Current Evidence on and Clinical Implications of Vitamin D Levels in Pain and Functional Management of Knee Osteoarthritis: A Systematic Review
Abstract
:1. Introduction
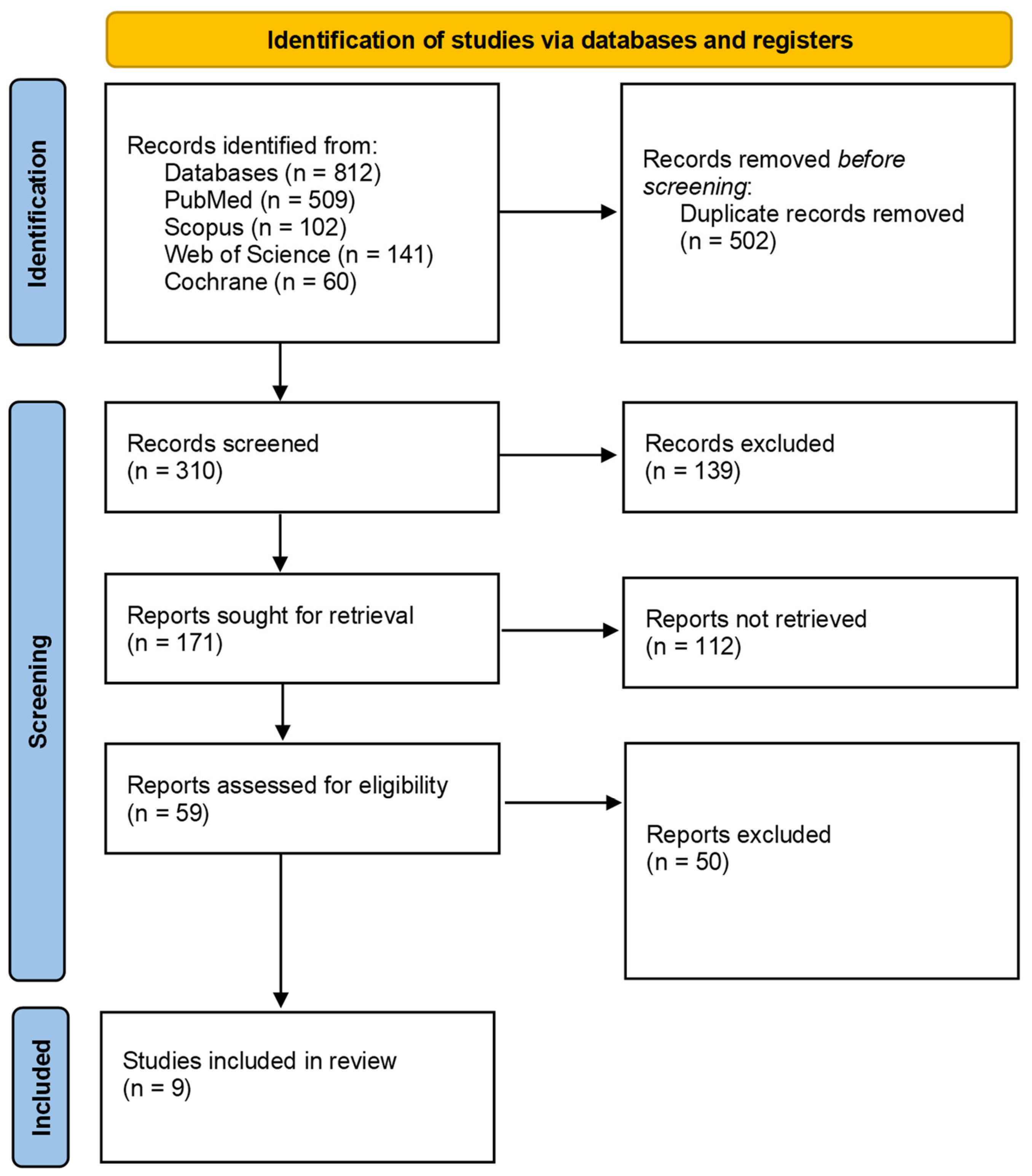
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kingsbury, S.R.; Gross, H.J.; Isherwood, G.; Conaghan, P.G. Osteoarthritis in Europe: Impact on health status, work productivity and use of pharmacotherapies in five European countries. Rheumatology 2014, 53, 937–947. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makaram, N.S.; Simpson, A.H.R.W. Disease-modifying agents in osteoarthritis: Where are we now and what does the future hold? Bone Jt. Res. 2023, 12, 654–656. [Google Scholar] [CrossRef]
- Felson, D.T. Osteoarthritis of the knee, Clinical Practice. N. Engl. J. Med. 2006, 354, 841–848. [Google Scholar] [CrossRef]
- Fontanella, C.G.; Belluzzi, E.; Pozzuoli, A.; Scioni, M.; Olivotto, E.; Reale, D.; Ruggieri, P.; De Caro, R.; Ramonda, R.; Carniel, E.L.; et al. Exploring Anatomo-Morphometric Characteristics of Infrapatellar, Suprapatellar Fat Pad, and Knee Ligaments in Osteoarthritis Compared to Post-Traumatic Lesions. Biomedicines 2022, 10, 1369. [Google Scholar] [CrossRef]
- Geng, R.; Li, J.; Yu, C.; Zhang, C.; Chen, F.; Chen, J.; Ni, H.; Wang, J.; Kang, K.; Wei, Z.; et al. Knee osteoarthritis: Current status and research progress in treatment (Review). Exp. Ther. Med. 2023, 26, 481. [Google Scholar] [CrossRef] [PubMed]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef]
- Battistelli, M.; Favero, M.; Burini, D.; Trisolino, G.; Dallari, D.; De Franceschi, L.; Goldring, S.R.; Goldring, M.B.; Belluzzi, E.; Filardo, G.; et al. Morphological and ultrastructural analysis of normal, injured and osteoarthritic human knee menisci. Eur. J. Histochem. 2019, 63, 2998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, X.; Chan, Y.T.; Yung, P.S.H.; Tuan Rocky, S.; Jiang, Y. Subchondral Bone Remodeling: A Therapeutic Target for Osteoarthritis. Front. Cell Dev. Biol. 2021, 8, 607764. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.T.; Riches, P.E.; Picard, F. The assessment of instability in the osteoarthritic knee. EFORT Open Rev. 2019, 4, 70–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghazwan, A.; Wilson, C.; Holt, C.A.; Whatling, G.M. Knee osteoarthritis alters peri-articular knee muscle strategies during gait. PLoS ONE. 2022, 17, e0262798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, N.; Yan, Z.P.; Chen, X.Y.; Ni, G.X. Infrapatellar Fat Pad and Knee Osteoarthritis. Aging Dis. 2020, 11, 1317–1328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bay-Jensen, A.C.; Reker, D.; Kjelgaard-Petersen, C.F.; Mobasheri, A.; Karsdal, M.A.; Ladel, C.; Henrotin, Y.; Thudium, C.S. Osteoarthritis year in review 2015: Soluble biomarkers and the BIPED criteria. Osteoarthr. Cartil. 2016, 24, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Convill, J.G.; Tawy, G.F.; Freemont, A.J.; Biant, L.C. Clinically Relevant Molecular Biomarkers for Use in Human Knee Osteoarthritis: A Systematic Review. Cartilage 2021, 13, 1511S–1531S. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tudorachi, N.B.; Totu, T.; Eva, I.; Bărbieru, B.; Totu, E.E.; Fifere, A.; Pinteală, T.; Sîrbu, P.-D.; Ardeleanu, V. Knee Osteoarthritis in Relation to the Risk Factors of the Metabolic Syndrome Components and Environment of Origin. J. Clin. Med. 2022, 11, 7302. [Google Scholar] [CrossRef]
- Previtali, D.; Andriolo, L.; Di Laura Frattura, G.; Boffa, A.; Candrian, C.; Zaffagnini, S.; Filardo, G. Pain Trajectories in Knee Osteoarthritis—A Systematic Review and Best Evidence Synthesis on Pain Predictors. J. Clin. Med. 2020, 9, 2828. [Google Scholar] [CrossRef]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Machrowska, A.; Karpiński, R.; Maciejewski, M.; Jonak, J.; Krakowski, P. Application of eemd-dfa algorithms and ann classification for detection of knee osteoarthritis using vibroarthrography. Appl. Comput. Sci. 2024, 20, 90–108. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M. Comparison of Selected Classification Methods Based On Machine Learning as A Diagnostic Tool for Knee Joint Cartilage Damage Based On Generated Vibroacoustic Processes. Appl. Comput. Sci. 2023, 19, 136–150. [Google Scholar] [CrossRef]
- Naoum, S. The Role of Vitamin D in the Development and Progression of Osteoarthritis. Rom. J. Mil. Med. 2023, 126, 298–305. [Google Scholar] [CrossRef]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bae, S.; Schmitt, L.C.; Burnett, Z.; Milliron, E.M.; Cavendish, P.A.; Magnussen, R.A.; Kaeding, C.C.; Flanigan, D.C.; Barker, T. Vitamin D Deficiency after Anterior Cruciate Ligament Reconstruction Associates with Knee Osteoarthritis: A Retrospective Study. Nutrients 2024, 16, 3029. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleet, J.C. Vitamin D-Mediated Regulation of Intestinal Calcium Absorption. Nutrients 2022, 14, 3351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Razzaque, M.S. Vitamin D and Phosphate Interactions in Health and Disease. Adv. Exp. Med. Biol. 2022, 1362, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Vranic, V.; Floranovic, M.; Petrovic, M.; Starcevic, S.; Supic, G. Vitamin D and Vitamin D Receptor Gene in Osteoarthritis. Exp. Appl. Biomed. Res. 2022, 23, 285–290. [Google Scholar] [CrossRef]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- Alharbi, S.S.; Albalawi, A.A., Sr.; Al Madshush, A.M.; Alsaidalani, W.M.H.; Aljohani, O.S.; Alaradi, A.R.; Alatawi, A.A.; Albalawi, R.S.; Alanazi, L.A.; Albalawi, H.S.; et al. Association Between Lower Levels of Vitamin D and Inflammation in the Geriatric Population: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e60892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, X.; Antony, B.; Wang, X.; Persson, M.S.; McAlindon, T.; Arden, N.K.; Srivastava, S.; Srivastava, R.; Van Middelkoop, M.; Bierma-Zeinstra, S.M.; et al. Effect of vitamin D supplementation on pain and physical function in patients with knee osteoarthritis (OA): An OA Trial Bank protocol for a systematic review and individual patient data (IPD) meta-analysis. BMJ Open 2020, 10, e035302. [Google Scholar] [CrossRef]
- Zhang, F.F.; Driban, J.B.; Lo, G.H.; Price, L.L.; Booth, S.; Eaton, C.B.; Lu, B.; Nevitt, M.; Jackson, B.; Garganta, C.; et al. Vitamin D deficiency is associated with progression of knee osteoarthritis. J. Nutr. 2014, 144, 2002–2008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tu, L.; Zheng, S.; Cicuttini, F.; Jin, X.; Han, W.; Zhu, Z.; Antony, B.; Winzenberg, T.; Jones, G.; Gu, J.; et al. Effects of Vitamin D Supplementation on Disabling Foot Pain in Patients With Symptomatic Knee Osteoarthritis. Arthritis Care Res. 2021, 73, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Singh, A.; Akhtar, M.; Najmi, A.K. Vitamin D supplementation for the management of knee osteoarthritis: A systematic review of randomized controlled trials. Rheumatol. Int. 2017, 37, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; He, Y.; Peng, L.H.; Luo, X.; Liu, M.; He, C.S.; Chen, J. Does vitamin D improve symptomatic and structural outcomes in knee osteoarthritis? A systematic review and meta-analysis. Aging Clin. Exp. Res. 2021, 33, 2393–2403. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Damen, J.; van Rijn, R.M.; Emans, P.J.; Hilberdink, W.K.H.A.; Wesseling, J.; Oei, E.H.G.; Bierma-Zeinstra, S.M.A. Prevalence and development of hip and knee osteoarthritis according to American College of Rheumatology criteria in the CHECK cohort. Arthritis Res. Ther. 2019, 21, 4. [Google Scholar] [CrossRef]
- Available online: https://www.hopkinsarthritis.org (accessed on 10 September 2024).
- Cueva, J.H.; Castillo, D.; Espinós-Morató, H.; Durán, D.; Díaz, P.; Lakshminarayanan, V. Detection and Classification of Knee Osteoarthritis. Diagnostics 2022, 12, 2362. [Google Scholar] [CrossRef]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar] [PubMed]
- Delgado, D.A.; Lambert, B.S.; Boutris, N.; McCulloch, P.C.; Robbins, A.B.; Moreno, M.R.; Harris, J.D. Validation of Digital Visual Analog Scale Pain Scoring With a Traditional Paper-based Visual Analog Scale in Adults. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2018, 2, e088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.zotero.org/ (accessed on 10 September 2024).
- Available online: https://www.riskofbias.info/welcome/robvis-visualization-tool (accessed on 10 September 2024).
- Heidari, B.; Javadian, Y.; Babaei, M.; Yousef-Ghahari, B. Restorative Effect of Vitamin D Deficiency on Knee Pain and Quadriceps Muscle Strength in Knee Osteoarthritis. Acta Med. Iran. 2015, 53, 466–470. [Google Scholar] [PubMed]
- Arden, N.K.; Cro, S.; Sheard, S.; Doré, C.J.; Bara, A.; Tebbs, S.A.; Hunter, D.J.; James, S.; Cooper, C.; O’Neill, T.W.; et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: A randomised controlled trial. Osteoarthr. Cartil. 2016, 24, 1858–1866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, X.; Jones, G.; Cicuttini, F.; Wluka, A.; Zhu, Z.; Han, W.; Antony, B.; Wang, X.; Winzenberg, T.; Blizzard, L.; et al. Effect of Vitamin D Supplementation on Tibial Cartilage Volume and Knee Pain Among Patients with Symptomatic Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2016, 315, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Manoy, P.; Yuktanandana, P.; Tanavalee, A.; Anomasiri, W.; Ngarmukos, S.; Tanpowpong, T.; Honsawek, S. Vitamin D Supplementation Improves Quality of Life and Physical Performance in Osteoarthritis Patients. Nutrients 2017, 9, 799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Cicuttini, F.; Jin, X.; Wluka, A.E.; Han, W.; Zhu, Z.; Blizzard, L.; Antony, B.; Winzenberg, T.; Jones, G.; et al. Knee effusion-synovitis volume measurement and effects of vitamin D supplementation in patients with knee osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Jin, X.; Cicuttini, F.; Wang, X.; Zhu, Z.; Wluka, A.; Han, W.; Winzenberg, T.; Antony, B.; Aitken, D.; et al. Maintaining Vitamin D Sufficiency Is Associated with Improved Structural and Symptomatic Outcomes in Knee Osteoarthritis. Am. J. Med. 2017, 130, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.A.; Parkes, M.J.; Hodgson, R.; Felson, D.T.; O’Neill, T.W.; Arden, N.K. Effect of Vitamin D supplementation on synovial tissue volume and subchondral bone marrow lesion volume in symptomatic knee osteoarthritis. BMC Musculoskelet. Disord. 2019, 20, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Divjak, A.; Jovanovic, I.; Matic, A.; Lucic, A.T.; Gajovic, N.; Jurisevic, M.; Skevin, A.J.; Veselinovic, M. The influence of vitamin D supplementation on the expression of mediators of inflammation in knee osteoarthritis. Immunol. Res. 2023, 71, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Saengsiwaritt, W.; Jittikoon, J.; Chaikledkaew, U.; Tawonsawatruk, T.; Honsawek, S.; Udomsinprasert, W. Effect of vitamin D supplementation on circulating level of autophagosome protein LC3A, inflammation, and physical performance in knee osteoarthritis. Clin. Transl. Sci. 2023, 16, 2543–2556, Erratum in: Clin. Transl. Sci. 2024, 17, e13856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heidari, B.; Babaei, M. Therapeutic and Preventive Potential of Vitamin D Supplementation in Knee Osteoarthritis. ACR Open Rheumatol. 2019, 1, 318–326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mabey, T.; Honsawek, S. Role of Vitamin D in Osteoarthritis: Molecular, Cellular, and Clinical Perspectives. Int. J. Endocrinol. 2015, 2015, 383918. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.J.; Lee, J.Y.; Kim, T.J.; Lee, J.W. Association between serum vitamin D status and health-related quality of life (HRQOL) in an older Korean population with radiographic knee osteoarthritis: Data from the Korean national health and nutrition examination survey (2010–2011). Health Qual Life Outcomes 2015, 13, 48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hung, M.; Bounsanga, J.; Voss, M.W.; Gu, Y.; Crum, A.B.; Tang, P. Dietary and Supplemental Vitamin C and D on Symptom Severity and Physical Function in Knee Osteoarthritis. J. Nutr. Gerontol. Geriatr. 2017, 36, 121–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alkan, G.; Akgol, G. Do vitamin D levels affect the clinical prognoses of patients with knee osteoarthritis? J. Back. Musculoskelet. Rehabil. 2017, 30, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Alabajos-Cea, A.; Herrero-Manley, L.; Suso-Martí, L.; Viosca-Herrero, E.; Cuenca-Martínez, F.; Varangot-Reille, C.; Blanco-Díaz, M.; Calatayud, J.; Casaña, J. The Role of Vitamin D in Early Knee Osteoarthritis and Its Relationship with Their Physical and Psychological Status. Nutrients 2021, 13, 4035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veronese, N.; Maggi, S.; Noale, M.; De Rui, M.; Bolzetta, F.; Zambon, S.; Corti, M.C.; Sartori, L.; Musacchio, E.; Baggio, G.; et al. Serum 25-Hydroxyvitamin D and Osteoarthritis in Older People: The Progetto Veneto Anziani Study. Rejuvenation Res. 2015, 18, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Cakar, M.; Ayanoglu, S.; Cabuk, H.; Seyran, M.; Dedeoglu, S.S.; Gurbuz, H. Association between vitamin D concentrations and knee pain in patients with osteoarthritis. PeerJ 2018, 6, e4670. [Google Scholar] [CrossRef]
- Javadian, Y.; Adabi, M.; Heidari, B.; Babaei, M.; Firouzjahi, A.; Ghahhari, B.Y.; Hajian-Tilaki, K. Quadriceps Muscle Strength Correlates with Serum Vitamin D and Knee Pain in Knee Osteoarthritis. Clin. J. Pain. 2017, 33, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Lee, J.E. Vitamin D and the characteristics associated with risk for knee pain among Korean older adults: Findings from a nationally representative survey. Geriatr. Gerontol. Int. 2017, 17, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Namutebi, F.; Kayima, J.; Kaddumukasa, M. Vitamin D and its association with symptom severity in knee osteoarthritis: A cross sectional study at a national referral hospital in Uganda. BMC Rheumatol. 2021, 5, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jacob, E.A.; Blum, L.; Bedair, H.S.; Freiberg, A.A.; Quraishi, S.A. The Association of Vitamin D Status and Pre-operative Physical Activity in Patients with Hip or Knee Osteoarthritis. J. Restor. Med. 2015, 4, 3–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Zhu, Z.; Pan, F.; Zheng, S.; Parameswaran, V.; Blizzard, L.; Ding, C.; Antony, B. Long-term effects of vitamin D supplementation and maintaining sufficient vitamin D on knee osteoarthritis over 5 years. Arthritis Res. Ther. 2023, 25, 178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, S.; Tu, L.; Cicuttini, F.; Han, W.; Zhu, Z.; Antony, B.; Wluka, A.; Winzenberg, T.; Meng, T.; Aitken, D.; et al. Effect of Vitamin D Supplementation on Depressive Symptoms in Patients With Knee Osteoarthritis. J. Am. Med. Dir. Assoc. 2019, 20, 1634–1640.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cicutinni, F.; Jin, X.; Antony, B.; Wluka, A.; Han, W.; Blizzard, L.; Winzenberg, T.; Jones, G.; Ding, C. Effect of Vitamin D on Effusion-Synovitis in Knee Osteoarthritis: A Randomized Controlled Trial. In Arthritis & Rheumatology; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Wallace, G.; Cro, S.; Doré, C.; King, L.; Kluzek, S.; Price, A.; Roemer, F.; Guermazi, A.; Keen, R.; Arden, N. Associations Between Clinical Evidence of Inflammation and Synovitis in Symptomatic Knee Osteoarthritis: A Cross-Sectional Substudy. Arthritis Care Res. 2017, 69, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Anari, H.; Enteshari-Moghaddam, A.; Abdolzadeh, Y. Association between serum Vitamin D deficiency and Knee Osteoarthritis. Mediterr. J. Rheumatol. 2020, 30, 216–219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montemor, C.N.; Fernandes, M.T.P.; Marquez, A.S.; Poli-Frederico, R.C.; da Silva, R.A.; Fernandes, K.B.P. Vitamin D deficiency, functional status, and balance in older adults with osteoarthritis. World J. Clin. Cases. 2021, 9, 9491–9499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naik, S.; Sahu, S.; Bandyopadhyay, D.; Tripathy, S. Serum levels of osteoprotegerin, RANK-L & vitamin D in different stages of osteoarthritis of the knee. Indian. J. Med. Res. 2021, 154, 491–496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tekeli, F.Y.; Tekeli, S.Ö.; Köse, Ö. Serum vitamin D receptor and fibroblast growth factor-23 levels in postmenopausal primary knee osteoarthritis patients. Turk. J. Biochem. 2022, 47, 788–794. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Gantaguru, A.; Nanda, S.N.; Velagada, S.; Srinivasan, A.; Mangaraj, M. Association of vitamin D and knee osteoarthritis in younger individuals. World J. Orthop. 2020, 11, 418–425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lotfi, A.; Abdel-Magied, R.A.; El-Shereef, R.R.; Saedii, A.A.; AbdelGawad, E.A. Relationship between serum 25-hydroxy vitamin D levels, knee pain, radiological osteoarthritis, and the Western Ontario and McMaster Universities Osteoarthritis Index in patients with primary osteoarthritis. Egypt Rheumatol. Rehabil. 2014, 41, 66–70. [Google Scholar] [CrossRef]
- Al-Jarallah, K.F.; Shehab, D.; Al-Awadhi, A.; Nahar, I.; Haider, M.Z.; Moussa, M.A. Are 25(OH)D levels related to the severity of knee osteoarthritis and function? Med. Princ. Pract. 2012, 21, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Mermerci Başkan, B.; Yurdakul, F.G.; Aydın, E.; Sivas, F.; Bodur, H. Effect of vitamin D levels on radiographic knee osteoarthritis and functional status. Turk. J. Phys. Med. Rehabil. 2017, 64, 1–7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, X.; Ding, C.; Hunter, D.J.; Gallego, B. Effectiveness of vitamin D supplementation on knee osteoarthritis—A target trial emulation study using data from the Osteoarthritis Initiative cohort. Osteoarthr. Cartil. 2022, 30, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Amini Kadijani, A.; Bagherifard, A.; Mohammadi, F.; Akbari, A.; Zandrahimi, F.; Mirzaei, A. Association of Serum Vitamin D with Serum Cytokine Profile in Patients with Knee Osteoarthritis. Cartilage 2021, 13, 1610S–1618S. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amirkhizi, F.; Asoudeh, F.; Hamedi-Shahraki, S.; Asghari, S. Vitamin D status is associated with inflammatory biomarkers and clinical symptoms in patients with knee osteoarthritis. Knee 2022, 36, 44–52. [Google Scholar] [CrossRef]
- Barker, T.; Henriksen, V.T.; Rogers, V.E.; Aguirre, D.; Trawick, R.H.; Lynn Rasmussen, G.; Momberger, N.G. Vitamin D deficiency associates with γ-tocopherol and quadriceps weakness but not inflammatory cytokines in subjects with knee osteoarthritis. Redox Biol. 2014, 2, 466–474. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Manoy, P.; Yuktanandana, P.; Tanavalee, A.; Anomasiri, W.; Honsawek, S. Decreased Serum Adiponectin Reflects Low Vitamin D, High Interleukin 6, and Poor Physical Performance in Knee Osteoarthritis. Arch. Immunol. Ther Exp. 2020, 68, 16. [Google Scholar] [CrossRef] [PubMed]
- Almalki, A.; Gharib, A.F.; Almehmadi, M.; Alharthi, A.; Alsalmi, O.; Alsulimani, A.H.; Alanazi, R.H.; AlWthenani, A.A.; Alotaibi, M.; AlZaidi, F.T. The Association of Vitamin D, Growth/Differentiation Factor 5 (GDF-5) Gene Polymorphism, and Serum GDF-5 Protein in Obese Patients With Knee Osteoarthritis. Cureus 2023, 15, e48350. [Google Scholar] [CrossRef]
- Ganguly, A. Role of Jumpstart Nutrition®, a Dietary Supplement, to Ameliorate Calcium-to-Phosphorus Ratio and Parathyroid Hormone of Patients with Osteoarthritis. Med. Sci. 2019, 7, 105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alayed Albarri, E.M.; Sameer Alnuaimi, A.; Abdelghani, D. Effectiveness of vitamin D2 compared with vitamin D3 replacement therapy in a primary healthcare setting: A retrospective cohort study. Qatar Med. J. 2022, 2022, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Risk of Bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall | ||
| Study | Heidari et al. [43] |  |  |  |  |  |  |  |  |
| Arden et al. [44] |  |  |  |  |  |  |  |  | |
| Jin et al. [45] |  |  |  |  |  |  |  |  | |
| Manoy et al. [46] |  |  |  |  |  |  |  |  | |
| Wang et al. [47] |  |  |  |  |  |  |  |  | |
| Zheng et al. [48] |  |  |  |  |  |  |  |  | |
| Perry et al. [49] |  |  |  |  |  |  |  |  | |
| Divjak et al. [50] |  |  |  |  |  |  |  |  | |
| Saengsiwaritt et al. [51] |  |  |  |  |  |  |  |  | |
| D1: Random sequence generation D2: Allocation concealment D3: Blinding of participants and personnel D4: Blinding of outcome assessment D5: Incomplete outcome data D6: Selective reporting D7: Other source of bias | Judgement:  High High  Unclear Unclear  Low Low | ||||||||
| PubMed | Scopus | Web of Science | Cochrane | Total | |
|---|---|---|---|---|---|
| Vitamin D AND knee osteoarthritis | 193 | 76 | 109 | 54 | 432 |
| Vitamin D AND knee pain | 129 | 20 | 22 | 6 | 177 |
| Cholecalciferol AND knee osteoarthritis | 25 | 0 | 0 | 0 | 25 |
| Cholecalciferol AND knee pain | 20 | 0 | 0 | 0 | 20 |
| 25-(OH)D AND knee osteoarthritis | 37 | 0 | 3 | 0 | 40 |
| 25-(OH)D AND knee pain | 18 | 0 | 0 | 0 | 18 |
| 25-Hydroxyvitamin D AND knee osteoarthritis | 56 | 3 | 3 | 0 | 62 |
| 25-Hydroxyvitamin D AND knee pain | 31 | 3 | 4 | 0 | 38 |
| TOTAL | 509 | 102 | 141 | 60 | 812 |
| Authors | Study Design | No. of Patients | Gender | Age (Years) | Dosage | Type of Vitamin | Timing of Treatment | Treatment Duration |
|---|---|---|---|---|---|---|---|---|
| Heidari et al. [43] | Prospective double-blind, placebo-controlled, randomized trial | 67 | 86.5% female | 50 ± 6.6 | 50,000 IU | D3 cholecalciferol | Weekly | 2 moths |
| Arden et al. [44] | Randomized double-blind, placebo-controlled trial | 474 | 61% female | 64.0 ± 8.0 | 800 IU | D3 cholecalciferol | Daily | 3 years |
| Jin et al. [45] | Randomized double-blind, placebo-controlled trial | 413 | 50% female | 63.2 | 50,000 IU | D3 cholecalciferol | Monthly | 2 years |
| Manoy et al. [46] | Controlled before–after study | 175 | 90% female | 64.58 ± 0.55 | 40,000 IU | D2 ergocalciferol | Weekly | 6 months |
| Wang et al. [47] | Post hoc analysis of a randomized double-blind, placebo-controlled trial | 413 | 50% female | 63 ± 7 | 50,000 IU | D3 cholecalfiferol | Monthly | 2 years |
| Zheng et al. [48] | Post hoc analysis of a randomized double-blind, placebo-controlled trial | 340 | 50% female | 63.2 | 50,000 IU | D3 cholecalciferol | Monthly | 2 years |
| Perry et al. [49] | Randomized double-blind, placebo-controlled trial | 50 | 74% female | 63.3 | 800 IU | D3 cholecalciferol | Daily | 2 years |
| Divjak et al. [50] | Open-label clinical trial | 80 | 58% female | 57.1 | 4000 IU | D3 cholecalciferol | Daily | 3 months |
| Saengsiwaritt et al. [51] | Prospective single-site, single-arm, nonrandomized interventional trial | 175 | 90.3% female | 65 | 40,000 IU | D2 ergocalciferol | Weekly | 6 months |
| Authors | Outcomes Measured | Results after Treatment |
|---|---|---|
| Heidari et al. (2014) [43] | - 25-Hydroxyvitamin D level - Knee pain: VAS, WOMAC pain scale - QMS: dynamometer | - 25-Hydroxyvitamin D level increased - WOMAC and VAS decreased - QMS increased |
| Arden et al. (2016) [44] | - Knee pain: VAS, WOMAC - JSN (X-rays) - 25-Hydroxyvitamin D level | - WOMAC pain decreased in vitamin D group and increased in placebo group - WOMAC stiffness decreased in both groups and WOMAC function increased in both groups - No significant change in JSN - 25-Hydroxyvitamin D level increased in vitamin D group and decreased in placebo group |
| Jin et al. (2016) [45] | - WOMAC pain score - Tibial cartilage volume (MRI) - Cartilage defects and BML (MRI) - 25-Hydroxyvitamin D level | - No significant differences in WOMAC pain score - No significant differences in change in tibial cartilage volume and BML - 25-Hydroxyvitamin D level increased more in the vitamin D group |
| Manoy et al. (2017) [46] | - WOMAC and VAS - Quality of life (SF-12) - Physical activity (PAQ-EJ) - Muscle strength (dynamometer) - Physical performance (4 m gait speed test, TUGT, STS, 6MWT); - 25-Hydroxyvitamin D level | - No significant change in WOMAC; VAS decreased - SF-12 improved significantly - No significant change in PAQ-EJ score - No significant improvement in knee extension force - Gait speed, TUGT, STS, and 6MWT improved - 25-Hydroxyvitamin D level increased significantly |
| Wang et al. (2017) [47] | - Volume of knee effusion synovitis (MRI) - 25-Hydroxyvitamin D level | - Clinical improvements in effusion synovitis volume - Serum 25-hydroxyvitamin D level increased in vitamin D group |
| Zheng et al. (2017) [48] | - WOMAC score - Structural changes in knee (MRI) | - No differences in WOMAC pain and stiffness - Significant differences in WOMAC physical function - Less loss of total tibial cartilage volume in participants with consistently sufficient vitamin D compared with participants with consistently insufficient vitamin D [33] |
| Perry et al. (2019) [49] | - STV and BML - 25-Hydroxyvitamin D level | - No significant changes in STV and subchondral BML - Serum vitamin D3 levels increased significantly in the vitamin D group and decreased significantly in placebo group |
| Divjak et al. (2023) [50] | - WOMAC and VAS - Measurement of cytokine levels - 25-Hydroxyvitamin D level | - In the vitamin D supplementation group, the VAS and WOMAC pain score decreased; the WOMAC pain score increased in the no-supplementation group; the WOMAC stiffness score increased in the vitamin D group and decreased in the no-supplementation group; the WOMAC function score decreased in the vitamin D group and decreased in the no-supplementation group; VAS decreased in the vitamin D group and in the no-supplementation group - IL-1β, IL-23, and IL-33 increased; TNF-α, IL-13, and IL-17 decreased - 25-Hydroxivitamin D level increased in the vitamin D supplementation group |
| Saengsiwaritt et al. (2023) [51] | - VAS and WOMAC - Muscle strength (dynamometer) - Physical performance - 25-Hydroxyvitamin D level | - VAS decreased significantly - Significant improvements in muscle strength (grip strength and knee extension force) - Physical performance increased (gait speed, TUGT, STS, and 6MWT) - 25-Hydroxyvitamin D level increased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgescu, B.; Cristea, A.E.; Oprea, D.; Lupu, A.A.; Stanciu, L.-E.; Borgazi, E.; Caraban, B.M.; Ciortea, V.M.; Irsay, L.; Iliescu, M.G. Current Evidence on and Clinical Implications of Vitamin D Levels in Pain and Functional Management of Knee Osteoarthritis: A Systematic Review. Clin. Pract. 2024, 14, 1997-2012. https://doi.org/10.3390/clinpract14050158
Georgescu B, Cristea AE, Oprea D, Lupu AA, Stanciu L-E, Borgazi E, Caraban BM, Ciortea VM, Irsay L, Iliescu MG. Current Evidence on and Clinical Implications of Vitamin D Levels in Pain and Functional Management of Knee Osteoarthritis: A Systematic Review. Clinics and Practice. 2024; 14(5):1997-2012. https://doi.org/10.3390/clinpract14050158
Chicago/Turabian StyleGeorgescu, Bianca, Adelina Elena Cristea, Doinița Oprea, Andreea Alexandra Lupu, Liliana-Elena Stanciu, Erdin Borgazi, Bogdan Marian Caraban, Viorela Mihaela Ciortea, Laszlo Irsay, and Mădălina Gabriela Iliescu. 2024. "Current Evidence on and Clinical Implications of Vitamin D Levels in Pain and Functional Management of Knee Osteoarthritis: A Systematic Review" Clinics and Practice 14, no. 5: 1997-2012. https://doi.org/10.3390/clinpract14050158






