Notalgia Paresthetica Review: Update on Presentation, Pathophysiology, and Treatment
Abstract
:1. Introduction
2. Epidemiology/Risk Factors
3. Pathophysiology
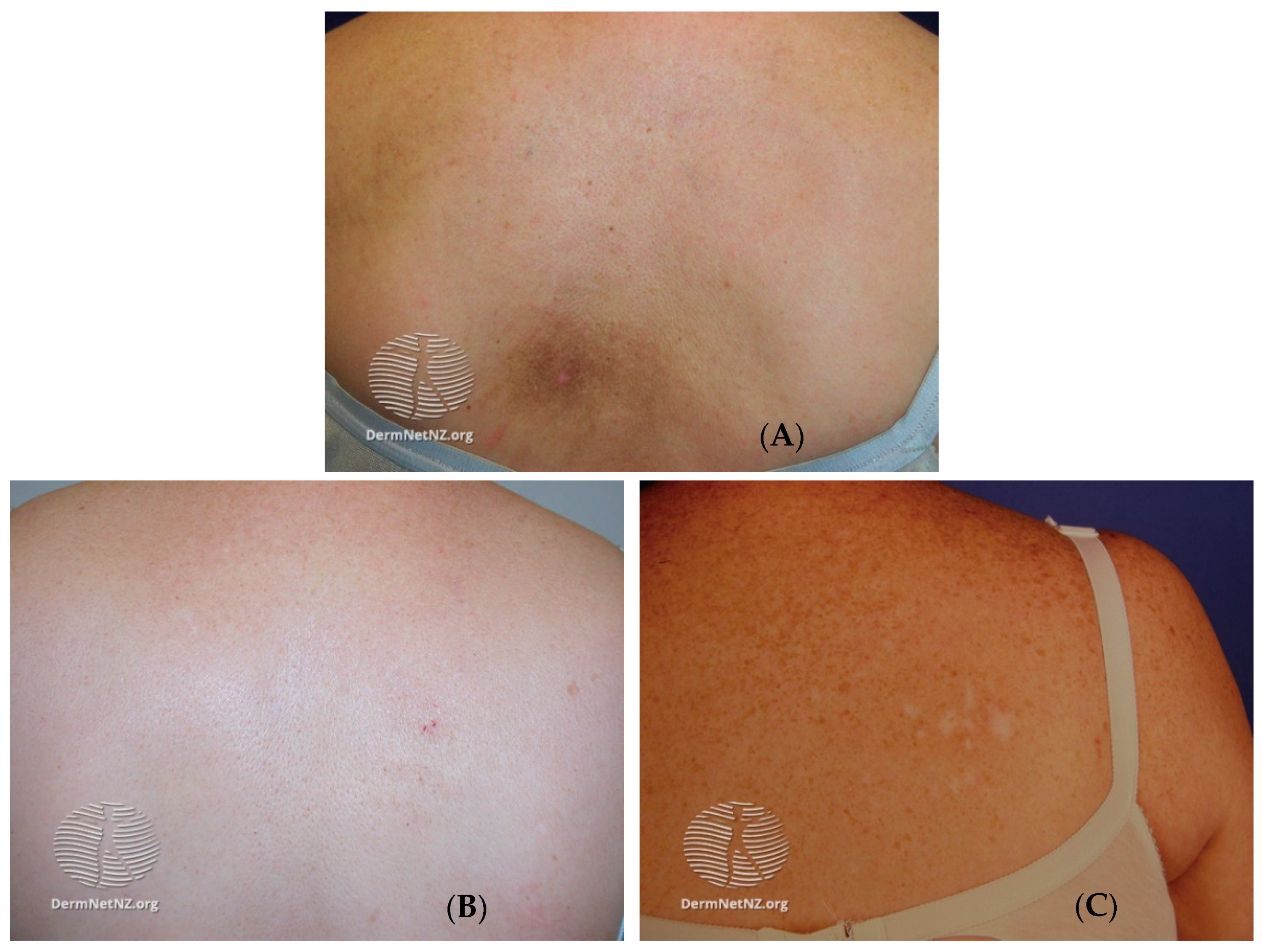
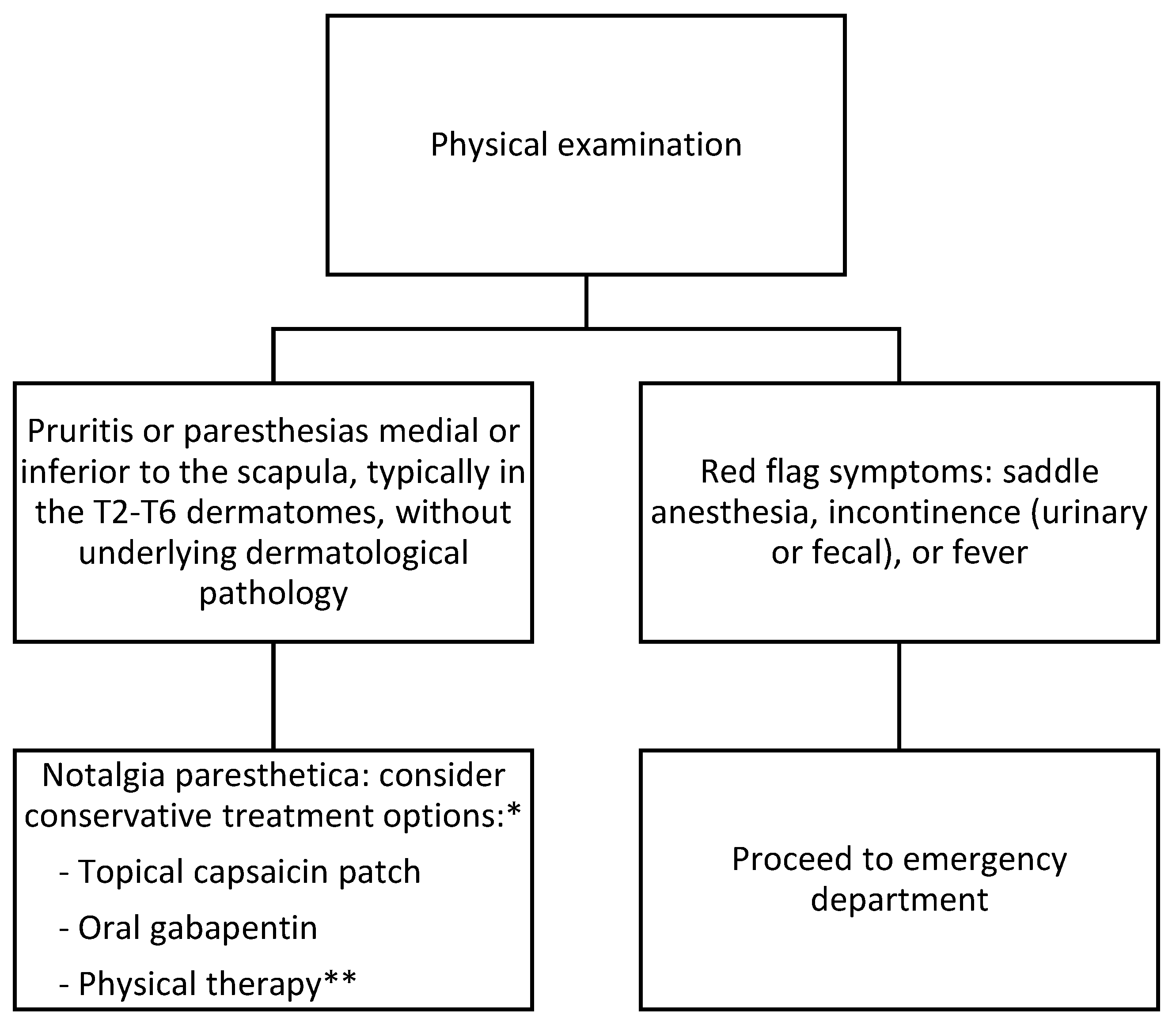
4. Presentation and Diagnosis
5. Differential Diagnosis
6. Treatment
7. Prognosis and Complications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Şenel, E.; Holt, S.; Sabancılar, E.; Sabancılar, Z.; Doğruer Şenel, S. The etiology of notalgia paresthetica: A descriptive study of 117 patients. Ir. J. Med. Sci. 2020, 189, 1311–1316. [Google Scholar] [CrossRef]
- Mülkoğlu, C.; Nacır, B. Notalgia paresthetica: Clinical features, radiological evaluation, and a novel therapeutic option. BMC Neurol. 2020, 20, 191. [Google Scholar] [CrossRef]
- Ellis, C. Notalgia paresthetica: The unreachable itch. Dermatol. Pract. Concept. 2013, 3, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Raison-Peyron, N.; Meunier, L.; Acevedo, M.; Meynadier, J. Notalgia paresthetica: Clinical, physiopathological and therapeutic aspects. A study of 12 cases. J. Eur. Acad. Dermatol. Venereol. 1999, 12, 215–221. [Google Scholar] [CrossRef]
- Andersen, H.H.; Sand, C.; Elberling, J. Considerable Variability in the Efficacy of 8% Capsaicin Topical Patches in the Treatment of Chronic Pruritus in 3 Patients with Notalgia Paresthetica. Ann. Dermatol. 2016, 28, 86–89. [Google Scholar] [CrossRef] [Green Version]
- Savk, O.; Savk, E. Investigation of spinal pathology in notalgia paresthetica. J. Am. Acad. Dermatol. 2005, 52, 1085–1087. [Google Scholar] [CrossRef]
- Şavk, E.; Savk, O.; Bolukbasi, O.; Culhaci, N.; Dikicioğlu, E.; Karaman, G.; Sendur, N. Notalgia paresthetica: A study on pathogenesis. Int. J. Dermatol. 2000, 39, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Pagliarello, C.; Fabrizi, G.; De Felici, B.; Casanova, D.; Feliciani, C.; Di Nuzzo, S. Notalgia paresthetica: Factors associated with its perceived severity, duration, side, and localization. Int. J. Dermatol. 2017, 56, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Weinstein, D.; Sami, N. Notalgia paresthetica: Treatment review and algorithmic approach. J. Dermatol. Treat. 2020, 31, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Bacci, E.D.; Currie, B.; Wilson, R.; Qian, J.; Munera, C.; Nograles, K. Understanding the patient experience of living with notalgia paresthetica: A qualitative interview study. JAAD Int. 2022, 8, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R. Notalgia Paresthetica: A Novel Approach to Treatment with Cryolipolysis. Cureus 2017, 9, e1719. [Google Scholar] [CrossRef] [Green Version]
- Howard, M.; Sahhar, L.; Andrews, F.; Bergman, R.; Gin, D. Notalgia paresthetica: A review for dermatologists. Int. J. Dermatol. 2018, 57, 388–392. [Google Scholar] [CrossRef]
- Šitum, M. Notalgia Paresthetica. Acta Clin. Croat. 2018, 57, 721–725. [Google Scholar] [CrossRef] [Green Version]
- Maciel AA, W.; Cunha, P.R.; Laraia, I.O.; Trevisan, F. Efficacy of gabapentin in the improvement of pruritus and quality of life of patients with notalgia paresthetica. An. Bras. Dermatol. 2014, 89, 570–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huesmann, T.; Cunha, P.; Osada, N.; Huesmann, M.; Zanelato, T.; Phan, N.Q.; Gontijo, G.A.; Marziniak, M.; Ständer, S. Notalgia Paraesthetica: A Descriptive Two-cohort Study of 65 Patients from Brazil and Germany. Acta Dermato Venereol. 2012, 92, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Massey, E.W.; Pleet, A.B. Electromyographic evaluation of notalgia paresthetica. Neurology 1981, 31, 642. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.; Meade, T.; Fleischer, A. Notalgia Paresthetica: Successful Treatment with Exercises. Acta Dermato Venereol. 2011, 91, 356–357. [Google Scholar] [CrossRef]
- da Cruz, C.M.; Antunes, F. Physical Medicine and Rehabilitation Role on Notalgia Paresthetica. Am. J. Phys. Med. Rehabil. 2018, 97, 929–932. [Google Scholar] [CrossRef]
- Ishizuka, K.; Sakai, H.; Tsuzuki, N.; Nagashima, M. Topographic anatomy of the posterior ramus of thoracic spinal nerve and surrounding structures. Spine 2012, 37, E817–E822. [Google Scholar] [CrossRef] [PubMed]
- Springall, D.R.; Karanth, S.S.; Kirkham, N.; Darley, C.R.; Polak, J.M. Symptoms of Notalgia Paresthetica May Be Explained by Increased Dermal Innervation. J. Investig. Dermatol. 1991, 97, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Şavk, E.; Dikicioğlu, E.; Çulhaci, N.; Karaman, G.; Şendur, N. Immunohistochemical Findings in Notalgia paresthetica. Dermatology 2002, 204, 88–93. [Google Scholar] [CrossRef]
- Alcántara, F.; Feito, M.; Albizuri, F.; Beato, M.; de Lucas, R. Notalgia Paresthetica and Multiple Endocrine Neoplasia Syndrome 2A: A Case Report. Pediatr. Dermatol. 2016, 33, e303–e305. [Google Scholar] [CrossRef] [PubMed]
- Papadatou-Pastou, M.; Ntolka, E.; Schmitz, J.; Martin, M.; Munafò, M.; Ocklenburg, S.; Paracchini, S. Human handedness: A meta-analysis. Psychol. Bull. 2020, 146, 481. [Google Scholar] [CrossRef]
- Weber, P.J.; Poulos, E.G. Notalgia paresthetica: Case reports and histologic appraisal. J. Am. Acad. Dermatol. 1988, 18, 25–30. [Google Scholar] [CrossRef]
- Riehl Melanosis—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557437/ (accessed on 17 February 2023).
- Chairatchaneeboon, M.; Thanomkitti, K.; Kim, E.J. Parapsoriasis—A Diagnosis with an Identity Crisis: A Narrative Review. Dermatol. Ther. 2022, 12, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Juarez, M.C.; Kwatra, S.G. A systematic review of evidence based treatments for lichen simplex chronicus. J. Dermatol. Treat. 2021, 32, 684–692. [Google Scholar] [CrossRef]
- Lichen Simplex Chronicus—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499991/ (accessed on 17 February 2023).
- Bandhlish, A.; Aggarwal, A.; Koranne, R. A Clinico-Epidemiological Study of Macular Amyloidosis from North India. Indian J. Dermatol. 2012, 57, 269. [Google Scholar] [CrossRef]
- Kibbi, A.-G.; Rubeiz, N.G.; Zaynoun, S.T.; Kurban, A.K. Primary localized cutaneous amyloidosis. Int. J. Dermatol. 1992, 31, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Chapter 133. Amyloidosis of the Skin|Fitzpatrick’s Dermatology in General Medicine, 8e|AccessMedicine|McGraw Hill Medical. Available online: https://accessmedicine.mhmedical.com/Content.aspx?bookid=392§ionid=41138854 (accessed on 17 February 2023).
- Sari Aslani, F.; Kargar, H.; Safaei, A.; Jowkar, F.; Hosseini, M.; Sepaskhah, M. Comparison of Immunostaining with Hematoxylin-Eosin and Special Stains in the Diagnosis of Cutaneous Macular Amyloidosis. Cureus 2020, 12, e7606. [Google Scholar] [CrossRef] [Green Version]
- Melo, B.L.A.; Costa, I.S.; de Assis Martins Goes, C.; Tigre, C.A.F.; André, N.F. An unusual presentation of macular amyloidosis. An. Bras. Dermatol. 2011, 86, 24–27. [Google Scholar] [CrossRef]
- Ponka, D.; Baddar, F. Wood lamp examination. Can. Fam. Physician 2012, 58, 976. [Google Scholar]
- Tinea Versicolor—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482500/ (accessed on 17 February 2023).
- Elmas, Ö.F.; Kızılyel, O.; Metin, M.S.; Atasoy, M.; Urlu, S.M.; Tuncer, K. An overlooked clinical entity: Notalgia paresthetica. Agri 2015, 27, 224–225. [Google Scholar] [PubMed]
- Chtompel, Y.; Eghtesadi, M.; Vargas-Schaffer, G. A Case Report of Refractory Notalgia Paresthetica Treated with Lidocaine Infusions. Am. J. Case Rep. 2017, 18, 1225–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibsohn, E. Treatment of notalgia paresthetica with capsaicin. Cutis 1992, 49, 335–336. [Google Scholar]
- Goulden, V.; Toomey, P.J.; Highet, A.S. Successful treatment of notalgia paresthetica with a paravertebral local anesthetic block. J. Am. Acad. Dermatol. 1998, 38, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Şavk, E.; Bolukbasi, O.; Akyol, A.; Karaman, G. Open pilot study on oxcarbazepine for the treatment of notalgia paresthetica. J. Am. Acad. Dermatol. 2001, 45, 630–632. [Google Scholar] [CrossRef]
- Ochi, H.; Tan, L.X.; Tey, H.L. Notalgia paresthetica: Treatment with topical tacrolimus. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 452–454. [Google Scholar] [CrossRef]
- Yeo, B.; Tey, H.L. Effective treatment of notalgia paresthetica with amitriptyline. J. Dermatol. 2013, 40, 505–506. [Google Scholar] [CrossRef]
- Poterucha, T.J.; Murphy, S.; Sandroni, P.; Rho, R.; Warndahl, R.; Weiss, W.; Davis, M. Topical amitriptyline combined with topical ketamine for the management of recalcitrant localized pruritus: A retrospective pilot study. J. Am. Acad. Dermatol. 2013, 69, 320–321. [Google Scholar] [CrossRef]
- Weinfeld, P.K. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch Dermatol. 2007, 143, 980–982. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pérez, L.; García-Gavín, J.; Allegue, F.; Caeiro, J.L.; Fabeiro, J.M.; Zulaica, A. Notalgia paresthetica: Treatment using intradermal botulinum toxin A. Actas Dermosifiliogr. 2014, 105, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Maari, C.; Marchessault, P.; Bissonnette, R. Treatment of notalgia paresthetica with botulinum toxin A: A double-blind randomized controlled trial. J. Am. Acad. Dermatol. 2014, 70, 1139–1141. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, L.; Allegue, F.; Fabeiro, J.M.; Caeiro, J.L.; Zulaica, A. Notalgia paresthesica successfully treated with narrow-band UVB: Report of five cases. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 730–732. [Google Scholar] [CrossRef]
- Şavk, E.; Şavk, Ö.; Şendur, F. Transcutaneous electrical nerve stimulation offers partial relief in notalgia paresthetica patients with a relevant spinal pathology. J. Dermatol. 2007, 34, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.H.; Rosson, G.D.; Elsamanoudi, I.; Lee Dellon, A. Surgical decompression for notalgia paresthetica: A case report. Microsurgery 2010, 30, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.M.; Cotterill, J.A. Notalgia paraesthetica--report of three cases and their treatment. Clin. Exp. Dermatol. 1991, 16, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Robbins, B.A.; Rayi, A.; Ferrer-Bruker, S.J. Notalgia Paresthetica. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]

| Author and Year | Groups Studied and Intervention | Results and Findings | Conclusions |
|---|---|---|---|
| Chtompel et al. 2017 [37] | One patient underwent IV lidocaine infusions. | Itch reduction in patient immediately following infusions with symptoms returning to baseline within one month. | Treatment was effective over the short term; however, larger studies and evaluation of long-term efficacy and safety are required. |
| Mülkoǧlu et al. 2020 [2] | Forty-five patients were treated with local intradermal lidocaine injections. | Significant reduction in pain and pruritis in patients lasting one to three months post injection. | Effective in the short term, but long-term efficacy and safety remain undetermined. |
| Andersen et al. 2016 [5] | Three patients were topically treated with 8% capsaicin patches. | Patients reported immediate symptom relief with duration varying from a few days to three months. | Clinical efficacy undetermined due to wide range of results. Further studies with a larger patient pool are necessary. |
| Leibsohn et al. 1992 [38] | Twenty-four patients were treated with 0.025% topical capsaicin patches for four months. | Seventy percent of patients achieved some degree of symptom relief but returned to baseline after cessation of therapy. | One of the first treatments for NP and relatively effective in some patients. Long-term effects beyond four months are unknown. |
| Maciel et al. 2014 [14] | Twenty patients divided into two groups were compared-one group treated with 0.025% topical capsaicin, and the second treated with oral 300 mg gabapentin. | Only gabapentin treatment group reported significant symptom reduction. | Gabapentin was well tolerated and effective. |
| Goulden et al.1998 [39] | One patient was treated with bilateral paravertebral block using bupivacaine (0.75%) and 40 mg methylprednisone. | Patient had completer resolution of symptoms for 12 months. | Effective treatment of NP, though limited by sample size and expertise required to perform blocks. |
| Savk et al. 2001 [40] | Four patients were treated with oral 300–900 mg oxcarbazepine twice a day. | Three of four patients reported improvement at one and six months of therapy. | Oxcarbazepine was well tolerated, with only one patient experiencing headache and dizziness. |
| Ochi et al. 2016 [41] | Seven patients were treated with 0.1% topical tacrolimus for six weeks. | Patients experienced decrease in mean itch score (0 to 10) from 6.6 +/− 1.9 to 4.6 +/− 2.1 (p < 0.02). | Topical treatment reduced pruritis intensity and/or frequency with return of symptoms after cessation. |
| Yeo et al. 2013 [42] | One patient was treated with oral 10 mg amitriptyline daily for three months. | Reduction of pruritis scores from 7/10 to 4/10 with sustained relief after discontinuation for one month. | Oral amitriptyline was effective in treating one patient without side effects. |
| Poterucha et al. 2013 [43] | Two patients were treated with amitriptyline/ketamine topical cream. | One of two patients experienced partial relief of pruritis with no relief in the second. | Topical amitriptyline/ketamine cream provided partial relief of pruritis. |
| Weinfeld et al. 2007 [44] | Two patients were treated with botulinum toxin A. First patient received 16 units, and second patient received 24 units, followed by a second dose of 48 units. | One patient had complete resolution after one injection for 18 months, and second patient required two injections to achieve complete resolution. | Botulinum toxin A injection produced resolution of NP, but dosing regimen is variable. |
| Perez-Perez et al. 2014 [45] | Five patients were treated with 48–56 units of botulinum toxin A. | Three patients had partial improvement of symptoms, and two had worsening of symptoms. | Variable and partial improvement with injection of botulinum toxin A. |
| Maari et al. 2014 [46] | Double-blinded randomized controlled trial consisting of 20 patients receiving either a mean dose of 142 units of botulinum toxin A or saline. | No significant difference was observed between the botulinum toxin A and saline group. | Botulinum toxin A was ineffective in treating NP. |
| Perez-Perez et al. 2010 [47] | Five patients received narrow-band UVB radiation with an average of 32.8 sessions with a mean cumulative dose of 33.75 J/cm2. | All five patients had reductions in symptoms, and two patients had complete resolution. | Narrow-band UVB radiation resulted in reduction of symptoms. |
| Fleischer et al. 2011 [17] | Two patients were treated with physical therapy. | Both patients achieved significant improvement in symptoms after strengthening and stretching exercises targeting scapular and pectoral muscles. | Physical therapy is an effective treatment in patients affected by atrophied paraspinal muscles or who report a shoulder with a reduced range of motion. |
| Savk et al. 2007 [48] | Fifteen patients were treated with TENS over 10 sessions. | Transient pruritis relief was achieved in some patients, but symptoms returned to baseline upon the cessation of treatment. | May be effective in some symptom relief and should be considered as part of a multi-modal therapy approach in treating NP. |
| Williams et al. 2010 [49] | One patient underwent surgical decompression. | Symptom relief was achieved in this patient postoperatively. | Surgical decompression successfully resulted in symptom relief. Further studies are required before implementation as a treatment for NP due to its invasiveness. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, C.; Downs, E.; De la Caridad Gomez, Y.; Nduaguba, C.; Woolley, P.; Varrassi, G.; Gill, J.; Simopoulos, T.T.; Viswanath, O.; Yazdi, C.A. Notalgia Paresthetica Review: Update on Presentation, Pathophysiology, and Treatment. Clin. Pract. 2023, 13, 315-325. https://doi.org/10.3390/clinpract13010029
Robinson C, Downs E, De la Caridad Gomez Y, Nduaguba C, Woolley P, Varrassi G, Gill J, Simopoulos TT, Viswanath O, Yazdi CA. Notalgia Paresthetica Review: Update on Presentation, Pathophysiology, and Treatment. Clinics and Practice. 2023; 13(1):315-325. https://doi.org/10.3390/clinpract13010029
Chicago/Turabian StyleRobinson, Christopher, Evan Downs, Yanet De la Caridad Gomez, Chinonso Nduaguba, Parker Woolley, Giustino Varrassi, Jatinder Gill, Thomas T. Simopoulos, Omar Viswanath, and Cyrus A. Yazdi. 2023. "Notalgia Paresthetica Review: Update on Presentation, Pathophysiology, and Treatment" Clinics and Practice 13, no. 1: 315-325. https://doi.org/10.3390/clinpract13010029
APA StyleRobinson, C., Downs, E., De la Caridad Gomez, Y., Nduaguba, C., Woolley, P., Varrassi, G., Gill, J., Simopoulos, T. T., Viswanath, O., & Yazdi, C. A. (2023). Notalgia Paresthetica Review: Update on Presentation, Pathophysiology, and Treatment. Clinics and Practice, 13(1), 315-325. https://doi.org/10.3390/clinpract13010029







