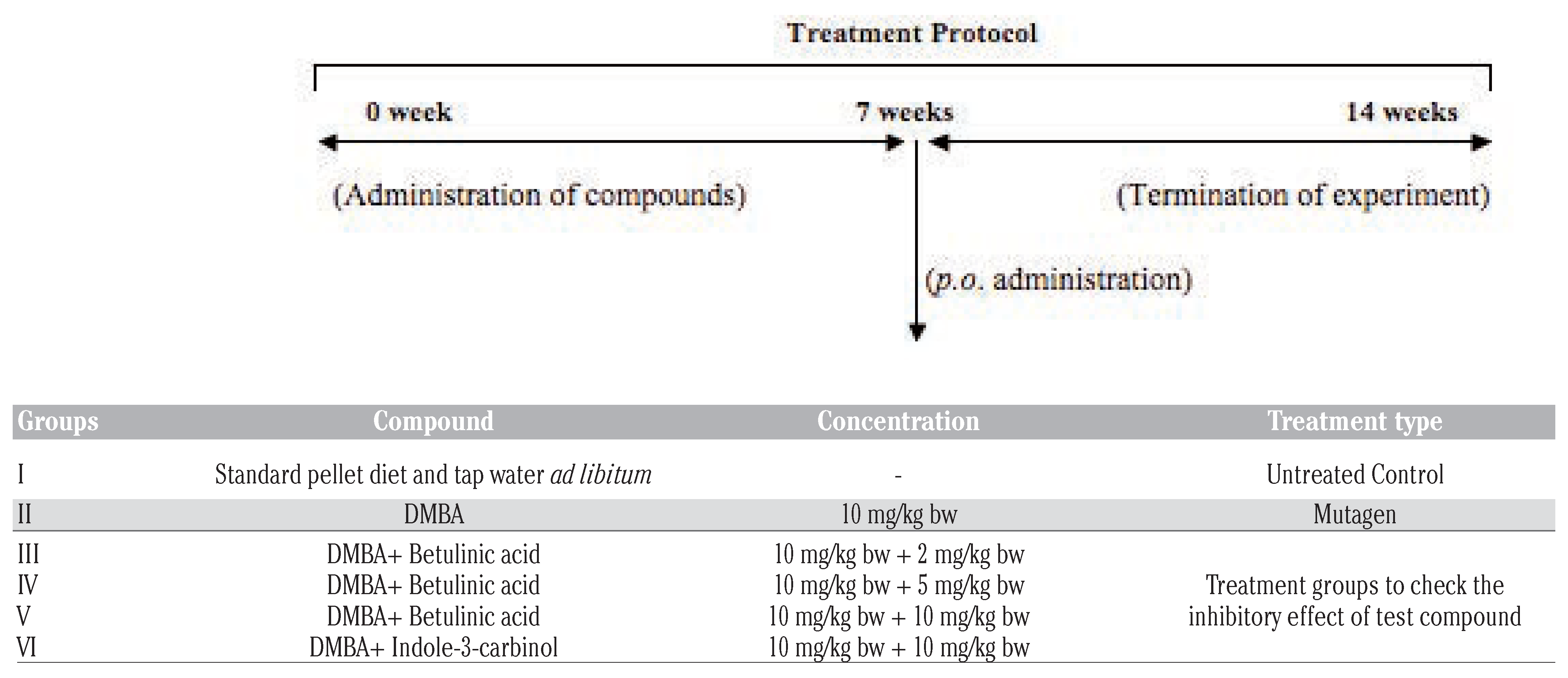
Ethical approval for animal research
Materials and Methods
Preparation of homogenate
Biochemical analysis
Estimation of membrane bound ATPases
Estimation of detoxification enzymes
Estimation of antioxidant enzymes
Estimation of oxidative stress parameters
Determination of protein content
Statistical analysis
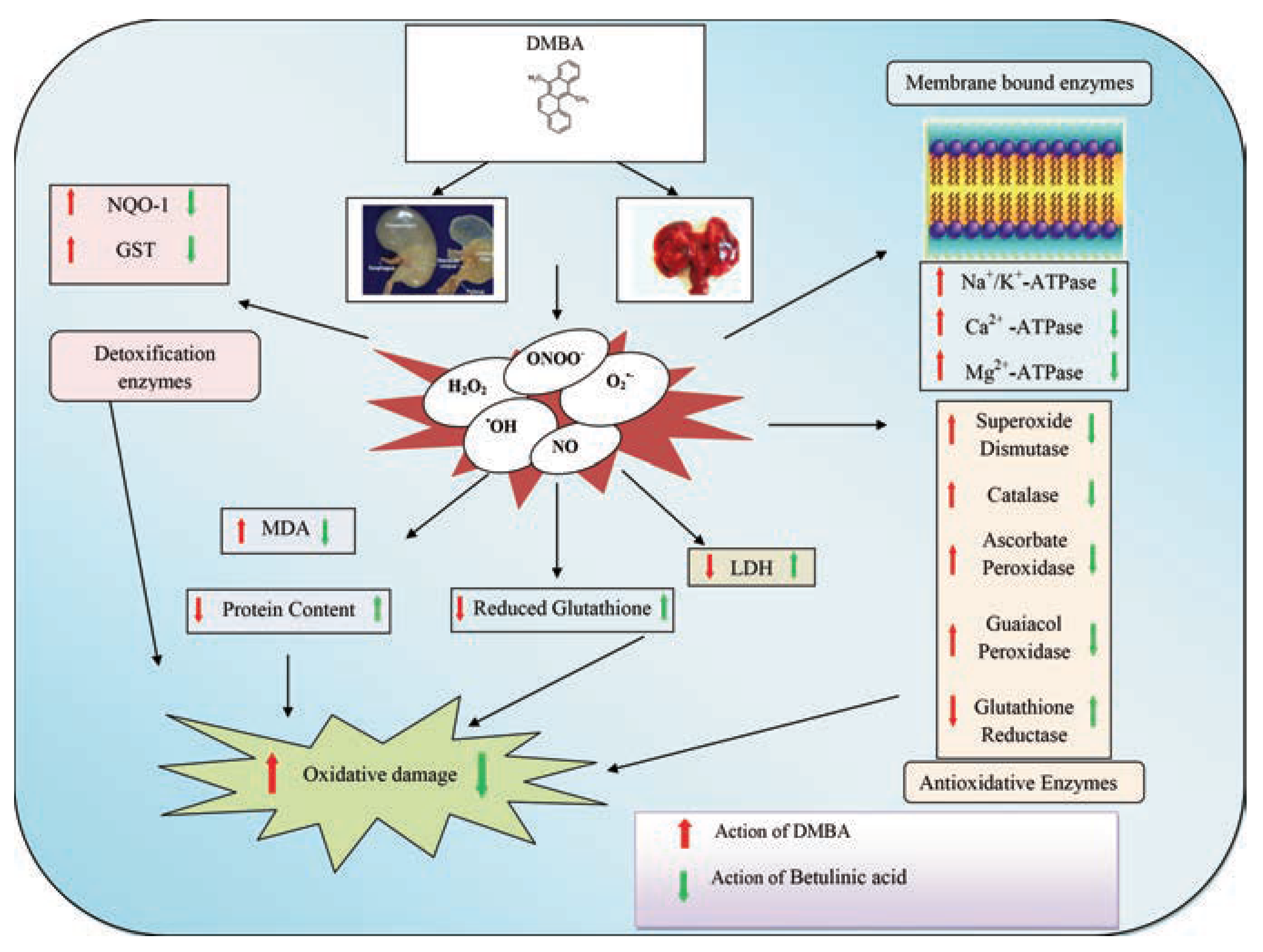
Results
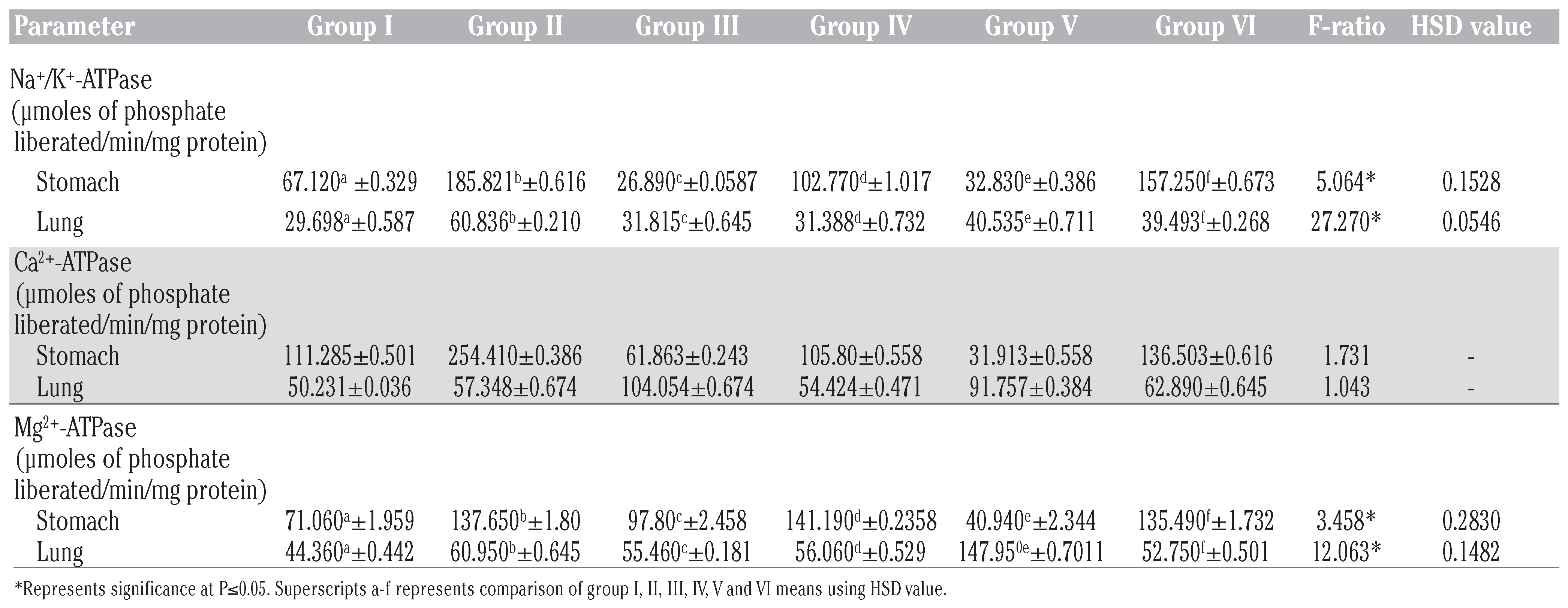
Betulinic acid modulates DMBA induced impairment of membrane bound ATPases
Betulinic acid modulates DMBA induced impairment of detoxification enzymes
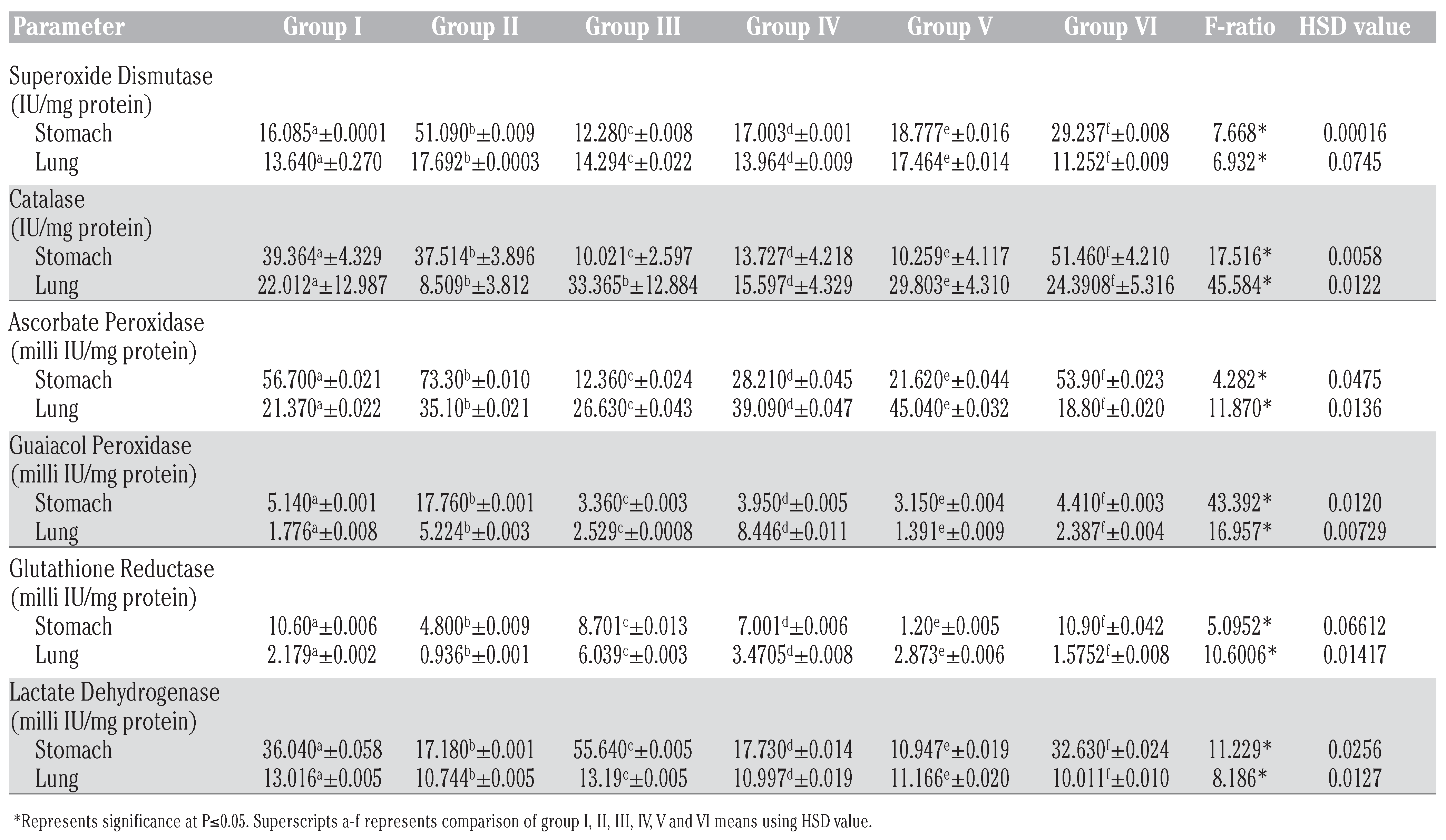
Betulinic acid modulates DMBA induced impairment of antioxidant enzymes
Betulinic acid modulates DMBA induced oxidative stress
Protein content analysis
Discussion
Conclusions
Research highlights
- -
- Extrahepatic organs cope up with scores of xenobiotics by acting as first line detoxification defense.
- -
- Betulinic acid neutralizes the oxidant radicals evoked by such xenobiotics (DMBA) in extrahepatic sites maintaining electrophysiological energetic and cellular homeostasis.
- -
- Thus, betulinic acid offers protection against tissue damage induced by DMBA.
Contributions
Acknowledgments
Conflicts of Interest
References
- Caldwell, J.; Gardner, I.; Swale, N. An introduction to drug disposition: the basic principles of absorption, distribution, metabolism and excretion. Toxicol Pathol 1995, 23, 102–14. [Google Scholar] [CrossRef] [PubMed]
- Robin Arora, S.; Vig, A.P. Inhibition of DNA oxidative damage and antimutagenic activity by dichloromethane extract of Brassica rapa var. rapa L. seeds. Ind Crops Prod 2015, 74, 585–91. [Google Scholar] [CrossRef]
- Kyegombe, D. Clinical and pharmacological significance of extrahepatic drug metabolism in man. Bahrain Med Bull 1990, 12, 29–32. [Google Scholar]
- Sampson, H.A. Food hypersensitivity: manifestations, digestions and natural history. Food Tech 1992, 41–144. [Google Scholar]
- Kashaw, V.; Nema, A.K.; Agarwal, A. Hepatoprotective prospective of herbal drugs and their vesicular carriers - a review. Int J Pharma Bio Sci 2011, 2, 360–74. [Google Scholar]
- Smyth, H.D.C.; Hickey, A.J. Controlled pulmonary drug delivery, Advances in delivery science and technology. New York: Springer-Verlag; 2011.
- Aoki, M.; Okudaira, K.; Haga, M.; Nishigaki, R.; Hayashi, M. Contribution of rat pulmonary metabolism to the elimination of lidocaine, midazolam and nifedipine. Drug Metab Dispos 2011, 38, 1183–8. [Google Scholar] [CrossRef] [PubMed]
- Yost, G.S. Sites of metabolism: lung. In: Woolf TF, ed. Handbook of drug metabolism. New York: Marcel Dekker Inc; 1999. pp 263-78.
- Perreault, S.; Dumont, L.; Villiere, V.; Ong, H.; Adam, A.; du Souich, P. Hepatic and extrahepatic metabolism of salbutamol in anesthetized rabbits. Drug Metab Dispos 1993, 21, 485–91. [Google Scholar]
- National Institute of Health. Division of occupational health and safety. 7,12-Dimethylbenz[a]anthracene safety datasheet; 1993.
- Grant, R. Cancer induction in the glandular stomach of rats at sites of implanted 7,12-Dimethylbenz[a]anthracene. J Natl Cancer Inst 1966, 37, 353–64. [Google Scholar] [CrossRef]
- Wattenberg, L.W. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst 1977, 58, 395–8. [Google Scholar] [CrossRef]
- Maltoni, C.; Prodi, G. Effect of 9,10-dimethyl-1,2-benzanthracene injected intraperitoneally into the rat. Tumori 1959, 45, 71–85. [Google Scholar]
- Dipple, A.; Bigger, C.A. Mechanism of action of food-associated polycyclic aromatic hydrocarbon carcinogens. Mutat Res 1991, 259, 263–6. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.C.; Giner, R.M.; Manez, S.; Gueho, J.; Julien, H.R.; Hostettmann, K.; Rios, J.L. Investigations on the steroidal antiinflammatory activity of triterpenoids from Diospyros leucomelas. Planta Med 1995, 61, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Martelanc, M.; Vovk, I.; Simonovska, B. Determination of three major triterpenoids in epicuticular wax of cabbage (Brassica oleracea L.) by high-performance liquid chromatography with UV and mass spectrometric detection. J Chromatogra A 2007, 1164, 145–52. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Mallick, S.; Vedasiromoni, J.R.; Pal, B.C. Anti-leukemic activity of Dillenia indica L. fruit extract and quantification of 3β, hydroxy-lup-20(29)-en-28-oic acid by HPLC. Phytomedicine 2010, 17, 431–5. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Saha, K.; Das, J.; Pal, M.; Saha, B.P. Studies on the anti-inflammatory activity of rhizomes of Nelumbo nucifera. Planta Med 1997, 63, 367–9. [Google Scholar] [CrossRef]
- Clercq, D.E. Antiviral therapy for human immunodeficiency virus infections. Clin Microbiol Rev 1995, 8, 200–39. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. 3β, hydroxy-lup-20(29)-en-28-oic acid: a natural product with anticancer activity. Mol Nutr Food Res 1995, 53, 140–6. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.; et al. Discovery of 3β, hydroxy-lup-20(29)-en-28-oic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med 1995, 1, 1046–51. [Google Scholar] [CrossRef]
- Hashimoto, F.; Kashiwada, Y.; Cosentino, L.M.; Chen, C.H.; Garrett, P.E.; Lee, K.H. Anti-AIDS agents-XXVII. Synthesis and anti-HIV activity of 3β, hydroxy-lup-20(29)-en-28-oic acid and dihyro3β, hydroxy-lup-20(29)-en-28-oic acid derivatives. Bioorg Med Chem 1997, 5, 2133–43. [Google Scholar] [CrossRef]
- Kessler, J.H.; Mullauer, F.B.; de Roo, G.M.; Medema, J.P. Broad in vitro efficacy of plant-derived 3β, hydroxy-lup-20(29)-en-28-oic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett 2007, 251, 132–45. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. 3β, hydroxy-lup-20(29)-en-28-oic acid induces apoptosis through a direct effect on mitochondria in neuroectodermal tumors. Med Pediatr Oncol 2000, 35, 616–8. [Google Scholar] [CrossRef] [PubMed]
- Syrovets, T.; Buchele, B.; Gedig, E.; Slupsky, J.R.; Simmet, T. Acetylboswellic acids are novel catalytic inhibitors of human topoisomerases I and II alpha. Mol Pharmacol 2000, 58, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Arora, S. Interactions of 3β, hydroxy-lup-20(29)-en-28-oic acid with xenobiotic metabolizing and antioxidative enzymes in 7,12-Dimethylbenz(a)anthracene-treated Sprague Dawley female rats. Free Radic Biol Med 2013, 65, 131–42. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.P.; Kool, H.J.M.; Mullenders, L.H.F.; Slater, R.; Zeeland, A.A.; Vrieling, H. 7,12-Dimethylbenz(a) anthracene-induced toxic and mutagenic responses vary dramatically between NER deficient Xpa, Xpc and Csb mice. Carcinogenesis 2001, 22, 1099–106. [Google Scholar] [CrossRef]
- Bonting, S.L. Sodium-potassium activated adenosine triphosphatase and cation transport. In: Bitter EE, ed. Membrane ion transport. Chichester: Inter Science; 1970. pp 257-63.
- Hjerten, S.; Pan, H. Purification and characterization of two forms of a low affinity Ca2+-ATPase from erythrocyte membranes. Biochim Biophys Acta 1983, 728, 281–8. [Google Scholar] [CrossRef]
- Ohnishi, T.; Suzuki, T.; Suzuki, Y.; Ozawa, K.A. A comparative study of plasma membrane Mg2+-ATPase activities in normal, regenerating and malignant cells. Biochim Biophys Acta 1982, 684, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Fiske, C.H.; Subbarow, Y. Calorimetric determination of phosphorous. J Biol Chem 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974, 249, 7130–9. [Google Scholar] [CrossRef]
- Ernster, L. DT-diaphorase. In: Estabrook RW, Pullman ME, eds. Methods in enzymology. New York: Academic Press; 1967. pp 309-17.
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 1978, 186, 189–95. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In: Colowick SP, Kaplan NO, eds. Methods in enzymology. New York, Academic Press; 1984. pp 121-6.
- Asada, K. Ascorbate peroxidase - a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant 1992, 85, 235–41. [Google Scholar] [CrossRef]
- Putter, J. Peroxidase. In: Bergmeyer HU, ed. Methods of enzymatic analysis. Weinhan, Verlag Chemie; 1974. pp 685- 90.
- Carlberg, I.; Mannervik, B. Glutathione reductase. In: Meister A, ed. Methods in enzymology. New York: Academic Press; 1975. pp 484-90.
- Kuznetsov, A.V.; Gnaiger, E. Laboratory protocol: Lactate dehydrogenase. Cytosolic marker enzyme. Mitochondr Physiol Network 2010, 18, 1–8. [Google Scholar]
- Devasagayam, T.P.; Boloor, K.K.; Ramasarma, T. Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J Biochem Biophys 2003, 40, 300–8. [Google Scholar] [PubMed]
- Jiang, Z.Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxides in low density lipoprotein. Anal Biochem 1992, 202, 384–9. [Google Scholar] [CrossRef]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. In: Meister A, ed. Methods in enzymology. New York: Academic Press;
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; et al. Measurement of protein using bicinchoninic acid. Anal Biochem 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Rauchova, H.; Ledvinkova, J.; Kalous, M.; Drahota, Z. The effect of lipid peroxidation on the activity of various membrane-bound ATPases in rat kidney. Int J Biochem Cell Biol 1995, 27, 251–5. [Google Scholar] [CrossRef]
- Franklin, C.S.; Kyegombe, D.B. The distribution and induction of some drugmetabolising enzymes in man. Br J Pharmacol 1973, 47, 616. [Google Scholar]
- Wacher, V.J.; Salphati, L.; Benet, L.Z. Active secretion and enterocytic drug metabolism barriers to drug absorption. Adv Drug Deliv Rev 2001, 46, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S.; Zhang, Q.Y.; Dunbar, D.; Kaminsky, L.S. Metabolic characterization of the major human small intestinal cytochrome p450s. Drug Metab Dispos 2001, 29, 347–52. [Google Scholar]
- Melander, A. Influence of food on the bioavailability of drugs. Clinical Pharmacokinet 1978, 3, 337–51. [Google Scholar] [CrossRef]
- Lanning, C.L.; Fine, R.L.; Sachs, C.W.; Rao, U.S.; Corcoran, J.J.; Abou-Donia, M.B. Chlorpyrifos oxon interacts with the mammalian multidrug resistance protein, P-glycoprotein. J Toxicol Environ Health 1996, 47, 395–407. [Google Scholar] [CrossRef]
- Rahimtula, A.D.; Zachariah, P.K.; Obrien, P.J. Differential effects of antioxidants, steroids and other compounds on benzo(a)pyrene 3-hydroxylase activity in various tissues of rat. Br J Cancer 1979, 40, 105–12. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.E.; Sriningsih, P.E.; Wuyung Ranasasmita, R. The influence of 7,12-Dimethylbenz(a)anthracene (7,12-dimethylbenz-[a]anthracene) regimen in the development of mammae carcinogenesis on Sprague Dawley female rat. Int J Canc Chemoprev 2010, 1, 60–6. [Google Scholar]
- Davis, R.K.; Stevenson, G.T.; Busch, K.A. Tumor incidence in normal spraguedawley female rats. Cancer Res 1956, 16, 194–7. [Google Scholar]
- Prejean, J.D.; Peckham, J.C.; Casey, A.E.; Griswold, D.P.; Weisburger, E.K.; Weisburger, J.H. Spontaneous tumors in sprague-dawley rats and swiss mice. Cancer Res 1973, 33, 2768–73. [Google Scholar]
- Glitsch, H.G. Electrophysiology of the sodium-potassium-ATPase in cardiac cells. Physiol Rev 2001, 81, 1791–826. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab 2010, 5, 7–18. [Google Scholar] [CrossRef]
- Levy, J.; Gavin, J.R.; Sowers, J.R. Diabetes mellitus: a disease of abnormal cellular calcium metabolism? Am J Med 1994, 96, 260–73. [Google Scholar] [CrossRef] [PubMed]
- Golfman, L.S.; Takeda, N.; Dhalla, N.S. Cardiac membrane Ca2+-transport in alloxan induced diabetes in rats. Diabetes Res Clin Pract 1996, 31, S73–7. [Google Scholar] [CrossRef]
- Tsakiris, S.; Marinou, K.; Schulpis, K.H. The in vitro effects of galactose and its derivatives on rat brain Mg2+-ATPase activity. Pharmacol Toxicol 2002, 91, 254–7. [Google Scholar] [CrossRef]
- Kabler, S.L.; Seidel, A.; Jacob, J.; Doehmer, J.; Morrow, C.S.; Townsend, A.J. Differential protection by human glutathione S-transferase P1 against cytotoxicity of benzo[a]pyrene, dibenzo[a,l]pyrene, or their dihydrodiol metabolites, in bi-transgenic cell lines that co-express rat versus human cytochrome P4501A1. Chem Biol Interact 2009, 179, 240–6. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 2010, 501, 116–23. [Google Scholar] [CrossRef]
- Balasenthil, S.; Saroja, M.; Ramachandran, C.R.; Nagini, S. Of humans and hamsters: comparative analysis of lipid peroxidation, glutathione and glutathione-dependent enzymes during oral carcinogenesis. Br J Oral Maxillofac Surg 2000, 38, 267–70. [Google Scholar] [CrossRef] [PubMed]
- Mikami, K.; Naito, M.; Ishiguro, T.; Yano, H.; Tomida, A.; Yamada, T.; et al. Immunological quantitation of DT-diaphorase in carcinoma cell lines and clinical colon cancers: advanced tumors express greater levels of DT-diaphorase. Jpn J Cancer Res 1998, 89, 910–5. [Google Scholar] [CrossRef]
- Flora, S.D.; Ramel, C. Mechanisms of inhibitors of mutagenesis and carcinogenesis. Classification and overview. Mutat Res 1988, 202, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hileman, E.O.; Plunkett, W.; Keating, M.J.; Huang, P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood 2003, 101, 4098–104. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B. Measurement of glutathione reductase activity. Curr Protoc Toxicol 2001. [CrossRef]
- Kaplan, N.O. Effect of hormones and environmental factors on lactic dehydrogenases. J Cell Physiol 1965, 66, 1–10. [Google Scholar] [CrossRef]
- Cassidy, W.M.; Reynolds, T.B. Serum lactic dehydrogenase in the differential diagnosis of acute hepatocellular injury. J Clin Gasteroenterol 1994, 19, 118–21. [Google Scholar] [CrossRef]
- Joshi, P.S.; Chougule, M.; Dudanakar, M.; Golgire, S. Comparison between salivary and serum lactate dehydrogenase levels in patients with oral leukoplakia and oral squamous cell carcinoma- a pilot study. Int J Oral Maxillofac Pathol 2012, 3, 7–12. [Google Scholar]
- Goyal, P.K.; Verma, P.; Sharma, P.; Parmar, J.; Agarwal, A. Evaluation of anti-cancer and anti-oxidative potential of Syzygium cumini against benzo(a)pyrene (BaP) induced gastric carcinogenesis in mice. Asian Pac J Cancer Prev 2010, 11, 753–8. [Google Scholar]
- Purushothaman, A.; Nandhakumar, E.; Sachdanandam, P. Modulation of lipid peroxidation and antioxidant status upon administration of ‘Shemamruthaa’ in 7,12-dimethylbenz[a]anthracene induced mammary carcinoma bearing rats. Asian Pac J Trop Biomed 2012, 2, S765–71. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage. Toxicol Pathol 2014, 38, 96–109. [Google Scholar] [CrossRef] [PubMed]



© P. Kaur et al., 2017 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BY-NC 3.0).
Share and Cite
Kaur, P.; Kaur, R.; Arora, R.; Arora, S. Effect of 3β, Hydroxy-lup-20(29)-en-28-oic Acid on 7,12-Dimethylbenz(a) Anthracene Impaired Cellular Homeostasis in Extrahepatic Organs of Sprague Dawley Rats. J. Xenobiot. 2017, 7, 6475. https://doi.org/10.4081/xeno.2017.6475
Kaur P, Kaur R, Arora R, Arora S. Effect of 3β, Hydroxy-lup-20(29)-en-28-oic Acid on 7,12-Dimethylbenz(a) Anthracene Impaired Cellular Homeostasis in Extrahepatic Organs of Sprague Dawley Rats. Journal of Xenobiotics. 2017; 7(1):6475. https://doi.org/10.4081/xeno.2017.6475
Chicago/Turabian StyleKaur, Pardeep, Rajbir Kaur, Rohit Arora, and Saroj Arora. 2017. "Effect of 3β, Hydroxy-lup-20(29)-en-28-oic Acid on 7,12-Dimethylbenz(a) Anthracene Impaired Cellular Homeostasis in Extrahepatic Organs of Sprague Dawley Rats" Journal of Xenobiotics 7, no. 1: 6475. https://doi.org/10.4081/xeno.2017.6475
APA StyleKaur, P., Kaur, R., Arora, R., & Arora, S. (2017). Effect of 3β, Hydroxy-lup-20(29)-en-28-oic Acid on 7,12-Dimethylbenz(a) Anthracene Impaired Cellular Homeostasis in Extrahepatic Organs of Sprague Dawley Rats. Journal of Xenobiotics, 7(1), 6475. https://doi.org/10.4081/xeno.2017.6475




