Pancreatic Carcinoma Metastatic to the Gingiva
Abstract
:1. Introduction
2. Case Report
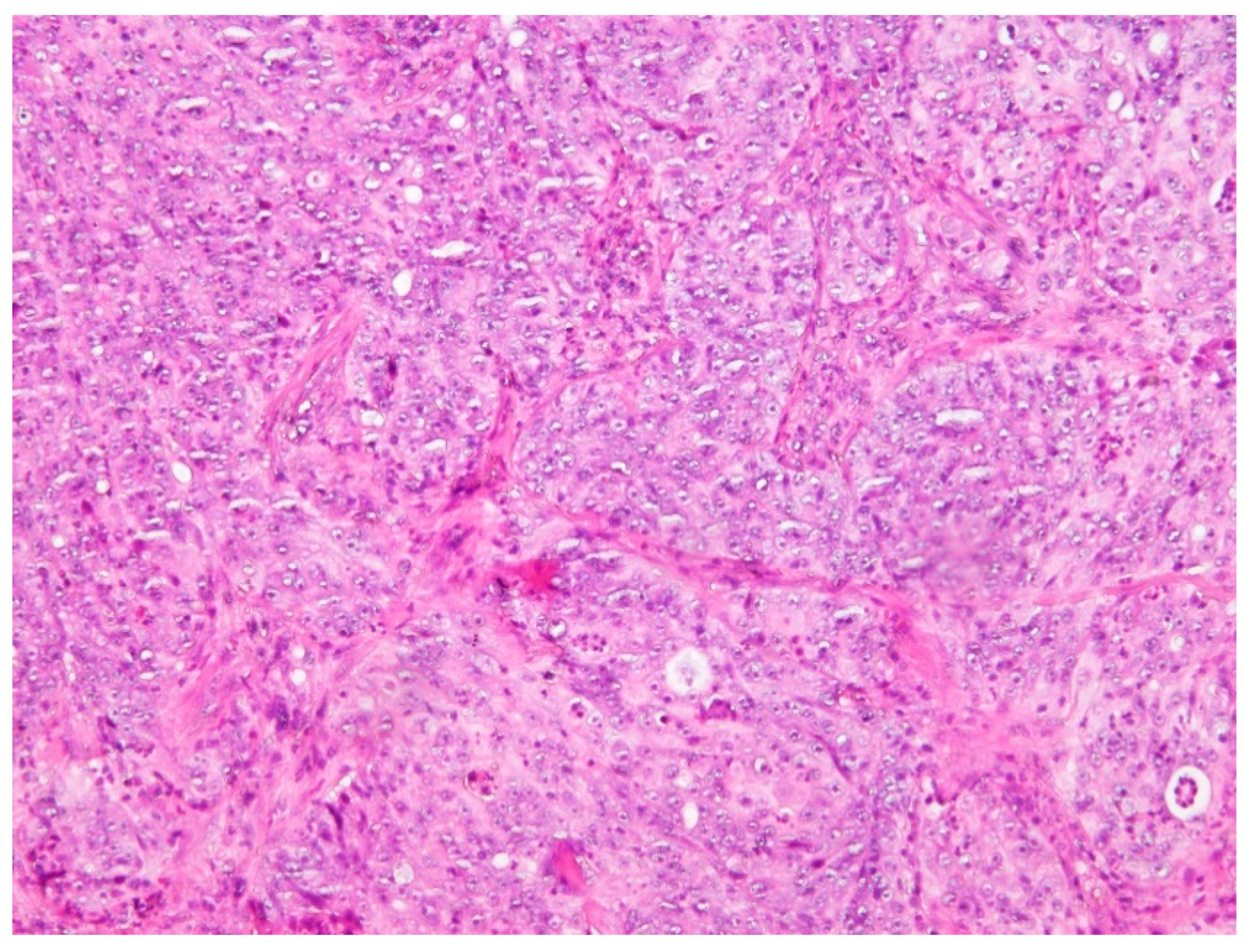
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirshberg, A.; Buchner, A. Metastatic tumours to the oral region. An overview. Eur. J. Cancer Part B 1995, 31, 355–360. [Google Scholar] [CrossRef]
- Hirshberg, A.; Shnaiderman-Shapiro, A.; Kaplan, I.; Berger, R. Metastatic tumours to the oral cavity—Pathogenesis and analysis of 673 cases. Oral Oncol. 2008, 44, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-Y.; Kim, S.-A.; Ahn, S.-G.; Kim, H.-K.; Hwang, H.-K.; Kim, B.-O.; Lee, S.-H.; Kim, J.-D.; Yoon, J.-H.; Kim, S.-G. Metastatic tumours to the jaws and oral soft tissues: A retrospective analysis of 41 Korean patients. Int. J. Oral Maxillofac. Surg. 2006, 35, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Yoshimura, H.; Kondo, S.; Sano, K. Temporomandibular dislocation caused by pancreatic cancer metastasis: A case report. Oncol. Lett. 2017, 14, 6053–6058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keane, M.G.; Horsfall, L.; Rait, G.; Pereira, S. A case-control study comparing the incidence of early symptoms in pancreatic and biliary tract cancer. BMJ Open 2014, 4, e005720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirshberg, A.; Leibovich, P.; Buchner, A. Metastatic tumors to the jawbones: Analysis of 390 cases. J. Oral Pathol. Med. 1994, 23, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Kolokythas, A.; Miloro, M.; Olsson, A.B. Metastatic pancreatic adenocarcinoma to the mandibular condyle: A rare clinical presentation. J. Oral Maxillofac. Surg. 2014, 72, 83–88. [Google Scholar] [CrossRef]
- Pires, F.R.; Sagarra, R.; Corrêa, M.E.P.; Pereira, C.M.; Vargas, P.A.; Lopes, M.A. Oral metastasis of a hepatocellular carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 359–368. [Google Scholar] [CrossRef]
- Stecher, J.A.; Mostofi, R.; True, L.; Indresano, A.T. Pancreatic carcinoma metastatic to the mandibular gingiva. J. Oral Maxillofac. Surg. 1985, 43, 385–390. [Google Scholar] [CrossRef]
- Kucuktulu, E.; Kucuktulu, U.; Guner, A.; Cobanoglu, H.B.; Mungan, S.; Mirik, A.O. Pancreatic tumor metastasis of the tongue. J. Case Rep. 2013, 3, 385–389. [Google Scholar] [CrossRef]
- Vähätalo, K.; Ekfors, T.; Syrjänen, S. Adenocarcinoma of the pancreas metastatic to the mandible. J. Oral Maxillofac. Surg. 2000, 58, 110–114. [Google Scholar] [CrossRef]
- Scipio, J.; Murti, P.; Al-Bayaty, H.; Matthews, R.; Scully, C. Metastasis of breast carcinoma to mandibular gingiva. Oral Oncol. 2001, 37, 393–396. [Google Scholar] [CrossRef]
- Miles, B.A.; Schwartz-Dabney, C.; Sinn, D.P.; Kessler, H.P. Bilateral metastatic breast adenocarcinoma within the temporomandibular joint: A case report. J. Oral Maxillofac. Surg. 2006, 64, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Muttagi, S.S.; D′cruz, A.; Chaukar, D.; Singh, B.; Chaturvedi, P.; Kane, S.; Pai, P.; Pawar, P. Metastatic tumors to the jaw bones: Retrospective analysis from an Indian tertiary referral center. Indian J. Cancer 2011, 48, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Shintaku, W.H.; Venturin, J.S.; Yepes, J.F. Application of advanced imaging modalities for the diagnosis of metastatic adenocarcinoma of the lungs in the temporomandibular joint. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, e37–e41. [Google Scholar] [CrossRef]
- Hirshberg, A.; Leibovich, P.; Buchner, A. Metastases to the oral mucosa: Analysis of 157 cases. J. Oral Pathol. Med. 1993, 22, 385–390. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Herford, A.S.; Romeo, U.; Bianchi, A.; Crimi, S.; D’Amico, C.; De Stefano, R.; Troiano, G.; Santoro, R.; et al. Molecular biomarkers related to oral carcinoma: Clinical trial outcome evaluation in a literature review. Dis. Markers 2019, 2019, 8040361. [Google Scholar] [CrossRef]
- Papa, F.; Ferrara, S.; Felicetta, L.; Lavorgna, G.; Matarazzo, M.; Staibano, S.; De Rosa, G.; Troisi, S.; Claudio, P.P. Mandibular metastatic hepatocellular carcinoma: Report of a case involving severe and uncontrollable hemorrhage. Anticancer Res. 2001, 21, 2121–2130. [Google Scholar]
- Favia, G.; Piattelli, A.; Favia, G. Hepatocellular carcinoma metastatic to the oral mucosa: Report of a case with multiple gingival localizations. J. Periodontol. 2000, 71, 641–645. [Google Scholar] [CrossRef]
- Maschino, F.; Guillet, J.; Curien, R.; Dolivet, G.; Bravetti, P.; Maschino, F.; Guillet, J.; Curien, R.; Dolivet, G.; Bravetti, P. Oral metastasis: A report of 23 cases. Int. J. Oral Maxillofac. Surg. 2013, 42, 164–168. [Google Scholar] [CrossRef]
- Cicciù, M.; Cervino, G.; Fiorillo, L.; D’Amico, C.; Oteri, G.; Troiano, G.; Zhurakivska, K.; Muzio, L.L.; Herford, A.S.; Crimi, S.; et al. Early diagnosis on oral and potentially oral malignant lesions: A systematic review on the VELscope® fluorescence method. Dent. J. 2019, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Authors | Year Reported | Patient Age | Patient Sex | Smoking | Previous Diseases of the Oral Cavity | Clinical Presentation | Site of Metastasis | PHD |
|---|---|---|---|---|---|---|---|---|
| Stechera et al. [9] | 1981 | 46 | Male | Yes | Chronic periodontitis | Teeth 21–27 were extremely mobile, attached to inflammed, edematous gingival tissue | Mandibular gingiva | Adenocarcinoma |
| Kucuktulu et al. [10] | 2013 | 72 | Male | Unknown | No | PET/CT evaluation revealed a 2 × 1 mm mass, hard in consistency in right anterior 1/3 of the tongue | Tongue | Adenocarcinoma |
| Maschino et al. [19] | 2013 | 71 | Male | Yes | Chronic periodontitis | Difficulties healing after oral surgery | Maxillar gingiva | Adenocarcinoma |
| Kim et al. [3] | 2005 | Unknown | Unknown | Unknown | Unknown | bleeding and increasing tooth mobility. | Unknown | Pleomorphic carcinoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubović, A.; Belušić-Gobić, M.; Harmicar, D.; Marušić, J.; Vučinić, D.; Zamolo, G. Pancreatic Carcinoma Metastatic to the Gingiva. Clin. Pract. 2021, 11, 58-64. https://doi.org/10.3390/clinpract11010010
Zubović A, Belušić-Gobić M, Harmicar D, Marušić J, Vučinić D, Zamolo G. Pancreatic Carcinoma Metastatic to the Gingiva. Clinics and Practice. 2021; 11(1):58-64. https://doi.org/10.3390/clinpract11010010
Chicago/Turabian StyleZubović, Arijan, Margita Belušić-Gobić, David Harmicar, Jasna Marušić, Damir Vučinić, and Gordana Zamolo. 2021. "Pancreatic Carcinoma Metastatic to the Gingiva" Clinics and Practice 11, no. 1: 58-64. https://doi.org/10.3390/clinpract11010010
APA StyleZubović, A., Belušić-Gobić, M., Harmicar, D., Marušić, J., Vučinić, D., & Zamolo, G. (2021). Pancreatic Carcinoma Metastatic to the Gingiva. Clinics and Practice, 11(1), 58-64. https://doi.org/10.3390/clinpract11010010





