Pushout Bond Strength of Root Fillings after Irrigation of Root Canals Utilizing Sodium Hypochlorite, Chlorhexidine, and Homeopathic Mother Tincture (Arnica Montana)
Abstract
1. Introduction
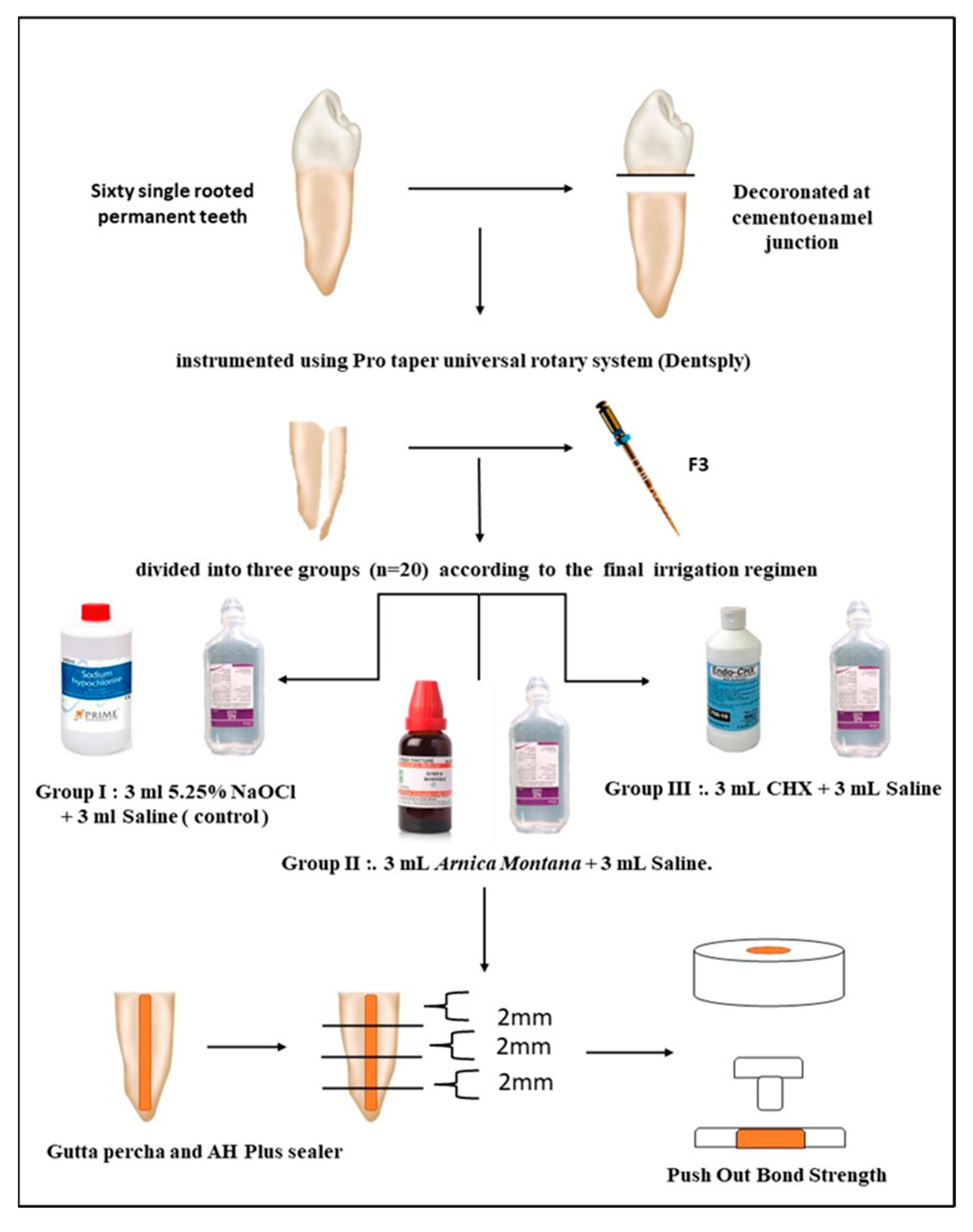
2. Materials and Methods
- Group I: 3 mL 5.25% NaOCl (Prime Dental Pvt. Ltd., Mumbai, India) followed by 3 mL saline (control).
- Group II: 3 mL Arnica montana followed by 3 mL saline.
- Group III: 3 mL 2% CHX (Prevest DenPro Ltd., Jammu, India) followed by 3 mL saline.
2.1. Root Canal Irrigation
2.2. Preparation of Arnica Montana Solution
- Adhesive failure between sealer and dentin (when the dentin was more than 75% free of root-filling material).
- Cohesive failure within sealer (when the percentage dropped below 25%).
- Mixed failure (when the percentage of exposed dentin was between 25% and 75%).
2.3. Statistical Analysis
3. Results
Intragroup Comparisons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, D.; Taneja, S.; Kumari, M. Efficacy of different irrigation regimes on the push-out bond strength of various resin-based sealers at different root levels: An in vitro study. J. Conserv. Dent. 2018, 21, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.; Gusman, H.; Gomes, B.P.F.A.; Simão, R.A. Scanning electron microscopic investigation of the effectiveness of phosphoric acid in smear layer removal when compared with EDTA and citric acid. J. Endod. 2011, 37, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Shivanna, V. The effect of different irrigating solutions on the push out bond strength of endodontic sealer to dentin and assessing the fracture modes: An in-vitro study. J. Int. Clin. Dent. Res. Organ. 2014, 6, 86–91. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in Endodontics. Br. Dent. J. 2014, 216, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, R.; Badaran, A.; Elghazawy, R.; El-Hady, S. Evaluation of antibacterial effect against E. Feacalis and smear layer removal ability of turmeric extract solution as a root canal irrigant for primary anterior teeth: An in-vitro study. Adv. Dent. J. 2022, 4, 187–197. [Google Scholar] [CrossRef]
- Melnyk, N.; Vlasova, I.; Skowrońska, W.; Bazylko, A.; Piwowarski, J.P.; Granica, S. Current knowledge on interactions of plant materials traditionally used in skin diseases in Poland and Ukraine with human skin microbiota. Int. J. Mol. Sci. 2022, 23, 9644. [Google Scholar] [CrossRef] [PubMed]
- Mawardi, H.; Elbadawi, L. A cross-sectional survey on dentist’s knowledge and attitude towards the application of Arnica Montana in dental practice. J. Clin. Diagn. Res. 2022, 16, 23–27. [Google Scholar] [CrossRef]
- Koo, H.; Gomes, B.P.F.A.; Rosalen, P.L.; Ambrosano, G.M.B.; Park, Y.K.; Cury, J.A. In vitro antimicrobial activity of propolis and Arnica Montana against oral pathogens. Arch. Oral Biol. 2000, 45, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Nabavizadeh, M.; Sobhnamayan, F.; Sedigh-Shams, M.; Liaghat, S. Comparison of the push-out bond strength of ah plus sealer to dentin after using different herbal irrigation solutions as the final rinse. PLoS ONE 2022, 17, e0276666. [Google Scholar] [CrossRef] [PubMed]
- Collares, F.M.; Portella, F.F.; Rodrigues, S.B.; Celeste, R.K.; Leitune, V.C.; Samuel, S.M. The influence of methodological variables on the push-out resistance to dislodgement of root filling materials: A meta-regression analysis. Int. Endod. J. 2015, 49, 836–849. [Google Scholar] [CrossRef]
- Üreyen Kaya, B.; Keçeci, A.D.; Orhan, H.; Belli, S. Micropush-out bond strengths of gutta-percha versus thermoplastic synthetic polymer-based systems—An ex vivo study. Int. Endod. J. 2008, 41, 211–218. [Google Scholar] [CrossRef]
- Dem, K.; Wu, Y.; Kaminga, A.C.; Dai, Z.; Cao, X.; Zhu, B. The push out bond strength of polydimethylsiloxane endodontic sealers to Dentin. BMC Oral. Health 2019, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Aamir, M.N.; Ahmad, M.; Ghafoor, N. Antibacterial activity of mother tinctures of Cholistan desert plants in Pakistan. Indian J. Pharm. Sci. 2012, 74, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.S.; Swartz, M.L.; Moore, B.K.; Rhodes, B.F. Influence of selected variables on adhesion testing. Dent. Mater. 1992, 8, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Yalgi, V.S.; Bhat, K.G. Antibacterial activity of homoeopathic tinctures on bacterial strains of streptococcus mutans and enterococcus faecalis: An in vitro study. J. Clin. Diagn. Res. 2019, 13, ZC01–ZC04. [Google Scholar] [CrossRef]
- Shivanand, S.; Patil, C.; Patil, A.C.; Choudhury, S.; Patil, S.A.; Doddwad, P.K. Evaluation of push-out bond strength of a resin sealer to dentin after a final flush of three irrigants. J. Contemp. Dent. Pract. 2020, 21, 982–985. [Google Scholar] [CrossRef]
- Teixeira, C.S.; Alfredo, E.; Thomé, L.H.; Gariba-Silva, R.; Silva-Sousa, Y.T.; Sousa-Neto, M.D. Adhesion of an endodontic sealer to dentin and gutta-percha: Shear and push-out bond strength measurements and Sem Analysis. J. Appl. Oral Sci. 2009, 17, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Gogos, C.; Economides, N.; Stavrianos, C.; Kolokouris, I.; Kokorikos, I. Adhesion of a new methacrylate resin-based sealer to human dentin. J. Endod. 2004, 30, 238–240. [Google Scholar] [CrossRef]
- Pawar, A.M.; Kfir, A.; Metzger, Z.; Bhardwaj, A.; Yohana, Y.; Wahjuningrun, D.A.; Luke, A.M.; Pawar, B.A. Can type of instrumentation and activation of the final Irrigant improve the obturation quality in oval root canals? A push-out bond strength study. Biology 2022, 11, 59. [Google Scholar] [CrossRef]
- Nagas, E.; Cehreli, Z.C.; Durmaz, V. Effect of light-emitting diode photopolymerization modes on the push-out bond strength of a methacrylate-based sealer. J. Endod. 2011, 37, 832–835. [Google Scholar] [CrossRef]
- Pawar, A.M.; Pawar, S.; Kfir, A.; Pawar, M.; Kokate, S. Push-out bond strength of root fillings made with c-point and BC sealer versus gutta-percha and AH plus after the instrumentation of oval canals with the self-adjusting file versus WaveOne. Int. Endod. J. 2015, 49, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Sritharan, A. Discuss that the coronal seal is more important than the apical seal for endodontic success. Aust. Endod. J. 2002, 28, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Ørstavik, D.A.G. Materials used for root canal obturation: Technical, biological and clinical testing. Endod. Top. 2005, 12, 25–38. [Google Scholar] [CrossRef]
- Estrela, C.; Estrela, C.R.A.; Barbin, E.L.; Spanó, J.C.; Marchesan, M.A.; Pécora, J.D. Mechanism of action of sodium hypochlorite. Braz. Dent. J. 2002, 13, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Daumer, K.M.; Khan, A.U.; Steinbeck, M.J. Chlorination of pyridinium compounds: Possible role of hypochlorite, N-chloramines, and chlorine in the oxidation of pyridinoline cross-links of articular cartilage collagen type II during acute inflammation. J. Biol. Chem. 2000, 275, 34681–34692. [Google Scholar] [CrossRef] [PubMed]
- Abuhaimed, T.S.; Abou Neel, E.A. Sodium hypochlorite irrigation and its effect on bond strength to dentin. BioMed Res. Int. 2017, 2017, 1930360. [Google Scholar] [CrossRef]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A plant of healing: Review. J. Pharm. Pharmacol. 2017, 69, 925–945. [Google Scholar] [CrossRef]
- Tuncel, B.; Nagas, E.; Cehreli, Z.; Uyanik, O.; Vallittu, P.; Lassila, L. Effect of endodontic chelating solutions on the bond strength of endodontic sealers. Braz. Oral Res. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Bhandi, S.; Alkahtani, A.; Reda, R.; Mashyakhy, M.; Boreak, N.; Maganur, P.C.; Vishwanathaiah, S.; Mehta, D.; Vyas, N.; Patil, V.; et al. Parathyroid Hormone Secretion and Receptor Expression Determine the Age-Related Degree of Osteogenic Differentiation in Dental Pulp Stem Cells. J. Pers. Med. 2021, 11, 349. [Google Scholar] [CrossRef]
- Dinesh, K.; Murthy, B.V.S.; Narayana, I.H.; Hegde, S.; Madhu, K.S.; Nagaraja, S. The effect of 2% chlorhexidine on the bond strength of two different obturating materials. J. Contemp. Dent. Pract. 2014, 15, 82–85. [Google Scholar] [CrossRef]
- Ordinola-Zapata, R.; Bramante, C.M.; Graeff, M.S.; del Carpio Perochena, A.; Vivan, R.R.; Camargo, E.J.; Garcia, R.B.; Bernardineli, N.; Gutmann, J.L.; de Moraes, I.G. Depth and percentage of penetration of endodontic sealers into dentinal tubules after root canal obturation using a lateral compaction technique: A confocal laser scanning microscopy study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Andriukaitiene, L.; Song, X.; Yang, N.; Lassila, L.V.; Vallittu, P.K.; Kerosuo, E. The effect of smear layer removal on E. faecalis leakage and bond strength of four resin-based root canal sealers. BMC Oral Health 2018, 18, 213. [Google Scholar] [CrossRef] [PubMed]
- Zanza, A.; Seracchiani, M.; Reda, R.; Miccoli, G.; Testarelli, L.; Di Nardo, D. Metallurgical Tests in Endodontics: A Narrative Review. Bioengineering 2022, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Almadi, K.H. Impact of antimicrobial photodynamic therapy on the bond-strength and penetration of endodontic sealers: A systematic review. Photodiagn. Photodyn. Ther. 2022, 41, 103249. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawi, A.-K.J. The effect of waterlase laser and herbal alternative, green tea and Salvadora Persica (Siwak) extract on push-out bond strength. J. Baghdad Coll. Dent. 2014, 26, 1–6. [Google Scholar] [CrossRef]
- Shweta, C.; Kimaya, K.K.; Chetana, J.; Alok, R.P.; Preetam, P.S.; Amol, H.K. Comparison of the effect of different irrigating solutions on bond strength of obturating materials: An in vitro study. Int. J. Sci. Study 2021, 9, 107–113. [Google Scholar]
| Section | Coronal | Middle | Apical |
|---|---|---|---|
| Group 1 | 1.7630 ± 0.44502 | 5.2480 ± 0.65287 | 4.9960 ± 0.67145 |
| Group 2 | 1.8730 ± 0.49376 | 4.4630 ± 0.85853 | 4.6010 ± 1.45383 |
| Group 3 | 1.7850 ± 0.39697 | 4.3110 ± 0.91307 | 4.2390 ± 0.82174 |
| p value (ANOVA) | 0.845 | 0.035 * | 0.282 |
| Post Hoc Test (Paired Wise Comparison) | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Coronal vs. Middle | 0.847 | 0.098 | 0.676 |
| Coronal vs. Apical | 0.993 | 0.041 * | 0.251 |
| Middle vs. Apical | 0.899 | 0.909 | 0.719 |
| Section | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Coronal | Adhesive 65 | Adhesive 00 | Adhesive 00 |
| Cohesive 10 | Cohesive 40 | Cohesive 35 | |
| Mixed 20 | Mixed 60 | Mixed 55 | |
| Middle | Adhesive 55 | Adhesive 20 | Adhesive 10 |
| Cohesive 25 | Cohesive 25 | Cohesive 25 | |
| Mixed 20 | Mixed 70 | Mixed 60 | |
| Apical | Adhesive 75 | Adhesive 20 | Adhesive 20 |
| Cohesive 15 | Cohesive 35 | Cohesive 55 | |
| Mixed 20 | Mixed 55 | Mixed 55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanvilkar, U.; Patil, H.; Bandekar, S.; Kshirsagar, S.; Pawar, A.M.; Wahjuningrum, D.A.; Pagnoni, F.; Reda, R.; Zanza, A.; Testarelli, L. Pushout Bond Strength of Root Fillings after Irrigation of Root Canals Utilizing Sodium Hypochlorite, Chlorhexidine, and Homeopathic Mother Tincture (Arnica Montana). Clin. Pract. 2023, 13, 305-314. https://doi.org/10.3390/clinpract13010028
Khanvilkar U, Patil H, Bandekar S, Kshirsagar S, Pawar AM, Wahjuningrum DA, Pagnoni F, Reda R, Zanza A, Testarelli L. Pushout Bond Strength of Root Fillings after Irrigation of Root Canals Utilizing Sodium Hypochlorite, Chlorhexidine, and Homeopathic Mother Tincture (Arnica Montana). Clinics and Practice. 2023; 13(1):305-314. https://doi.org/10.3390/clinpract13010028
Chicago/Turabian StyleKhanvilkar, Unmesh, Hitesh Patil, Siddhesh Bandekar, Shirin Kshirsagar, Ajinkya M. Pawar, Dian Agustin Wahjuningrum, Francesco Pagnoni, Rodolfo Reda, Alessio Zanza, and Luca Testarelli. 2023. "Pushout Bond Strength of Root Fillings after Irrigation of Root Canals Utilizing Sodium Hypochlorite, Chlorhexidine, and Homeopathic Mother Tincture (Arnica Montana)" Clinics and Practice 13, no. 1: 305-314. https://doi.org/10.3390/clinpract13010028
APA StyleKhanvilkar, U., Patil, H., Bandekar, S., Kshirsagar, S., Pawar, A. M., Wahjuningrum, D. A., Pagnoni, F., Reda, R., Zanza, A., & Testarelli, L. (2023). Pushout Bond Strength of Root Fillings after Irrigation of Root Canals Utilizing Sodium Hypochlorite, Chlorhexidine, and Homeopathic Mother Tincture (Arnica Montana). Clinics and Practice, 13(1), 305-314. https://doi.org/10.3390/clinpract13010028










