Carcinogenic Effects of Areca Nut and Its Metabolites: A Review of the Experimental Evidence
Abstract
:1. Introduction
2. Different Mechanisms of Carcinogenicity
2.1. Areca Nut Extract
2.2. Areca Alkaloids
2.3. Effect of Areca Nut Extracts on Molecular Carcinogenesis
2.4. Areca Nuts-Related ROS Production and Inflammation
2.5. AN-Induced Cell Motility and Epithelial-Mesenchymal Transition (EMT)
2.6. Areca Nut Stimulates Autophagy and Restrains Tumor Suppressors
2.7. Areca Nut Consumption Evokes Genotoxicity, Cytotoxicity, Cell Cycle Arresting, and Apoptosis
2.8. Areca Nut Promotes Malignant Transformation by Inducing Tissue Hypoxia
2.9. Areca Nut Metabolites on the Oral Microbiome
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reichart, P.A.; Warnakulasuriya, S. Oral lichenoid contact lesions induced by areca nut and betel quid chewing: A mini review. J. Investig. Clin. Dent. 2012, 3, 163–166. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr. Eval. Carcinog. Risks Hum. 2004, 85, 1. [Google Scholar]
- Gupta, P.C.; Warnakulasuriya, S. Global Epidemiology of Areca Nut Usage. Addict. Biol. 2002, 7, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Reichart, P.A. A review of Betel quid chewing, oral cancer and precancer in Mainland China. Oral Oncol. 2007, 43, 424–430. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chang, J.T.-C.; Liao, C.-T.; Wang, H.-M.; Yen, T.-C.; Chiu, C.-C.; Lu, Y.-C.; Li, H.-F.; Cheng, A.-J. Head and neck cancer in the betel quid chewing area: Recent advances in molecular carcinogenesis. Cancer Sci. 2008, 99, 1507–1514. [Google Scholar] [CrossRef]
- Chen, P.-H.; Mahmood, Q.; Mariottini, G.L.; Chiang, T.-A.; Lee, K.-W. Adverse health effects of betel quid and the risk of oral and pharyngeal cancers. BioMed. Res. Int. 2017, 2017, 3904098. [Google Scholar] [CrossRef] [Green Version]
- Sharan, R.N.; Mehrotra, R.; Choudhury, Y.; Asotra, K. Association of Betel Nut with carcinogenesis: Revisit with a clinical perspective. PLoS ONE 2012, 7, e42759. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.; Johnson, N.W. Systematic Review and meta-analysis of association of Smokeless Tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PLoS ONE 2014, 9, e113385. [Google Scholar] [CrossRef]
- Liao, C.-T.; Wallace, C.G.; Lee, L.-Y.; Hsueh, C.; Lin, C.-Y.; Fan, K.-H.; Wang, H.-M.; Ng, S.-H.; Lin, C.-H.; Tsao, C.-K.; et al. Clinical evidence of field cancerization in patients with oral cavity cancer in a betel quid chewing area. Oral Oncol. 2014, 50, 721–731. [Google Scholar] [CrossRef]
- Garg, A.; Chaturvedi, P.; Gupta, P.C. A review of the systemic adverse effects of areca nut or betel nut. Indian J. Med. Paediatr. Oncol. 2014, 35, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Chen, L.Y.; Wang, H.L.; Chen, B.H. Quantification of salivary arecoline, arecaidine and n-methylnipecotic acid levels in volunteers by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2015, 39, 714–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, S.; Vickers, E.R.; Ghu, S.; Zoellner, H. Salivary arecoline levels during areca nut chewing in human volunteers. J. Oral Pathol. Med. 2010, 39, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Kamath, V.V.; Maria, S.; Satelur, K.; Rajkumar, K. Evaluation of transforming growth factor beta1 gene in oral submucous fibrosis induced in Sprague-Dawley rats by injections of areca nut and Pan Masala (commercial areca nut product) extracts. J. Cancer Res. Ther. 2016, 12, 379. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-H.; Chen, P.-H.; Chen, Y.-K.; Chen, C.-H.; Ho, M.-L.; Wang, Y.-H. Characterization of a novel dermal fibrosis model induced by areca nut extract that mimics oral submucous fibrosis. PLoS ONE 2016, 11, e0166454. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.-H.; Wu, C.-C.; Lee, L.-Y.; Li, Y.-C.; Liu, H.-P.; Hsu, C.-W.; Lu, Y.-C.; Chang, J.T.; Cheng, A.-J. Proteomics analysis reveals involvement of KRT17 in areca nut-induced oral carcinogenesis. J. Proteome Res. 2016, 15, 2981–2997. [Google Scholar] [CrossRef]
- Kuo, T.-M.; Luo, S.-Y.; Chiang, S.-L.; Yeh, K.-T.; Hsu, H.-T.; Wu, C.-T.; Lu, C.-Y.; Tsai, M.-H.; Chang, J.-G.; Ko, Y.-C. Fibrotic effects of arecoline n-oxide in oral potentially malignant disorders. J. Agric. Food Chem. 2015, 63, 5787–5794. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hu, C.-C.; Lii, C.-K.; Tai, K.-W.; Yang, S.-H.; Chou, M.-Y. Cytotoxicity and arecoline mechanisms in human gingival fibroblasts in vitro. Clin. Oral Investig. 2001, 5, 51–56. [Google Scholar] [CrossRef]
- Jeng, J.-H.; Hahn, L.-J.; Lin, B.-R.; Hsieh, C.-C.; Chan, C.-P.; Chang, M.-C. Effects of areca nut, inflorescence piper betle extracts and arecoline on cytotoxicity, total and unscheduled DNA synthesis in cultured gingival keratinocytes. J. Oral Pathol. Med. 2007, 28, 64–71. [Google Scholar] [CrossRef]
- Tsai, Y.-S.; Lee, K.-W.; Huang, J.-L.; Liu, Y.-S.; Juo, S.-H.H.; Kuo, W.-R.; Chang, J.-G.; Lin, C.-S.; Jong, Y.-J. Arecoline, a major alkaloid of areca nut, inhibits p53, represses DNA repair, and triggers DNA damage response in human epithelial cells. Toxicology 2008, 249, 230–237. [Google Scholar] [CrossRef]
- Wary, K.K.; Sharan, R.N. Aqueous extract of betel-nut of north-east india induces DNA-strand breaks and enhances rate of cell proliferation in vitro. J. Cancer Res. Clin. Oncol. 1988, 114, 579–582. [Google Scholar] [CrossRef]
- Sharan, R.N.; Wary, K.K. Study of unscheduled DNA synthesis following exposure of human cells to arecoline and extracts of betel nut in vitro. Mutat. Res. Genet. Toxicol. 1992, 278, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-Y.; Chang, K.-W.; Liu, C.-J.; Tseng, Y.-H.; Lu, H.-H.; Lee, S.-Y.; Lin, S.-C. Ripe areca nut extract induces G 1 phase arrests and senescence-associated phenotypes in normal human oral keratinocyte. Carcinogenesis 2006, 27, 1273–1284. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-C.; Chang, M.-C.; Chang, H.-H.; Wang, T.-M.; Tseng, W.-Y.; Tai, T.-F.; Yeh, H.-W.; Yang, T.-T.; Hahn, L.-J.; Jeng, J.-H. Areca nut-induced micronuclei and cytokinesis failure in Chinese hamster ovary cells is related to reactive oxygen species production and actin filament deregulation. Environ. Mol. Mutagen. 2009, 50, 367–374. [Google Scholar] [CrossRef]
- Chiang, S.-L.; Jiang, S.-S.; Wang, Y.-J.; Chiang, H.-C.; Chen, P.-H.; Tu, H.-P.; Ho, K.-Y.; Tsai, Y.-S.; Chang, I.-S.; Ko, Y.-C. Characterization of arecoline-induced effects on cytotoxicity in normal human gingival fibroblasts by global gene expression profiling. Toxicol. Sci. 2007, 100, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.-C.; Lee, T.-C. Genetic damage in cultured human keratinocytes stressed by long-term exposure to areca nut extracts. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2006, 599, 66–75. [Google Scholar] [CrossRef]
- Illeperuma, R.P.; Kim, D.K.; Park, Y.J.; Son, H.K.; Kim, J.Y.; Kim, J.; Lee, D.Y.; Kim, K.Y.; Jung, D.W.; Tilakaratne, W.M.; et al. Areca nut exposure increases secretion of tumor-promoting cytokines in gingival fibroblasts that trigger dna damage in oral keratinocytes. Int. J. Cancer 2015, 137, 2545–2557. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-C.; Wu, H.-L.; Lee, J.-J.; Lee, P.-H.; Chang, H.-H.; Hahn, L.-J.; Lin, B.-R.; Chen, Y.-J.; Jeng, J.-H. The induction of prostaglandin E2 production, interleukin-6 production, cell cycle arrest, and cytotoxicity in primary oral keratinocytes and KB cancer cells by areca nut ingredients is differentially regulated by MEK/Erk Activation. J. Biol. Chem. 2004, 279, 50676–50683. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-C.; Chen, Y.-J.; Chang, H.-H.; Chan, C.-P.; Yeh, C.-Y.; Wang, Y.-L.; Cheng, R.-H.; Hahn, L.-J.; Jeng, J.-H. Areca nut components affect COX-2, cyclin B1/cdc25c and keratin expression, PGE2 production in keratinocyte is related to reactive oxygen species, CYP1A1, SRC, EGFR and Ras Signaling. PLoS ONE 2014, 9, e101959. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Yang, L.-C.; Hu, F.-W.; Peng, C.-Y.; Yu, C.-H.; Yu, C.-C. Elevation of Twist expression by arecoline contributes to the pathogenesis of oral submucous fibrosis. J. Formos. Med. Assoc. 2016, 115, 311–317. [Google Scholar] [CrossRef]
- Pant, I.; Kumar, N.; Khan, I.; Rao, S.G.; Kondaiah, P. Role of areca nut induced TGF-β and epithelial-mesenchymal interaction in the pathogenesis of oral submucous fibrosis. PLoS ONE 2015, 10, e0129252. [Google Scholar] [CrossRef]
- Panigrahi, G.B.; Rao, A.R. Induction of in vivo sister chromatid exchanges by arecaidine, a betel nut alkaloid, in mouse bone-marrow cells. Cancer Lett. 1984, 23, 189–192. [Google Scholar] [CrossRef]
- Wu, M.; Xing, G.; Qi, X.; Feng, C.; Liu, M.; Gong, L.; Luan, Y.; Ren, J. Assessment of the mutagenic potential of arecoline in GPT Delta transgenic mice. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 748, 65–69. [Google Scholar] [CrossRef]
- Chou, W.-W.; Guh, J.-Y.; Tsai, J.-F.; Hwang, C.-C.; Chen, H.-C.; Huang, J.-S.; Yang, Y.-L.; Hung, W.-C.; Chuang, L.-Y. Arecoline-induced growth arrest and P21WAF1 expression are dependent on p53 in rat hepatocytes. Toxicology 2008, 243, 1–10. [Google Scholar] [CrossRef]
- Dave, B.J.; Trivedi, A.H.; Adhvaryu, S.G. In vitro genotoxic effects of areca nut extract and Arecoline. J. Cancer Res. Clin. Oncol. 1992, 118, 283–288. [Google Scholar] [CrossRef]
- Huang, J.L.; McLeish, M.J. High-performance liquid chromatographic determination of the alkaloids in betel nut. J. Chromatogr. A 1989, 475, 447–450. [Google Scholar] [CrossRef]
- Tilakaratne, W.M.; Ekanayaka, R.P.; Warnakulasuriya, S. Oral submucous fibrosis: A historical perspective and a review on etiology and pathogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Trakoli, A. IARC monographs on the evaluation of carcinogenic risks to humans. volume 99: Some aromatic amines, organic dyes, and related exposures. International Agency for Research on Cancer. Occup. Med. 2012, 62, 232. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-H.; Chou, M.-Y.; Chang, Y.-C. The up-regulation of cyclooxygenase-2 expression in human buccal mucosal fibroblasts by Arecoline: A possible role in the pathogenesis of oral submucous fibrosis. J. Oral Pathol. Med. 2003, 32, 146–153. [Google Scholar] [CrossRef]
- Nair, U.J.; Floyd, R.A.; Nair, J.; Bussachini, V.; Friesen, M.; Bartsch, H. Formation of reactive oxygen species and of 8-hydroxydeoxyguanosine in DNA in vitro with betel quid ingredients. Chem. Biol. Interact. 1987, 63, 157–169. [Google Scholar] [CrossRef]
- Prokopczyk, B.; Rivenson, A.; Bertinato, P.; Brunnemann, K.D.; Hoffmann, D. 3-(Methylnitrosamino) propionitrile: Occurrence in saliva of betel quid chewers, carcinogenicity, and DNA methylation in F344 rats. Cancer Res. 1987, 47, 467–471. [Google Scholar]
- Moon, S.H.; Liu, X.; Cedars, A.M.; Yang, K.; Kiebish, M.A.; Joseph, S.M.; Kelley, J.; Jenkins, C.M.; Gross, R.W. Cytotoxic and genotoxic effects of areca nut-related compounds in cultured human buccal epithelial cells. Cancer Res. 1989, 49, 5294–5298. [Google Scholar]
- Lin, L.M.; Chen, Y.K.; Lai, D.L.; Huang, Y.L. Minimal arecaidine concentrations showing a promotion effect during DMBA-induced hamster cheek pouch carcinogenesis. J. Oral Pathol. Med. 1996, 25, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.-W.; Pei, R.-J.; Tseng, H.-C.; Yeh, K.-T.; Chan, H.-C.; Lee, M.-R.; Lin, C.; Hsieh, W.-T.; Kao, M.-C.; Tsai, M.-H.; et al. Co-treating with Arecoline and 4-Nitroquinoline 1-oxide to establish a mouse model mimicking oral tumorigenesis. Chem. Biol. Interact. 2010, 183, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-Y.; Chen, H.-M.; Chang, M.-C.; Kok, S.-H.; Lee, J.-J.; Chang, B.-E.; Jeng, P.-Y.; Chan, C.-P.; Jeng, J.-H. Cytotoxicity and transformation of C3H10T1/2 cells induced by areca nut components. J. Formos. Med. Assoc. 2016, 115, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Wary, K.K.; Sharan, R.N. Cytotoxic and cytostatic effects of arecoline and sodium nitrite on human cellsin vitro. Int. J. Cancer 1991, 47, 396–400. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Liu, Y.-C.; Huang, W.-T.; Huang, G.-C.; Chen, T.-C.; Lin, M.-H. Up-regulation of matrix metalloproteinase-8 by betel quid extract and arecoline and its role in 2D motility. Oral Oncol. 2007, 43, 1026–1033. [Google Scholar] [CrossRef]
- Yang, C.Y.; Meng, C.L.; van der Bijl, P.; Lee, H.K. The effect of betel nut extract on cell growth and prostaglandin endoperoxide synthase in human epidermoid carcinoma cells. Prostaglandins Other Lipid Mediat. 2002, 67, 181–195. [Google Scholar] [CrossRef]
- Ho, T.-J.; Chiang, C.-P.; Hong, C.-Y.; Kok, S.-H.; Kuo, Y.-S.; Yen-Ping Kuo, M. Induction of the c-jun protooncogene expression by areca nut extract and arecoline on oral mucosal fibroblasts. Oral Oncol. 2000, 36, 432–436. [Google Scholar] [CrossRef]
- Jeng, J.H.; Kuo, M.L.; Hahn, L.J.; Kuo, M.Y.P. Genotoxic and non-genotoxic effects of betel quid ingredients on oral mucosal fibroblasts in vitro. J. Dent. Res. 1994, 73, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-C.; Lin, S.-C.; Chiang, W.-F.; Yen, C.-Y.; Lin, C.-H.; Liu, S.-Y. Areca nut extract treatment elicits the fibroblastoid morphological changes, actin re-organization and signaling activation in oral keratinocytes. J. Oral Pathol. Med. 2003, 32, 600–605. [Google Scholar] [CrossRef]
- Jeng, J.-H.; Lan, W.-H.; Hahn, L.-J.; Hsieh, C.-C.; Kuo, M. Inhibition of the migration, attachment, spreading, growth and collagen synthesis of human gingival fibroblasts by Arecoline, a major areca alkaloid, in vitro. J. Oral Pathol. Med. 1996, 25, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C. Areca nut extract and arecoline induced the cell cycle arrest but not apoptosis of cultured oral KB epithelial cells: Association of glutathione, reactive oxygen species and mitochondrial membrane potential. Carcinogenesis 2001, 22, 1527–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.-H.; Chang, M.-C.; Chang, W.-H.; Wang, T.-M.; Wang, Y.-J.; Hahn, L.-J.; Ho, Y.-S.; Lin, C.-Y.; Jeng, J.-H. Prolonged exposure to arecoline arrested human KB epithelial cell growth: Regulatory mechanisms of cell cycle and apoptosis. Toxicology 2006, 220, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-S.; Lin, C.-S.; Chiang, S.-L.; Lee, C.-H.; Lee, K.-W.; Ko, Y.-C. Areca nut induces mir-23a and inhibits repair of DNA double-strand breaks by targeting FANCG. Toxicol. Sci. 2011, 123, 480–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.-H.; Liu, C.-J.; Liu, T.-Y.; Kao, S.-Y.; Lin, S.-C.; Chang, K.-W. Areca-treated fibroblasts enhance tumorigenesis of oral epithelial cells. J. Dent. Res. 2008, 87, 1069–1074. [Google Scholar] [CrossRef]
- Lin, K.-H.; Lin, C.-Y.; Liu, C.-C.; Chou, M.-Y.; Lin, J.-K. Arecoline N-oxide: Its mutagenicity and possible role as ultimate carcinogen in areca oral carcinogenesis. J. Agric. Food Chem. 2011, 59, 3420–3428. [Google Scholar] [CrossRef]
- Balachandran, B.; Sharan, R.N. Induction of mutations by different extracts of betel nut and radiation: Their implication in carcinogenesis. Radiat. Res. 1995, 1, 165. [Google Scholar]
- Sharan, R.N. Association of betel nut with carcinogenesis. Cancer J. 1996, 9, 13–19. [Google Scholar]
- Sharan, R.N. Biochemical investigation of carcinogenic potency of betel nut (Kwai) of north-east India. Oral Oncol. 1994, 3, 190–193. [Google Scholar]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, T.; Touyz, R. Redox signaling in hypertension. Cardiovasc. Res. 2006, 71, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, S18–S25. [Google Scholar] [CrossRef]
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011, 572634. [Google Scholar] [CrossRef] [Green Version]
- Haigis, M.C.; Yankner, B.A. The aging stress response. Mol. Cell 2010, 40, 333–344. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J.-I. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef] [Green Version]
- Stich, H.F.; Anders, F. The involvement of reactive oxygen species in oral cancers of Betel Quid/tobacco chewers. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1989, 214, 47–61. [Google Scholar] [CrossRef]
- Nair, U.J.; Nair, J.; Friesen, M.D.; Bartsch, H.; Ohshima, H. ortho- and meta-tyrosine formation from phenylalanine in human saliva as a marker of hydroxyl radical generation during betel quid chewing. Carcinogenesis 1995, 16, 1195–1198. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Pan, T.-S.; Ting, C.-C.; Liang, S.-S.; Huang, S.-H.; Liu, H.-Y.; Ko, E.C.-C.; Wu, C.-W.; Tang, J.-Y.; Chen, P.-H. Cytochrome P450 metabolism of betel quid-derived compounds: Implications for the development of prevention strategies for oral and pharyngeal cancers. Sci. World J. 2013, 2013, 618032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, Y.-T.; Chen, P.S.; Wu, C.-H.; Tseng, Y.-T.; Wu, Y.-C.; Lo, Y.-C. Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system. Free. Radic. Biol. Med. 2010, 49, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-Y.; Lin, M.-H.; Liu, S.-Y.; Chiang, W.-F.; Hsieh, W.-F.; Cheng, Y.-C.; Hsu, K.-C.; Liu, Y.-C. Arecoline-mediated inhibition of AMP-activated protein kinase through reactive oxygen species is required for apoptosis induction. Oral Oncol. 2011, 47, 345–351. [Google Scholar] [CrossRef]
- Kumpawat, K.; Deb, S.; Ray, S.; Chatterjee, A. Genotoxic effect of raw betel-nut extract in relation to endogenous glutathione levels and its mechanism of action in mammalian cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 538, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-C.; Huang, L.-W.; Su, S.-J.; Hsieh, B.-S.; Cheng, H.-L.; Hu, Y.-C.; Chen, Y.-H.; Hwang, C.-C.; Chang, K.-L. Hemeoxygenase-1 expression in response to arecoline-induced oxidative stress in human umbilical vein endothelial cells. Int. J. Cardiol. 2011, 151, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-Y.; Wan, H.-C.; Lai, Y.-L.; Kuo, Y.-F.; Liu, T.-Y.; Chen, Y.-T.; Hung, S.-L. Areca nut extracts increased expression of inflammatory cytokines, tumor necrosis factor-α, interleukin-1β, interleukin-6 and interleukin-8, in peripheral blood mononuclear cells. J. Periodontal Res. 2009, 44, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-Y.; Wan, H.-C.; Lai, Y.-L.; Liu, T.-Y.; Hung, S.-L. Enhancing effects of areca nut extracts on the production of interleukin-6 and interleukin-8 by peripheral blood mononuclear cells. J. Periodontol. 2006, 77, 1969–1977. [Google Scholar] [CrossRef]
- Hung, S.-L.; Lin, Y.-J.; Chien, E.J.; Liu, W.-G.; Chang, H.-W.; Liu, T.-Y.; Chen, Y.-T. Areca nut extracts-activated secretion of leukotriene B4, and phosphorylation of p38 mitogen-activated protein kinase and elevated intracellular calcium concentrations in human polymorphonuclear leukocytes. J. Periodontal Res. 2007, 42, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-L.; Wu, C.-Y.; Lee, Y.-Y.; Chang, H.-W.; Liu, T.-Y.; Hung, S.-L. Stimulatory effects of areca nut extracts on prostaglandin E2production by human Polymorphonuclear Leukocytes. J. Periodontol. 2010, 81, 758–766. [Google Scholar] [CrossRef]
- Haque, M.F.; Meghji, S.; Khitab, U.; Harris, M. Oral submucous fibrosis patients have altered levels of cytokine production. J. Oral Pathol. Med. 2000, 29, 123–128. [Google Scholar] [CrossRef]
- Haque, M.F.; Harris, M.; Meghji, S.; Barrett, A.W. Immunolocalization of cytokines and growth factors in oral submucous fibrosis. Cytokine 1998, 10, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Malhotra, P.S.; Thomas, G.R.; Ondrey, F.G.; Duffey, D.C.; Smith, C.W.; Enamorado, I.; Yeh, N.T.; Kroog, G.S.; Rudy, S.; et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin. Cancer Res. 1999, 5, 1369–1379. [Google Scholar]
- Rhodus, N.L.; Cheng, B.; Myers, S.; Miller, L.; Ho, V.; Ondrey, F. The feasibility of monitoring NF-κB associated cytokines: TNF-α, IL-1α, IL-6, and IL-8 in whole saliva for the malignant transformation of oral lichen planus. Mol. Carcinog. 2005, 44, 77–82. [Google Scholar] [CrossRef]
- Chang, M.-C.; Chan, C.-P.; Wang, W.-T.; Chang, B.-E.; Lee, J.-J.; Tseng, S.-K.; Yeung, S.-Y.; Hahn, L.-J.; Jeng, J.-H. Toxicity of areca nut ingredients: Activation of Chk1/CHK2, induction of cell cycle arrest, and regulation of MMP-9 and TIMPs production in SAS epithelial cells. Head Neck 2012, 35, 1295–1302. [Google Scholar] [CrossRef]
- Lin, W.-W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef] [Green Version]
- Parsonne, J. Molecular mechanisms for inflammation-promoted pathogenesis of cancer—The Sixteenth International Symposium of the Sapporo Cancer Seminar. Cancer Res. 1997, 57, 3620–3624. [Google Scholar]
- Trinchieri, G. Innate inflammation and cancer: Is it time for cancer prevention? F1000 Med. Rep. 2011, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, K.; Ishikawa, I. The roles of cyclooxygenase-2 and prostaglandin E2in periodontal disease. Periodontol. 2000 2007, 43, 85–101. [Google Scholar] [CrossRef]
- Van Dyke, T.E. Control of inflammation and periodontitis. Periodontol. 2000 2007, 45, 158–166. [Google Scholar] [CrossRef]
- Telagi, N.; Ahmed Mujib, B.R.; Kulkarni, P.G.; Naik, R. The master switch: Comparative study of mast cell in oral epithelial dysplasia, oral submucous fibrosis and oral squamous cells carcinoma and their association with inflammation and angiogenesis. J. Oral Maxillofac. Pathol. 2015, 19, 25. [Google Scholar] [CrossRef] [Green Version]
- Shieh, D.-H.; Chiang, L.-C.; Shieh, T.-Y. Augmented mrna expression of tissue inhibitor of metalloproteinase-1 in buccal mucosal fibroblasts by arecoline and safrole as a possible pathogenesis for oral submucous fibrosis. Oral Oncol. 2003, 39, 728–735. [Google Scholar] [CrossRef]
- Chang, L.-Y.; Wan, H.-C.; Lai, Y.-L.; Chou, I.-C.; Chen, Y.-T.; Hung, S.-L. Areca nut extracts increased the expression of cyclooxygenase-2, prostaglandin E2 and interleukin-1α in human immune cells via oxidative stress. Arch. Oral Biol. 2013, 58, 1523–1531. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Lin, M.-H.; Liu, S.-Y.; Chiang, W.-F.; Chen, L.-L.; Chen, T.-C.; Cheng, Y.-C.; Hsu, K.-C.; Cheng, P.-C.; Lee, C.-H.; et al. Possible mechanism of betel-quid-extract-induced expression of matrix metalloproteinase-2. J. Formos. Med. Assoc. 2010, 109, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Uehara, O.; Takimoto, K.; Morikawa, T.; Harada, F.; Takai, R.; Adhikari, B.R.; Itatsu, R.; Nakamura, T.; Yoshida, K.; Matsuoka, H.; et al. Upregulated expression of MMP-9 in gingival epithelial cells induced by prolonged stimulation with arecoline. Oncol. Lett. 2017, 14, 1186–1192. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.-C.; Chen, B.-H.; Hour, T.-C.; Chiang, W.-F.; Wu, Y.-J.; Chen, C.-Y.; Chen, H.-R.; Chan, P.-T.; Liu, S.-Y.; Chen, J.Y.-F. Betel quid extract promotes oral cancer cell migration by activating a muscarinic M4 receptor-mediated signaling cascade involving sfks and ERK1/2. Biochem. Biophys. Res. Commun. 2010, 399, 60–65. [Google Scholar] [CrossRef]
- Li, Y.-C.; Chang, J.T.; Chiu, C.; Lu, Y.-C.; Li, Y.-L.; Chiang, C.-H.; You, G.-R.; Lee, L.-Y.; Cheng, A.-J. Areca nut contributes to oral malignancy through facilitating the conversion of cancer stem cells. Mol. Carcinog. 2015, 55, 1012–1023. [Google Scholar] [CrossRef]
- Liu, S.Y.; Lin, M.H.; Yang, S.C.; Huang, G.C.; Chang, L.; Chang, S.; Yen, C.Y.; Chiang, W.F.; Lee, C.H.; Kuo, Y.Y.; et al. Areca quid chewing enhances the expression of salivary matrix metalloproteinase-9. J. Formos. Med. Assoc. Taiwan Yi Zhi 2005, 104, 113–119. [Google Scholar]
- Chapman, H.A. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu. Rev. Physiol. 2011, 73, 413–435. [Google Scholar] [CrossRef]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1419. [Google Scholar] [CrossRef] [Green Version]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. Erratum: The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.C.; Tsai, C.H.; Lai, Y.L.; Yu, C.C.; Chi, W.Y.; Li, J.J.; Chang, W.W. Arecoline-induced myofibroblast transdifferentiation from human buccal mucosal fibroblasts is mediated byzeb1. J. Cell. Mol. Med. 2014, 18, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Hsia, S.M.; Hsieh, P.L.; Liao, Y.W.; Peng, C.Y.; Wu, C.Z.; Lin, K.C.; Tsai, L.L.; Yu, C.C. Slug mediates myofibroblastic differentiation to promote fibrogenesis in buccal mucosa. J. Cell. Physiol. 2018, 234, 6721–6730. [Google Scholar] [CrossRef]
- Sume, S.S.; Kantarci, A.; Lee, A.; Hasturk, H.; Trackman, P.C. Epithelial to mesenchymal transition in gingival overgrowth. Am. J. Pathol. 2010, 177, 208–218. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Chang, C.-S.; Liu, T.-Y.; Kao, S.-Y.; Chang, K.-W.; Lin, S.-C. Areca nut extract treatment down-regulates involucrin in normal human oral keratinocyte through P13K/Akt Activation. Oral Oncol. 2007, 43, 670–679. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Yang, C.-C.; Lin, S.-C.; Cheng, C.-C.; Lin, S.-H.; Liu, C.-J.; Chang, K.-W. Areca nut extract upregulates vimentin by activating PI3K/Akt signaling in oral carcinoma. J. Oral Pathol. Med. 2010, 40, 160–166. [Google Scholar] [CrossRef]
- Ho, C.M.; Hu, F.-W.; Lee, S.-S.; Shieh, T.-M.; Yu, C.-H.; Lin, S.-S.; Yu, C.-C. ZEB1 as an indicator of tumor recurrence for areca quid chewing-associated oral squamous cell carcinomas. J. Oral Pathol. Med. 2014, 44, 693–698. [Google Scholar] [CrossRef]
- Wang, T.Y.; Peng, C.-Y.; Lee, S.-S.; Chou, M.-Y.; Yu, C.-C.; Chang, Y.-C. Acquisition cancer stemness, mesenchymal transdifferentiation, and chemoresistance properties by chronic exposure of oral epithelial cells to Arecoline. Oncotarget 2016, 7, 84072–84081. [Google Scholar] [CrossRef]
- Fan, C.-C.; Wang, T.-Y.; Cheng, Y.-A.; Jiang, S.S.; Cheng, C.-W.; Lee, A.Y.-L.; Kao, T.-Y. Expression of E-cadherin, twist, and p53 and their prognostic value in patients with oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2013, 139, 1735–1744. [Google Scholar] [CrossRef]
- White, E. The role for autophagy in cancer. J. Clin. Investig. 2015, 125, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.-H.; Liu, Y.-C.; Liu, S.-Y.; Chen, F.-C.; Yang, P.-J.; Li, G.-H.; Liu, P.-Y.; Yen, C.-Y. Clathrin-mediated endocytosis is required for ANE 30-100k-induced autophagy. J. Oral Pathol. Med. 2017, 47, 25–31. [Google Scholar] [CrossRef]
- Li, Y.-C.; Cheng, A.-J.; Lee, L.-Y.; Huang, Y.-C.; Chang, J.T.-C. Multifaceted mechanisms of areca nuts in oral carcinogenesis: The molecular pathology from precancerous condition to malignant transformation. J. Cancer 2019, 10, 4054–4062. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.-Y.; Chiang, W.-F.; Liu, S.-Y.; Lin, C.-C.; Liao, K.-A.; Lin, C.-Y.; Hsieh, W.-F.; Cheng, Y.-C.; Hsu, K.-C.; Lin, P.-Y.; et al. Impacts of autophagy-inducing ingredient of areca nut on tumor cells. PLoS ONE 2015, 10, e0128011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, W.-T.; Lee, C.-I.; Chen, J.Y.-F.; Cheng, Y.-P.; Yang, S.-R.; Chen, J.-H.; Chen, H.-R. Areca nut extract induces pyknotic necrosis in serum-starved oral cells via increasing reactive oxygen species and inhibiting gsk3β: An implication for cytopathic effects in betel quid chewers. PLoS ONE 2013, 8, e63295. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-H.; Kao, S.-Y.; Liu, T.-Y.; Liu, S.-T.; Huang, W.-P.; Chang, K.-W.; Lin, S.-C. Areca nut extract induced oxidative stress and upregulated hypoxia inducing factor leading to autophagy in oral cancer cells. Autophagy 2010, 6, 725–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, W.-T.; Yang, S.-R.; Chen, J.Y.-F.; Cheng, Y.-P.; Lee, Y.-R.; Chiang, M.-K.; Chen, H.-R. Arecoline downregulates levels of p21 and p27 through the reactive oxygen species/mTOR complex 1 pathway and may contribute to oral squamous cell carcinoma. Cancer Sci. 2012, 103, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, G.B.; Rao, A.R. Chromosome-breaking ability of arecoline, a major betel-nut alkaloid, in mouse bone-marrow cells in vivo. Mutat. Res. Lett. 1982, 103, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Stich, H.F.; Stich, W.; Lam, P.P.S. Potentiation of genotoxicity by concurrent application of compounds found in betel quid: Arecoline, eugenol, quercetin, chlorogenic acid and MN2+. Mutat. Res. Genet. Toxicol. 1981, 90, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Shirname, L.P.; Menon, M.M.; Bhide, S.V. Mutagenicity of betel quid and its ingredients using mammalian test systems. Carcinogenesis 1984, 5, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.H.; Ho, Y.S.; Chan, C.P.; Wang, Y.J.; Hahn, L.J.; Lei, D.; Hsu, C.C.; Chang, M.C. Areca nut extract up-regulates prostaglandin production, cyclooxygenase-2 mRNA and protein expression of human oral keratinocytes. Carcinogenesis 2000, 21, 1365–1370. [Google Scholar] [CrossRef]
- Tseng, S.-K.; Chang, M.-C.; Su, C.-Y.; Chi, L.-Y.; Chang, J.Z.-C.; Tseng, W.-Y.; Yeung, S.-Y.; Hsu, M.-L.; Jeng, J.-H. Arecoline induced cell cycle arrest, apoptosis, and cytotoxicity to human endothelial cells. Clin. Oral Investig. 2011, 16, 1267–1273. [Google Scholar] [CrossRef]
- Nojima, H. Protein kinases that regulate chromosome stability and their downstream targets. Genome Dis. 2006, 1, 131–148. [Google Scholar]
- Wang, Y.-C.; Tsai, Y.-S.; Huang, J.-L.; Lee, K.-W.; Kuo, C.-C.; Wang, C.-S.; Huang, A.-M.; Chang, J.-Y.; Jong, Y.-J.; Lin, C.-S. Arecoline arrests cells at prometaphase by deregulating mitotic spindle assembly and spindle assembly checkpoint: Implication for carcinogenesis. Oral Oncol. 2010, 46, 255–262. [Google Scholar] [CrossRef]
- Jeng, J.H.; Chang, M.C.; Hahn, L.J. Role of areca nut in Betel Quid-associated chemical carcinogenesis: Current awareness and future perspectives. Oral Oncol. 2001, 37, 477–492. [Google Scholar] [CrossRef]
- Lowe, S.W. Activation of p53 by oncogenes. Endocr. Relat. Cancer 1999, 6, 45–48. [Google Scholar] [CrossRef]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: New developments in an old debate. J. Cell. Biochem. 2015, 116, 696–703. [Google Scholar] [CrossRef]
- Bredell, M.G.; Ernst, J.; El-Kochairi, I.; Dahlem, Y.; Ikenberg, K.; Schumann, D.M. Current relevance of hypoxia in head and neck cancer. Oncotarget 2016, 7, 50781–50804. [Google Scholar] [CrossRef] [Green Version]
- Pereira, K.M.; Chaves, F.N.; Viana, T.S.; Carvalho, F.S.; Costa, F.W.; Alves, A.P.; Sousa, F.B. Oxygen metabolism in oral cancer: HIF and Gluts (review). Oncol. Lett. 2013, 6, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Radhakrishnan, R. Limited mouth opening in oral submucous fibrosis: Reasons, ramifications, and remedies. J. Oral Pathol. Med. 2016, 46, 424–430. [Google Scholar] [CrossRef]
- Block, K.I.; Gyllenhaal, C.; Lowe, L.; Amedei, A.; Amin, A.R.M.R.; Amin, A.; Aquilano, K.; Arbiser, J.; Arreola, A.; Arzumanyan, A.; et al. Designing a broad-spectrum integrative approach for cancer prevention and treatment. Semin. Cancer Biol. 2015, 35, S276–S304. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Kujan, O.; Shearston, K.; Farah, C.S. The role of hypoxia in oral cancer and potentially malignant disorders: A Review. J. Oral Pathol. Med. 2016, 46, 246–252. [Google Scholar] [CrossRef]
- Schofield, C.J.; Ratcliffe, P.J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004, 5, 343–354. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Yang, S.-F.; Lee, S.-S.; Chang, Y.-C. Regulation of hypoxia-inducible factor-1α in human buccal mucosal fibroblasts stimulated with arecoline. J. Formos. Med. Assoc. 2017, 116, 484–487. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Lee, S.-S.; Chang, Y.-C. Hypoxic regulation of plasminogen activator inhibitor-1 expression in human buccal mucosa fibroblasts stimulated with arecoline. J. Oral Pathol. Med. 2014, 44, 669–673. [Google Scholar] [CrossRef]
- Yen, C.-Y.; Chiang, W.-F.; Liu, S.-Y.; Cheng, P.-C.; Lee, S.-Y.; Hong, W.-Z.; Lin, P.-Y.; Lin, M.-H.; Liu, Y.-C. Long-term stimulation of areca nut components results in increased chemoresistance through elevated autophagic activity. J. Oral Pathol. Med. 2013, 43, 91–96. [Google Scholar] [CrossRef]
- Duan, Y.; He, Q.; Yue, K.; Si, H.; Wang, J.; Zhou, X.; Wang, X. Hypoxia induced bcl-2/TWIST1 complex promotes tumor cell invasion in oral squamous cell carcinoma. Oncotarget 2016, 8, 7729–7739. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Hijioka, H.; Kume, K.; Miyawaki, A.; Nakamura, N. Notch signaling induces EMT in OSCC cell lines in a hypoxic environment. Oncol. Lett. 2013, 6, 1201–1206. [Google Scholar] [CrossRef] [Green Version]
- De Lima, P.O.; Jorge, C.C.; Oliveira, D.T.; Pereira, M.C. Hypoxic condition and prognosis in oral squamous cell carcinoma. Anticancer Res. 2014, 34, 605–612. [Google Scholar]
- Zhang, Z.; Han, H.; Rong, Y.; Zhu, K.; Zhu, Z.; Tang, Z.; Xiong, C.; Tao, J. Hypoxia potentiates gemcitabine-induced stemness in pancreatic cancer cells through AKT/Notch1 signaling. J. Exp. Clin. Cancer Res. 2018, 37, 291. [Google Scholar] [CrossRef] [PubMed]
- Takeda, D.; Hasegawa, T.; Ueha, T.; Iwata, E.; Harada, R.; Sakakibara, A.; Kawamoto, T.; Minamikawa, T.; Sakai, Y.; Komori, T. Induced pluripotent-stem-cell related genes contribute to de-differentiation in oral squamous cell carcinoma. Anticancer Res. 2017, 37, 1075–1082. [Google Scholar] [PubMed]
- Belstrøm, D. The oral microbiota as part of the human microbiota—Links to general health. Nor. Tannlegeforen. Tid. 2020, 130, 114–120. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol. Cancer Res. Treat. 2019, 18, 153303381986735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Yuan, F.; Chen, S.; Li, X.; Kong, L.; Zhang, W. Potential role of host microbiome in areca nut-associated carcinogenesis and addiction. Molecules 2022, 27, 8171. [Google Scholar] [CrossRef]
- Rowińska, I.; Szyperska-Ślaska, A.; Zariczny, P.; Pasławski, R.; Kramkowski, K.; Kowalczyk, P. The influence of diet on oxidative stress and inflammation induced by bacterial biofilms in the human oral cavity. Materials 2021, 14, 1444. [Google Scholar] [CrossRef]
- Gallimidi, A.B.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens porphyromonas gingivalis and fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, B.Y.; Zhu, X.; Goodman, M.T.; Gatewood, R.; Mendiola, P.; Quinata, K.; Paulino, Y.C. Betel nut chewing, oral premalignant lesions, and the oral microbiome. PLoS ONE 2017, 12, e0172196. [Google Scholar] [CrossRef] [Green Version]
- Zhong, X.; Lu, Q.; Zhang, Q.; He, Y.; Wei, W.; Wang, Y. Oral microbiota alteration associated with oral cancer and areca chewing. Oral Dis. 2020, 27, 226–239. [Google Scholar] [CrossRef] [PubMed]
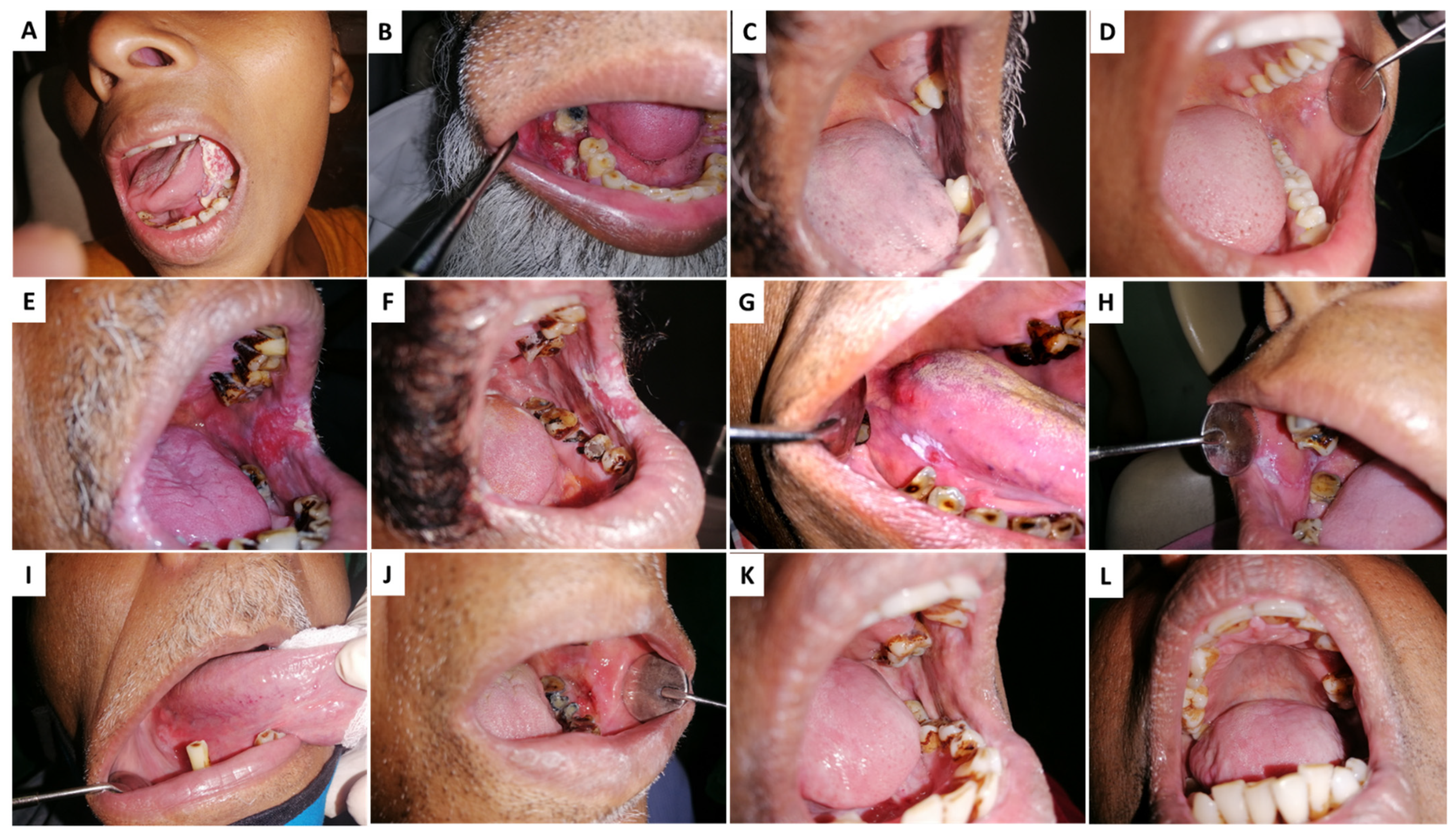




| Nature of Extract(s) | Type of Experiment | Analyses Conducted | Main Observations | Reference |
|---|---|---|---|---|
| Aqueous AN and pan masala extracts | Injection into buccal mucosa of Sprague-Dawley rats | Histological analysis and TGF-beta1 gene by RT-PCR | Epithelial atrophy and collagen accumulation, significant upregulation of TGF beta1 gene | [13] |
| AN extract | Subcutaneous injection into BALB/C mice | Histological analysis, immunohistochemical staining, and immunoblotting | Increase of collagen deposition, higher expression of α-smooth muscle actin, and connective tissue growth factors compared to control group | [14] |
| Arecoline | Smearing in the inner mouth area of C57BL/6 mice followed by administration via drinking | Examination of tongue tissue, Krt17 protein expression analysis | Malignant lesions observed, and upregulation of Krt17 compared to control group | [15] |
| Arecoline | In vitro exposure of arecoline on human gingival fibroblasts | Analysis of cytotoxicity, mitochondrial activity, and cell cycle analysis | DNA inhibition, decrease of mitochondrial activity, and cell cycle arrest at the G2/M phase in a dose-dependent manner | [17] |
| AN extract and arecoline | In vitro exposure on human gingival tissue | Cytotoxicity, total and unscheduled DNA synthesis | AN extract caused cell growth suppression, and induction of total and unscheduled DNA synthesis at lower concentrations than arecoline | [18] |
| Aqueous AN and aqueous arecoline extracts | In vitro exposure on mouse kidney cells | Cell growth and DNA strand break analysis | Suppression of cell growth and enhanced DNA strand breaks caused by exposure to AN or arecoline compared to control group | [20] |
| Aqueous, acetic acid, hydrochloric acid, and ethanol extracts of AN | In vitro treatment on Hep 2 cells | Cell viability and unscheduled DNA synthesis | Reduction of cell viability and increase of unscheduled DNA synthesis observed, with aqueous and acetic acid extracts showing a higher effect than other extracts | [21] |
| AN extract | In vitro treatment on normal human oral keratinocytes | Cell viability and proliferation, p38MAPK and repair enzymes, cell cycle, NF-κB, and IκBα activation | Inhibition of cell viability and proliferation, p38MAPK activation, cell cycle arrest at G1 phase, induction of NF-κB and IκBα | [22] |
| Aqueous AN extract | In vitro treatment on Chinese hamster ovary cells | Cytotoxicity, intracellular ROS production and micronuclei formation, cell cycle analysis, evaluation of actin filament distribution and nucleus number | Increased MN frequency, G2/M arrest, cytokinesis failure, and accumulation of hyperploid/aneuploid cells increased intracellular H2O2 levels and actin filament disorganization | [23] |
| Arecoline extracts | In vitro exposure of arecoline on human gingival fibroblasts | Cytotoxicity assay and gene expression profiling | Increased cytotoxicity in a dose-dependent manner, the genes AKR1A1, CYP26B1, S100A12, ALDH9A1, MAOA, UGCGL1, and GSS, LCMT1, and NAT8 were all repressed by arecoline. Gene related to DNA damage signaling (DDIT4) was moderately induced. DNA repair-related genes BRCA1 repressed, and RAD50 were induced by arecoline | [24] |
| AN extract, arecoline and arecaidine | In vitro exposure of AN extract on human keratinocytes | Cytotoxicity assay, apoptosis, ROS analysis, and hypoxanthine phosphoribosyltransferase (HPRT) mutation. | Increased HPRT mutations, intracellular ROS generation, and apoptosis | [25] |
| AN extract | In vitro exposure of AN extract on human gingival fibroblasts | Cytokinin secretion, ROS production, oxidative DNA damage, DNA double-strand breaks, gene silencing | GRO-α, IL-6, and IL-8 cytokinin production was enhanced. Results indicate NOX1 and NOX4 gene-mediated cytokine-induced oxidative DNA damage by regulating ROS production | [26] |
| AN extract and arecoline | In vitro exposure of AN extract and arecoline on human keratinocytes compared to KB carcinoma cells | mRNA expression, extracellular signal-regulated kinase (ERK) phosphorylation via RT-PCR, flow cytometry, Western blotting, and ELISA | Induced c-Fos mRNA expression and PGE2 and IL-6 production by cells and stimulation of ERK-1/ERK2 phosphorylation | [27] |
| AN extract | In vitro exposure of AN extract on human gingival keratinocytes | Cytotoxicity, mRNA and protein expression, and ELISA | Extract stimulated PGE2/PGF2α production, and upregulated expression of cyclooxygenase-2 (COX-2), cytochrome P450 1A1 (CYP1A1) and hemeoxygenase-1 (HO-1) | [28] |
| Arecoline | In vitro exposure of arecoline extract on human buccal mucosal fibroblasts | Gene expression, collagen contraction, and migration capability | Increased Twist expression transcript and protein levels; myofibroblast activity, including collagen gel contraction and migration capability | [29] |
| AN extract | In vitro exposure of AN extract on human gingival fibroblasts and epithelial cells compared to TGF-β treatment | Transcriptome profiling | AN and TGF-β enhanced fibroblast activation in both types of cells. Both significantly common and unique gene expression patterns were identified in both types of cells. Action of AN on fibroblasts is enhanced by epithelial-mesenchymal interaction via TGF-β | [30] |
| Arecaidine | Intraperitoneal injection into Swiss albino mice | Sister chromatid exchange analysis | Sister chromatid exchange frequency increased dose-dependently | [31] |
| Arecoline | Organ-specific mutagenic potential in gpt delta transgenic mice | Genomic DNA analysis from the oral tissues and liver tissues | G:C to T:A transversions (in oral tissues) and G:C to A:T transitions (in oral tissues and liver tissues) were observed | [32] |
| Arecoline | Cytotoxic and genotoxic effects of arecoline in normal rat hepatocytes | Cell cycle analysis, DNA damage, TGF-β1 mRNA expression, protein expression, phosphorylation of p53 | Arecoline induces cell cycle arrest, and DNA damage, increasing TGF-β1 mRNA expression and transcription. Also, arecoline increased p21WAF1 protein expression and p53 phosphorylation and gene transcription | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senevirathna, K.; Pradeep, R.; Jayasinghe, Y.A.; Jayawickrama, S.M.; Illeperuma, R.; Warnakulasuriya, S.; Jayasinghe, R.D. Carcinogenic Effects of Areca Nut and Its Metabolites: A Review of the Experimental Evidence. Clin. Pract. 2023, 13, 326-346. https://doi.org/10.3390/clinpract13020030
Senevirathna K, Pradeep R, Jayasinghe YA, Jayawickrama SM, Illeperuma R, Warnakulasuriya S, Jayasinghe RD. Carcinogenic Effects of Areca Nut and Its Metabolites: A Review of the Experimental Evidence. Clinics and Practice. 2023; 13(2):326-346. https://doi.org/10.3390/clinpract13020030
Chicago/Turabian StyleSenevirathna, Kalpani, Roshan Pradeep, Yovanthi Anurangi Jayasinghe, Shalindu Malshan Jayawickrama, Rasika Illeperuma, Saman Warnakulasuriya, and Ruwan Duminda Jayasinghe. 2023. "Carcinogenic Effects of Areca Nut and Its Metabolites: A Review of the Experimental Evidence" Clinics and Practice 13, no. 2: 326-346. https://doi.org/10.3390/clinpract13020030






