Is It Possible to Prevent the Thanatogenetic Processes in Premature Babies?
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohuma, E.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and worldwide estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Hug, L.; You, D.; Blencowe, H.; Mishra, A.; Wang, Z.; Fix, M.J.; Wakefield, J.; Moran, A.C.; Gaigbe-Togbe, V.; Suzuki, E.; et al. Global, regional, and national estimates and trends in stillbirths from 2000 to 2019: A systematic assessment. Lancet 2021, 398, 772–785. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; pp. 94–96. [Google Scholar]
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; E Black, R.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000–19: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc. Health 2022, 6, 106–115, Erratum in Lancet Child Adolesc. Health 2022, 6, e4. [Google Scholar] [CrossRef]
- United Nations. The Millennium Development Goals Report 2015; United Nations: New York, NY, USA, 2015; Available online: https://www.un.org/millenniumgoals/2015_MDG_Report/pdf/MDG%202015%20rev%20(July%201).pdf (accessed on 1 February 2024).
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development. September 2015. Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld (accessed on 1 February 2024).
- Ramaswamy, V.V.; Abiramalatha, T.; Bandyopadhyay, T.; Shaik, N.B.; Bandiya, P.; Nanda, D.; Pullattayil, S.A.K.P.; Murki, S.; Roehr, C.C. ELBW and ELGAN outcomes in developing nations-Systematic review and meta-analysis. PLoS ONE 2021, 16, e0255352. [Google Scholar] [CrossRef]
- WHO, March of Dimes, PMNCH, Save the Children. 15 million preterm births: Priorities for action based on national, regional and global estimates. In Born Too Soon: The Global Action Report on Preterm Birth; Howson, C.P., Kinney, M.V., Lawn, J., Eds.; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Santana, D.S.; Silveira, C.; Costa, M.L.; Souza, R.T.; Surita, F.G.; Souza, J.P.; Mazhar, S.B.; Jayaratne, K.; Qureshi, Z.; Sousa, M.H.; et al. Perinatal outcomes in twin pregnancies complicated by maternal morbidity: Evidence from the WHO Multicountry Survey on Maternal and Newborn Health. BMC Pregnancy Childbirth 2018, 18, 449. [Google Scholar] [CrossRef]
- Gao, L.; Lyu, S.P.; Zhao, X.R.; Wu, Y.; Hua, R.Y.; Wang, S.; Zhang, Y.; Wang, Y.-L. Systematic management of twin pregnancies to reduce pregnancy complications. Chin. Med. J. 2020, 133, 1355–1357. [Google Scholar] [CrossRef] [PubMed]
- Walani, S.R. Global burden of preterm birth. Int. J. Gynecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Sustainable Development Goal 3. Ensure Healthy Lives an Promote Well-Being for All at All Ages. Available online: https://www.un.org/sustainabledevelopment/health/ (accessed on 5 February 2024).
- Adugna, D.G. Prevalence and associated risk factors of preterm birth among neonates in referral hospitals of Amhara Region, Ethiopia. PLoS ONE 2022, 17, e0276793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Ma, Y.; Liu, L.; Xia, Q.; Fan, D.; Ai, W. Mode of delivery and preterm birth in subsequent births: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0213784. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.; Monet, B.; Ducruet, T.; Chaillet, N.; Audibert, F. Effect of maternal age on the risk of preterm birth: A large cohort study. PLoS ONE 2018, 13, e0191002. [Google Scholar] [CrossRef] [PubMed]
- Capra, L.; Tezza, G.; Mazzei, F.; Boner, A.L. The origins of health and disease: The influence of maternal diseases and lifestyle during gestation. Ital. J. Pediatr. 2013, 39, 7. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Irvin-Choy, N.; Hoffman, M.K.; Gleghorn, J.P.; Day, E.S. Diseases and conditions that impact maternal and fetal health and the potential for nanomedicine therapies. Adv. Drug Deliv. Rev. 2021, 170, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. CMAJ 2021, 193, E540–E548. [Google Scholar] [CrossRef] [PubMed]
- Karnati, S.; Kollikonda, S.; Abu-Shaweesh, J. Late preterm infants—Changing trends and continuing challenges. Int. J. Pediatr. Adolesc. Med. 2020, 7, 36–44. [Google Scholar] [CrossRef]
- Cutland, C.L.; Lackritz, E.M.; Mallett-Moore, T.; Bardaji, A.; Chandrasekaran, R.; Lahariya, C.; Nisar, M.I.; Tapia, M.D.; Pathirana, J.; Kochhar, S.; et al. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017, 35, 6492–6500. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Zainiyah, S.Y.; Lye, M.S.; Zalilah, M.S.; Heidarzadeh, M. Comparison of maternal characteristics in low birth weight and normal birth weight infants. East Mediterr. Health J. 2013, 19, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. How Many Children Were Born in the EU in 2021? Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/w/ddn-20230309-1 (accessed on 9 March 2023).
- UNICEF. Available online: https://data.unicef.org/topic/child-survival/stillbirths/ (accessed on 31 January 2023).
- Arnaez, J.; Ochoa-Sangrador, C.; Caserío, S.; Gutiérrez, E.P.; del Pilar Jiménez, M.; Castañón, L.; Benito, M.; Peña, A.; Hernández, N.; Hortelano, M.; et al. Lack of changes in preterm delivery and stillbirths during COVID-19 lockdown in a European region. Eur. J. Pediatr. 2021, 180, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.C. Therapeutic trajectory for improving survival and outcomes of very low birth weight (VLBW) preterm in-fants. Pediatr. Neonatol. 2023, 64, 493–494. [Google Scholar] [CrossRef]
- Khasawneh, W.; Khriesat, W. Assessment and comparison of mortality and short-term outcomes among premature infants before and after 32-week gestation: A cross-sectional analysis. Ann. Med. Surg. 2020, 60, 44–49. [Google Scholar] [CrossRef]
- Been, J.V.; Burgos Ochoa, L.; Bertens, L.C.M.; Schoenmakers, S.; Steegers, E.A.P.; Reiss, I.K.M. Impact of COVID-19 mit-igation measures on the incidence of preterm birth: A national quasi-experimental study. Lancet Public Health 2020, 5, e604–e611. [Google Scholar] [CrossRef] [PubMed]
- Hedermann, G.; Hedley, P.L.; Baekvad-Hansen, M.; Hjalgrim, H.; Rostgaard, K.; Poorisrisak, P.; Breindahl, M.; Melbye, M.; Hougaard, D.M.; Christiansen, M.; et al. Danish premature birth rates during the COVID-19 lockdown. Arch. Dis. Child Fetal Neonatal Ed. 2020, 106, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.K.; Purtill, H.; Reidy, E.; Daly, M.; Imcha, M.; McGrath, D.; O’Connell, N.H.; Dunne, C.P. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: A ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob. Health 2020, 5, e003075. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, M.; Zhu, Z.; Liu, Y. Coronavirus disease 2019 (COVID-19) and pregnancy: A systematic review. J. Matern. Fetal Neonatal Med. 2022, 35, 1619–1622. [Google Scholar] [CrossRef]
- Alcover, N.; Regiroli, G.; Benachi, A.; Vauloup-Fellous, C.; Vivanti, A.J.; De Luca, D. Systematic review and synthesis of stillbirths and late miscarriages following SARS-CoV-2 infections. Am. J. Obstet. Gynecol. 2023, 229, 118–128. [Google Scholar] [CrossRef] [PubMed]
- van Baar, J.A.C.; Kostova, E.B.; Allotey, J.; Thangaratinam, S.; Zamora, J.R.; Bonet, M.; Kim, C.R.; Mofenson, L.M.; Kunst, H.; Khalil, A.; et al. COVID-19 in pregnant women: A systematic review and meta-analysis on the risk and prevalence of pregnancy loss. Hum. Reprod. Update 2024, 30, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, C.S.; Hirakata, V.N.; de Souza Buriol, V.C.; Nunes, M.; Goldani, M.Z.; da Silva, C.H. The relationship between the different low birth weight strata of newborns with infant mortality and the influence of the main health determinants in the extreme south of Brazil. Popul. Health Metr. 2019, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Ballot, D.E.; Chirwa, T.F.; Cooper, P.A. Determinants of survival in very low birth weight neonates in a public sector hospital in Johannesburg. BMC Pediatr. 2010, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Cnattingius, S.; Johansson, S.; Razaz, N. Apgar Score and Risk of Neonatal Death among Preterm Infants. N. Engl. J. Med. 2020, 383, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Chang, Y.S.; Ahn, S.Y.; Sung, S.I.; Park, W.S. Predicting mortality in extremely low birth weight infants: Comparison between gestational age, birth weight, Apgar score, CRIB II score, initial and lowest serum albumin levels. PLoS ONE 2018, 13, e0192232. [Google Scholar] [CrossRef]
- Santos, C.L.; Costa, K.M.M.; Dourado, J.E.; Lima, S.B.G.; Dotto, L.M.G.; Schirmer, J. Maternal factors associated with prematurity in public maternity hospitals at the Brazilian Western Amazon. Midwifery 2020, 85, 102670. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; McClure, E.M.; Saleem, S.; Reddy, U.M. Infection-related stillbirths. Lancet 2010, 375, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- McClure, E.M.; Silver, R.M.; Kim, J.; Ahmed, I.; Kallapur, M.; Ghanchi, N.; Nagmoti, M.B.; Dhaded, S.; Aceituno, A.; Tikmani, S.S.; et al. Maternal infection and stillbirth: A review. J. Matern. Fetal Neonatal Med. 2022, 35, 4442–4450. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.; Koller-Smith, L.; Lui, K.; Bajuk, B.; Bolisetty, S.; New South Wales and Australian Capital Territory Neonatal Intensive Care Units’ Data Collection. Causes of death in very preterm infants cared for in neonatal intensive care units: A population-based retrospective cohort study. BMC Pediatr. 2017, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Honein, M.A.; Kirby, R.S.; Meyer, R.E.; Xing, J.; Skerrette, N.I.; Yuskiv, N.; Marengo, L.; Petrini, J.R.; Davidoff, M.J.; Mai, C.T.; et al. The Association Between Major Birth Defects and Preterm Birth. Matern. Child Health J. 2009, 13, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Truttmann, A.C.; Ginet, V.; Puyal, J. Current Evidence on Cell Death in Preterm Brain Injury in Human and Preclinical Models. Front. Cell Dev. Biol. 2020, 8, 27. [Google Scholar] [CrossRef]
- Grafe, M.R.; Kinney, H.C. Neuropathology associated with stillbirth. Semin. Perinatol. 2002, 26, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Suhag, A.; Berghella, V. Intrauterine Growth Restriction (IUGR): Etiology and Diagnosis. Curr. Obstet. Gynecol. Rep. 2013, 2, 102–111. [Google Scholar] [CrossRef]
- Novac, M.V.; Iliescu, D.G.; Tudorache, S.; Manolea, M.; Meetescu, R.E.; Vrabie, S.; Novac, M.B.; Alexandru, D.O.; Dijmarescu, L. Ultrasound Evaluation of Fetal Biometry and Doppler Parameters in the Third Trimester of Pregnancy Suspected of Intrauterine Growth Restriction. Curr. Health Sci. J. 2018, 44, 23–28. [Google Scholar] [CrossRef]
- Fardiazar, Z.; Atashkhouei, S.; Yosefzad, Y.; Goldust, M.; Torab, R. Comparison of fetal middle cerebral arteries, umbilical and uterin artery color Doppler ultrasound with blood gas analysis in pregnancy complicated by IUGR. Iran. J. Reprod. Med. 2013, 11, 47–51. [Google Scholar] [PubMed]
- Ramanlal, R.; Gupta, V. Physiology, Vasodilation. [Updated 2023 Jan 23]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557562/ (accessed on 5 February 2024).
- Cohen, E.; Baerts, W.; van Bel, F. Brain-Sparing in Intrauterine Growth Restriction: Considerations for the Neonatologist. Neonatology 2015, 108, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P. Intraventricular hemorrhage in premature infants: Mechanism of disease. Pediatr. Res. 2010, 67, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Tian, X. Analysis of risk factors of early intraventricular hemorrhage in very-low-birth-weight premature infants: A single center retrospective study. BMC Pregnancy Childbirth 2022, 22, 890. [Google Scholar] [CrossRef]
- Jain, K.; Sankar, M.J.; Nangia, S.; Ballambattu, V.B.; Sundaram, V.; Ramji, S.; Plakkal, N.; Kumar, P.; Jain, A.; Sivanandan, S.; et al. Causes of death in preterm neonates (<33 weeks) born in tertiary care hospitals in India: Analysis of three large prospective multicentric cohorts. J. Perinatol. 2019, 39, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.; Walls, M.; McGuire, W. Respiratory complications of preterm birth. BMJ. 2004, 329, 962–965. [Google Scholar] [CrossRef]
- Neeha, S.; Kattimani, S.R.; Mahanta, A.A.; Patil, A.G. An autopsy based descriptive study of the spectrum of pulmonary lesions encountered in fetal deaths at a tertiary care center. Indian J. Pathol. Microbiol. 2018, 61, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Drakeley, A.J.; Roberts, D.; Alfirevic, Z. Cervical stitch (cerclage) for preventing pregnancy loss in women. Cochrane Database Syst. Rev. 2003, 2003, CD003253. [Google Scholar] [CrossRef] [PubMed]
- Henderickx, J.G.E.; Zwittink, R.D.; Renes, I.B.; van Lingen, R.A.; van Zoeren-Grobben, D.; Groot Jebbink, L.J.; Boeren, S.; van Elburg, R.M.; Knol, J.; Belzer, C. Maturation of the preterm gastrointestinal tract can be defined by host and microbial markers for digestion and barrier defense. Sci. Rep. 2021, 11, 12808. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.C.; Bellanger, A.; Ménard, O.; Pladys, P.; Le Gouar, Y.; Dirson, E.; Kroell, F.; Dupont, D.; Deglaire, A.; Bourlieu, C. Impact of human milk pasteurization on gastric digestion in preterm infants: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, D.; Li, X.; Zheng, A.; Wang, F. Spontaneous massive fetomaternal hemorrhage: Two case reports and a lit-erature review of placental pathology. BMC Pregnancy Childbirth 2023, 23, 530. [Google Scholar] [CrossRef]
- O’Leary, B.D.; Walsh, C.A.; Fitzgerald, J.M.; Downey, P.; McAuliffe, F.M. The contribution of massive fetomaternal hemorrhage to antepartum stillbirth: A 25-year cross-sectional study. Acta Obstet. Gynecol. Scand. 2015, 94, 1354–1358. [Google Scholar] [CrossRef]
- Khowaja, W.H.; Leghari, A.L.; Hussain, A.S.; Ariff, S.; Khan, I.A. Frequency and Early Complications of Late Preterm Infants: A Descriptive Analysis from Two Secondary-care Hospitals of Karachi. Cureus 2019, 11, e5789. [Google Scholar] [CrossRef] [PubMed]
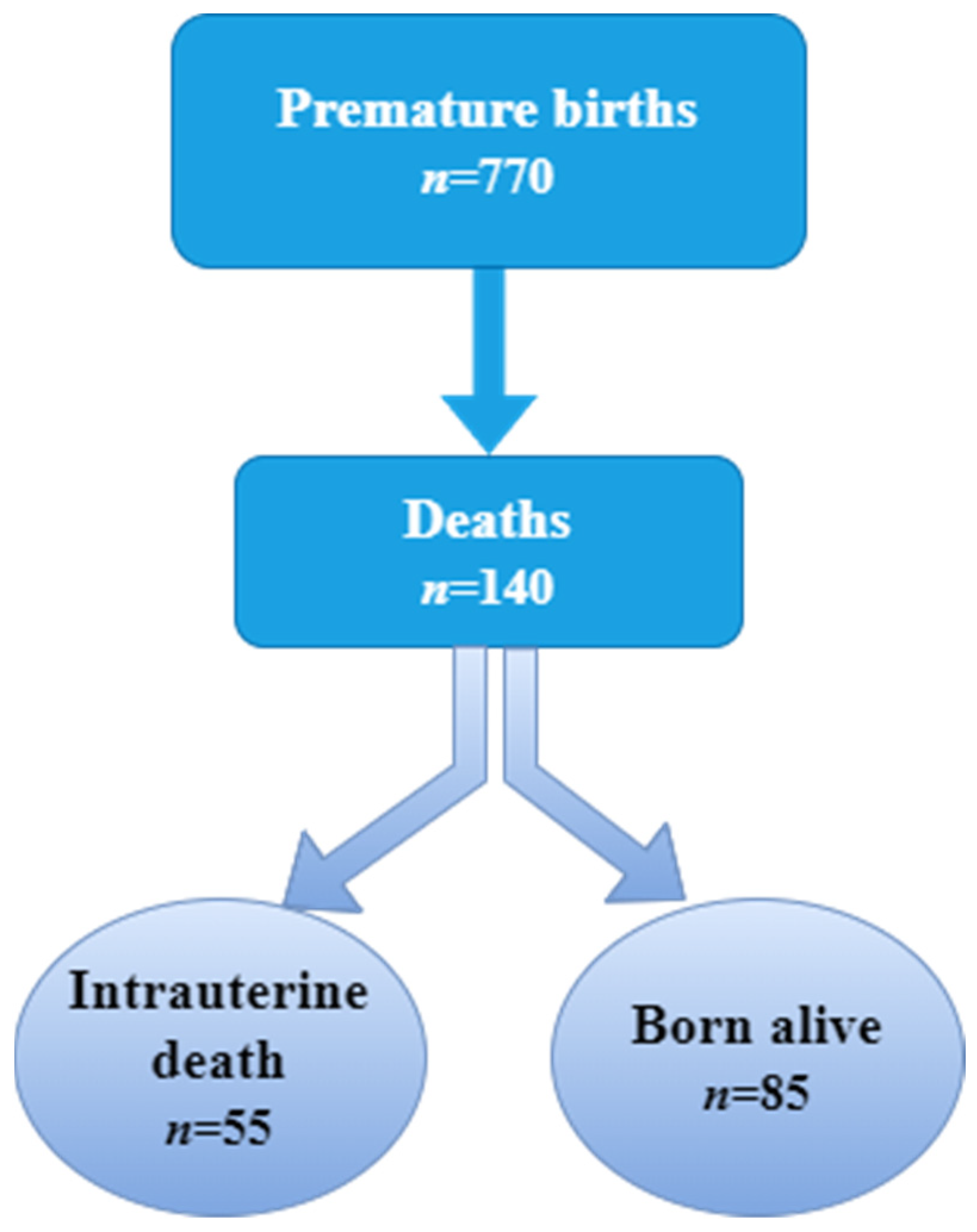
| Born Alive | Total | ||||||
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| Deaths (n) | Mortality Rate (%) | Deaths (n) | Mortality Rate (%) | Deaths (n) | Mortality Rate (%) | ||
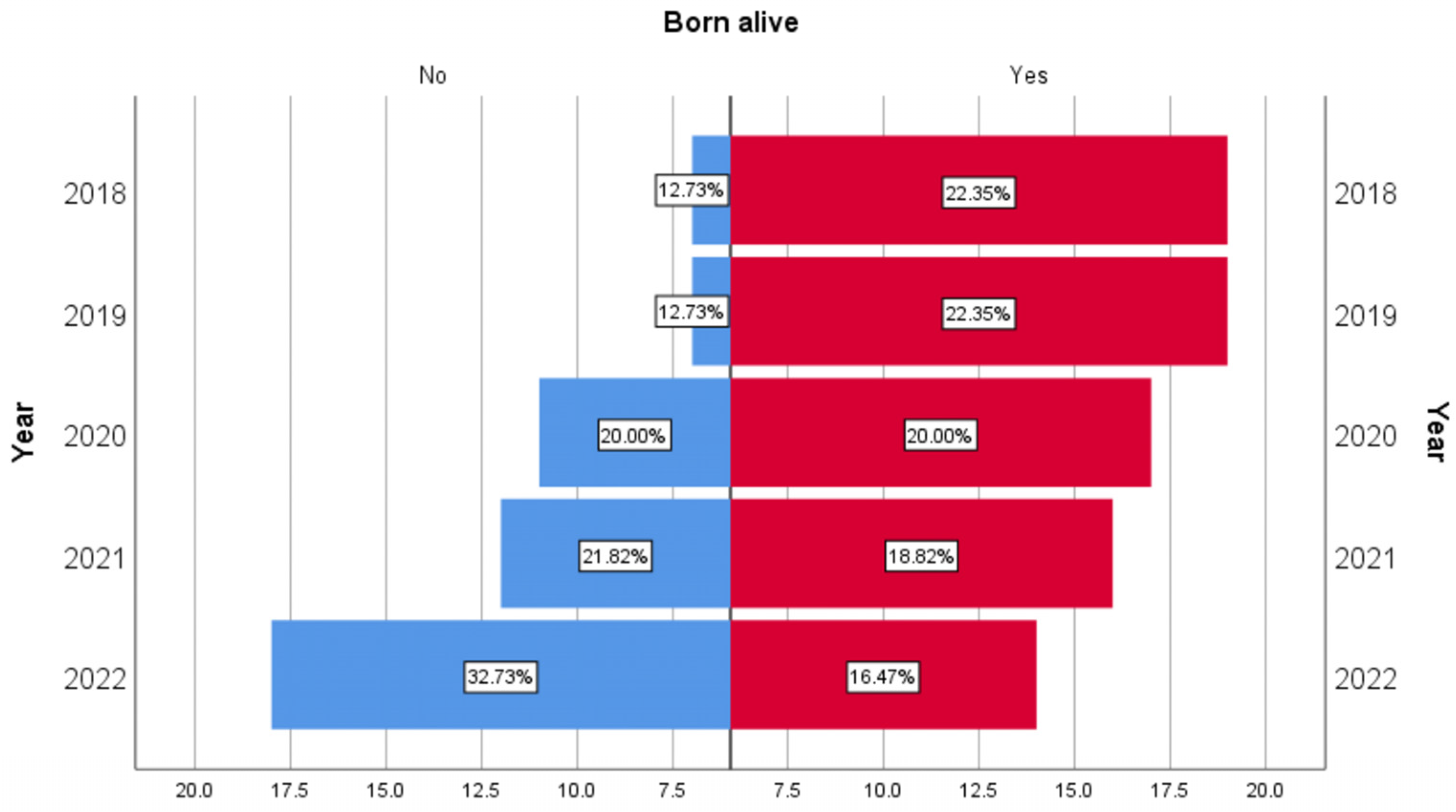
| Years | 2018 | 19 | 10.92 | 7 | 4.02 | 26 | 14.94 |
| 2019 | 19 | 12.18 | 7 | 4.49 | 26 | 16.67 | |
| 2020 | 17 | 10.49 | 11 | 6.79 | 28 | 17.28 | |
| 2021 | 16 | 9.94 | 12 | 7.45 | 28 | 17.39 | |
| 2022 | 14 | 11.97 | 18 | 15.38 | 32 | 27.35 | |
| Premature Born Alive | Premature Stillborns | p-Value | |
|---|---|---|---|
| (n = 85) | (n = 55) | ||
| Gestational age (weeks) | <0.001 | ||
| Average ± SE (min–max) | 27.45 ± 0.37 (22–35) | 30.73 ± 0.49 (22–35) | |
| Degree of prematurity according to gestational age (n, %) | <0.001 | ||
| Extremely premature infants | 17, 20% | 4, 7.27% | |
| Very premature infants | 55, 64.71% | 23, 41.82% | |
| Medium preterm infants | 7, 8.24% | 12, 21.82% | |
| Late preterm infants | 6, 7.06% | 16, 29.09% | |
| Sex (n, %) | 0.080 | ||
| Female | 30, 35.29% | 28, 50.91% | |
| Male | 55, 64.71% | 27, 49.09% | |
| Weight (grams) | <0.001 | ||
| Average ± SE (min–max) | 1224 ± 84.54 (300–3975) | 1632.36 ± 88.63 (290–3880) | |
| Degree of prematurity according to weight (n, %) | <0.001 | ||
| Extremely low birth weight | 50, 58.82% | 8, 14.55% | |
| Very low birth weight | 13, 15.29% | 17, 30.91% | |
| Low birth weight | 15, 17.65% | 23, 41.82% | |
| Normal birth weight | 7, 8.24% | 7, 12.73% | |
| Length (cm) | <0.001 | ||
| Average ± SE (min–max) | 36.79 ± 0.65 (28–54) | 40.45 ± 0.84 (24–54) | |
| Cranial circumference (cm) | 0.001 | ||
| Average ± SE (min–max) | 26.31 ± 0.52 (19–46) | 28.3 ± 0.53 (16–39) | |
| Chest circumference (cm) | 0.006 | ||
| Average ± SE (min–max) | 23.88 ± 0.49 (12–38) | 28.86 ± 0.63 (14–39) | |
| Abdominal circumference (cm) | 0.113 | ||
| Average ± SE (min–max) | 21.7 ± 0.47 (14–38) | 22.33 ± 0.53 (11–31) |
| Survival (h *, Mean ± SE) | p-Value | APGAR Score (Median) | p-Value | |
|---|---|---|---|---|
| ELBW (n = 50) ** | 230.98 ± 88.27 | 0.046 | 1 (1–7) | 0.035 |
| VLBW (n = 13) | 123.38 ± 36.02 | 2 (1–6) | ||
| LBW (n = 15) | 539.07 ± 278.91 | 3 (1–8) | ||
| NBW (n = 7) | 630.14 ± 451.01 | 2 (1–8) | ||
| EPT (n = 17) *** | 595.53 ± 302.15 | 0.981 | 1 (1–6) | 0.011 |
| VPT (n = 55) | 252.33 ±80.81 | 2 (1–8) | ||
| MPT (n = 7) | 14.29 ± 64.89 | 1 (1–4) | ||
| LPT (n = 6) | 109.83 ± 32.20 | 4 (2–8) |
| Premature Born Alive | Premature Stillborns | p-Value | |
|---|---|---|---|
| (n = 85) | (n = 55) | ||
| Mother’s age (years) | 0.831 | ||
| Average ± SE (min–max) | 28.05 ± 0.74 (16–45) | 27.82 ± 0.93 (16–46) | |
| Gestations | 0.920 | ||
| Average ± SE (min–max) | 3.20 ± 0.28 (1–12) | 3.25 ± 0.37 (1–12) | |
| Parity | 0.254 | ||
| Average ± SE (min–max) | 1.92 ± 0.15 (1–9) | 2.11 ± 0.19 (1–7) | |
| Maternal conditions (n, %) | 0.006 | ||
| No | 60, 70.59% | 25, 45.45% | |
| Hypertension | 7, 8.24% | 15, 27.27% | |
| Diabetes | 3, 3.53% | 1, 1.82% | |
| Cicatricial uterus | 4, 4.71% | 6, 10.91% | |
| Cervical insufficiency | 6, 7.06% | 1, 1.82% | |
| Cervico-isthmic incompetence | 2, 2.35% | 2, 3.64% | |
| Infectious causes | 3, 3.52% | 5, 9.09% | |
| Diagnosis at admission (n, %) | <0.001 | ||
| Fetal distress | 21, 24.71% | 0, 0% | |
| Rupture of the amniotic membrane | 17, 20% | 5, 9.09% | |
| Painful uterine contractions | 15, 17.65% | 5, 9.09% | |
| Absence of fetal movements | 1, 1.18% | 17, 30.91% | |
| Intact amniotic membrane | 7, 8.24% | 12, 21.82% | |
| Placental abruption | 7, 8.24% | 7, 12.73% | |
| Preeclampsia | 3, 3.53% | 2, 3.64% | |
| Metrorrhagia | 2, 2.35% | 3, 5.45% | |
| Other diagnoses | 12, 14.10% | 4, 7.27% |
| Thanatogenesis | Diagnostics | Frequency (n, %) |
|---|---|---|
| Direct causes of death (I A) | Acute cardiorespiratory failure | 72, 87.80% |
| Sepsis | 4, 4.88% | |
| Hemorrhagic disease of the newborn | 2, 2.44% | |
| Multiple organ failure | 2, 2.44% | |
| Cerebral intraventricular hemorrhage | 2, 2.44% | |
| Intermediate morbid conditions (I B) | Cerebral hemorrhage | 10, 27.03% |
| Pulmonary hemorrhage | 9, 24.32% | |
| Cerebral edema | 4, 10.81% | |
| Cerebral intraventricular hemorrhage | 3, 8.11% | |
| Hydrocephalus | 2, 5.41% | |
| Other diagnoses | 9, 24.32% | |
| Initial morbid conditions (I C) | Plurivisceral hemorrhages | 22, 25.88% |
| Cerebral hemorrhage | 16, 18.82% | |
| Pulmonary hemorrhage | 10, 11.76% | |
| Bronchopneumonia | 7, 8.24% | |
| Pneumonia | 7, 8.24% | |
| Other diagnoses | 23, 27.06% | |
| Other initial morbid conditions (I D) | Prematurity | 72, 90% |
| Plurimalformative syndrome | 2, 2.50% | |
| Disseminated coagulation syndrome | 1, 1.25% | |
| Congenital cardiac malformation | 1, 1.25% | |
| Other diagnoses | 4, 5% | |
| Other important morbid conditions (II) | Prematurity | 6, 27.27% |
| Plurivisceral hemorrhages | 4, 18.18% | |
| Enterocolitis | 2, 9.09% | |
| Pneumonia | 2, 9.09% | |
| Adrenal hemorrhages | 2, 9.09% | |
| Other diagnoses | 6, 27.30% |
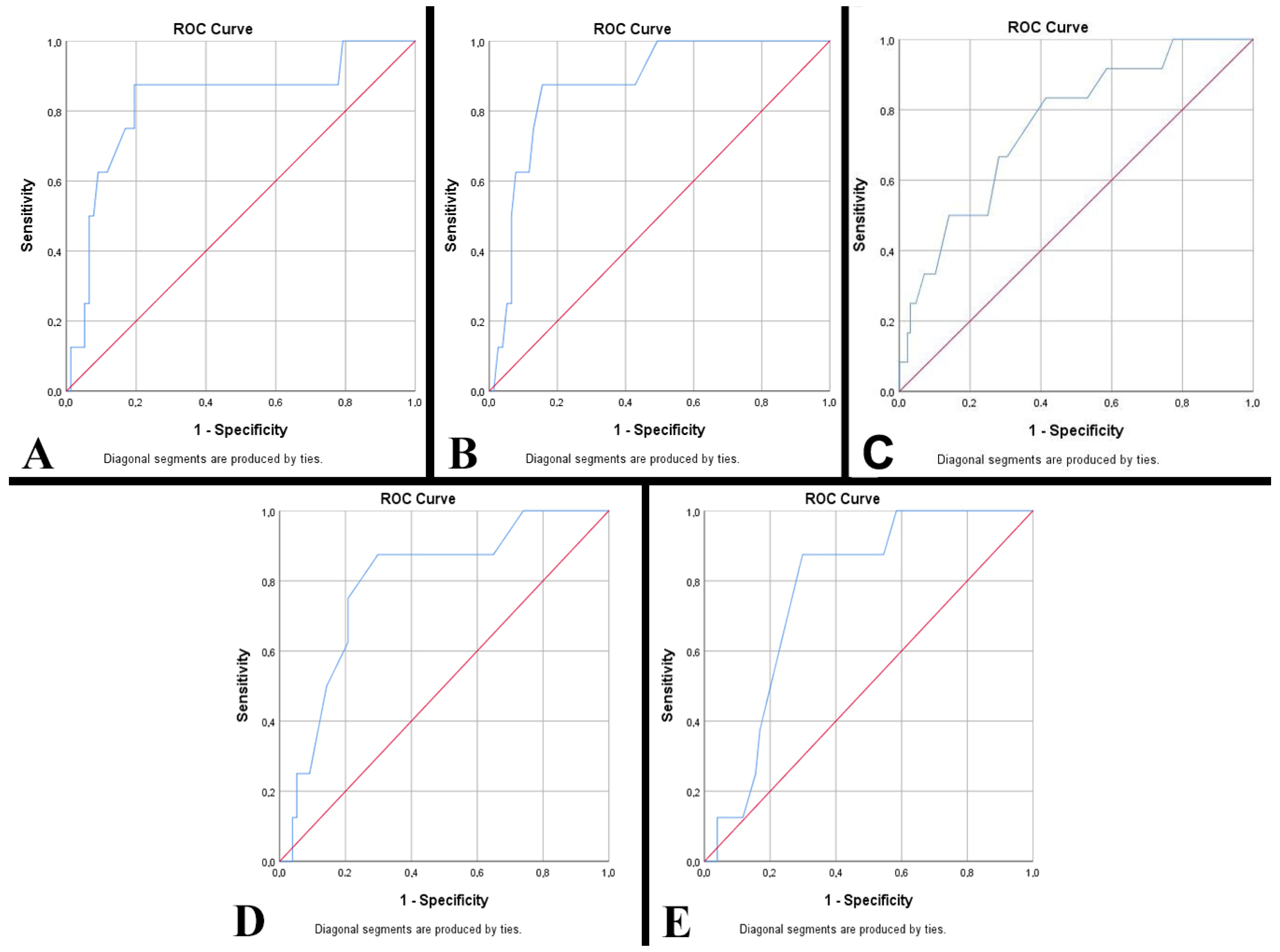
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | CI 95% | p-Value | HR | CI 95% | p-Value | |
| Lungs | ||||||
| Normal | 1 | 1 | ||||
| Pneumonia | 4.418 | 0.539–36.245 | 0.166 | 5.966 | 0.473–75.162 | 0.167 |
| Bronchopneumonia | 2.762 | 0.327–23.365 | 0.351 | 5.579 | 0.404–77.049 | 0.199 |
| Hemorrhage | 7.791 | 0.957–63.435 | 0.055 | 12.874 | 1.029–161.009 | 0.047 |
| Congestion | 5.023 | 0.286–88.147 | 0.270 | 6.585 | 0.261–166.141 | 0.252 |
| Atelectasis | 54.391 | 5.531–534.821 | 0.001 | 66.583 | 4.457–994.680 | 0.002 |
| Pulmonary immaturity | 5.421 | 0.444–66.231 | 0.186 | 8.880 | 0.503–156.911 | 0.136 |
| Acute respiratory distress syndrome | 7.754 | 0.440–136.718 | 0.162 | 14.173 | 0.582–344.865 | 0.104 |
| Heart | ||||||
| Normal | 1 | 1 | ||||
| Atrial septal defect | 0.209 | 0.028–1.575 | 0.129 | 0.160 | 0.013–1.954 | 0.151 |
| Ventricular septal defect | 1.607 | 0.219–11.819 | 0.641 | 1.346 | 0.182–9.961 | 0.771 |
| Dystrophy | 2.850 | 1.092–7.442 | 0.032 | 3.190 | 1.085–9.377 | 0.035 |
| Hemorrhage | 1.261 | 0.797–1.996 | 0.322 | 1.367 | 0.840–2.225 | 0.208 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghitoi, S.A.; Deacu, M.; Aschie, M.; Enciu, M.; Mitroi, A.F.; Cozaru, G.C.; Nicolau, A.A.; Orasanu, C.I.; Ursica, O.A.; Voda, R.I. Is It Possible to Prevent the Thanatogenetic Processes in Premature Babies? Clin. Pract. 2024, 14, 1801-1817. https://doi.org/10.3390/clinpract14050144
Ghitoi SA, Deacu M, Aschie M, Enciu M, Mitroi AF, Cozaru GC, Nicolau AA, Orasanu CI, Ursica OA, Voda RI. Is It Possible to Prevent the Thanatogenetic Processes in Premature Babies? Clinics and Practice. 2024; 14(5):1801-1817. https://doi.org/10.3390/clinpract14050144
Chicago/Turabian StyleGhitoi, Sinziana Andra, Mariana Deacu, Mariana Aschie, Manuela Enciu, Anca Florentina Mitroi, Georgeta Camelia Cozaru, Antonela Anca Nicolau, Cristian Ionut Orasanu, Oana Andreea Ursica, and Raluca Ioana Voda. 2024. "Is It Possible to Prevent the Thanatogenetic Processes in Premature Babies?" Clinics and Practice 14, no. 5: 1801-1817. https://doi.org/10.3390/clinpract14050144
APA StyleGhitoi, S. A., Deacu, M., Aschie, M., Enciu, M., Mitroi, A. F., Cozaru, G. C., Nicolau, A. A., Orasanu, C. I., Ursica, O. A., & Voda, R. I. (2024). Is It Possible to Prevent the Thanatogenetic Processes in Premature Babies? Clinics and Practice, 14(5), 1801-1817. https://doi.org/10.3390/clinpract14050144






